Secondary Metabolites from Fungi Microsphaeropsis spp.: Chemistry and Bioactivities
Abstract
:1. Introduction
2. Types of Chemical Structures
2.1. Polyketones
2.2. Terpenoids
2.3. Macrolide
2.4. Nitrogen Compounds
2.5. Other Classes
3. Biological Activities
3.1. Antifungal Activity
3.2. Antibacterial Activity
3.3. Cytotoxic Activity
3.4. Other Biological Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, H.; Gao, Z.Q.; Liu, S.Y.; Xu, H.; Chen, J.; Yang, L.J. Subcutaneous phaeohyphomycosis caused by Microsphaeropsis arundinis: A case report and review of the literatures. Chin. J. Mycol. 2022, 17, 220–224. [Google Scholar]
- Mohamed-Azni, I.N.A.; Sritharan, K.; Ho, S.-H.; Roslan, N.D.; Arulandoo, X.; Sundram, S. Isolation, identification and pathogenicity of fungi associated with leaf blotches in Tenera x Tenera (TxT) variety of oil palm in Malaysia. J. Plant Pathol. 2022, 104, 167–177. [Google Scholar] [CrossRef]
- Webster, J. Coelomycetes VI. Nomenclature of generic names proposed for Coelomycetes. By BC Sutton. Trans. Br. Mycol. Soc. 1978, 70, 489. [Google Scholar]
- Sutton, B.C. The Coelomycetes. Fungi imperfecto with pycnidia, acervuli and stromata. Australas. Plant Pathol. 1980, 9, 120–121. [Google Scholar]
- Zeng, H.T.; Ye, L.; Yi, W.T.; Chen, J.Y.; Xu, S.S.; Zhao, L.J.; Zeng, X. Study on secondary metabolites of endophytic fungus Talaromyces sp. from Uncaria rhynchophylla. Nat. Prod. Res. Dev. 2022, 34, 1707–1712. [Google Scholar]
- Schmit, J.P.; Mueller, G.M. An estimate of the lower limit of global fungal diversity. Biodivers. Conserv. 2007, 16, 99–111. [Google Scholar] [CrossRef]
- Qin, S.-H.; Zhao, L.-X.; Yang, Y.-B.; Hu, M.; Ding, Z.-T. A New Isochroman Derivative from the Endophytic Microsphaeropsis arundinis. Chem. Nat. Compd. 2017, 53, 877–879. [Google Scholar] [CrossRef]
- Botero, W.B.; De Amorim, M.; Carlos, I.; Polesi, M.; Dos Santos, L. Aromatic Polyketides and Macrolides from Microsphaeropsis arundinis. J. Braz. Chem. Soc. 2020, 31, 364–369. [Google Scholar] [CrossRef]
- Höller, U.; König, G.M.; Wright, A.D. Three New Metabolites from Marine-Derived Fungi of the Genera Coniothyrium and Microsphaeropsis. J. Nat. Prod. 1999, 62, 114–118. [Google Scholar] [CrossRef]
- Fukami, A.; Taniguchi, Y.; Nakamura, T.; Rho, M.-C.; Kawaguchi, K.; Hayashi, M.; Komiyama, K.; Omura, S. New members of the macrosphelides from Microsphaeropsis sp. FO-5050IV. J. Antibiot. 1999, 52, 501–504. [Google Scholar] [CrossRef]
- Brauers, G.; Edrada, R.A.; Ebel, R.; Proksch, P.; Wray, V.; Berg, A.; Gräfe, U.; Schächtele, C.; Totzke, F.; Finkenzeller, G.; et al. Anthraquinones and Betaenone Derivatives from the Sponge-Associated Fungus Microsphaeropsis Species: Novel Inhibitors of Protein Kinases. J. Nat. Prod. 2000, 63, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Seephonkai, P.; Isaka, M.; Kittakoop, P.; Palittapongarnpim, P.; Kamchonwongpaisan, S.; Tanticharoen, M.; Thebtaranonth, Y. Evaluation of Antimycobacterial, Antiplasmodial and Cytotoxic Activities of Preussomerins Isolated from the Lichenicolous Fungus Microsphaeropsis sp. BCC 3050. Planta Med. 2002, 68, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-F.; Cai, S.-Y.; Hao, X.-M.; Cao, F.; Zhu, H.-J. Alkaloids and Butyrolactones from a Marine-Derived Microsphaeropsis sp. Fungus. Chem. Nat. Compd. 2018, 54, 402–404. [Google Scholar] [CrossRef]
- Luo, J.; Liu, X.; Li, E.; Guo, L.; Che, Y. Arundinols A–C and Arundinones A and B from the Plant Endophytic Fungus Microsphaeropsis arundinis. J. Nat. Prod. 2013, 76, 107–112. [Google Scholar] [CrossRef]
- Sommart, U.; Rukachaisirikul, V.; Tadpetch, K.; Sukpondma, Y.; Phongpaichit, S.; Hutadilok-Towatana, N.; Sakayaroj, J. Modiolin and phthalide derivatives from the endophytic fungus Microsphaeropsis arundinis PSU-G18. Tetrahedron 2012, 68, 10005–10010. [Google Scholar] [CrossRef]
- Yoganathan, K.; Cao, S.; Crasta, S.C.; Aitipamula, S.; Whitton, S.R.; Ng, S.; Buss, A.D.; Butler, M.S. Microsphaerins A–D, four novel benzophenone dimers with activity against MRSA from the fungus Microsphaeropsis sp. Tetrahedron 2008, 64, 10181–10187. [Google Scholar] [CrossRef]
- Hormazabal, E.; Astudillo, L.; Schmeda-Hirschmann, G.; Rodríguez, J.; Theoduloz, C. Metabolites from Microsphaeropsis olivacea, an Endophytic Fungus of Pilgerodendron uviferum. Z. Naturforschung C 2005, 60, 11–21. [Google Scholar] [CrossRef]
- Dai, J.; Krohn, K.; Elsässer, B.; Flörke, U.; Draeger, S.; Schulz, B.; Pescitelli, G.; Salvadori, P.; Antus, S.; Kurtán, T. Metabolic products of the endophytic fungus Microsphaeropsis sp. from Larix decidua. Eur. J. Org. Chem. 2007, 2007, 4845–4854. [Google Scholar] [CrossRef]
- Hussain, H.; Ahmed, I.; Schulz, B.; Draeger, S.; Flörke, U.; Pescitelli, G.; Krohn, K. Solid-state circular dichroism and hydrogen bonding: Absolute configuration of massarigenin A from Microsphaeropsis sp. Chirality 2011, 23, 617–623. [Google Scholar] [CrossRef]
- Krohn, K.; Kouam, S.F.; Kuigoua, G.M.; Hussain, H.; Cludius-Brandt, S.; Flörke, U.; Kurtán, T.; Pescitelli, G.; Di Bari, L.; Draeger, S.; et al. Xanthones and oxepino[2, 3-b]chromones from three endophytic fungi. Chem. Eur. J. 2009, 15, 12121–12132. [Google Scholar] [CrossRef]
- Liu, Y.F.; Zhang, Y.H.; Shao, C.L.; Cao, F.; Wang, C.Y. Microketides A and B, polyketides from a gorgonian-derived Microsphaeropsis sp. fungus. J. Nat. Prod. 2020, 83, 1300–1304. [Google Scholar] [CrossRef]
- Fukami, A.; Nakamura, T.; Kim, Y.-P.; Shiomi, K.; Hayashi, M.; Nagai, T.; Yamada, H.; Komiyama, K.; Omura, S. A new anti-influenza virus antibiotic, 10-norparvulenone from Microsphaeropsis sp. FO-5050. J. Antibiot. 2000, 53, 1215–1218. [Google Scholar] [CrossRef] [PubMed]
- Quang, T.H.; Kim, D.C.; Van Kiem, P.; Van Minh, C.; Nhiem, N.X.; Tai, B.H.; Yen, P.H.; Ngan, N.T.T.; Kim, H.J.; Oh, H. Macrolide and phenolic metabolites from the marine-derived fungus Paraconiothyrium sp. VK-13 with anti-inflammatory activity. J. Antibiot. 2018, 71, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Kim, Y.-P.; Hiraoka, H.; Natori, M.; Takamatsu, S.; Kawakubo, T.; Masuma, R.; Komiyama, K.; Omura, S. Macrosphelide, a Novel Inhibitor of Cell-cell Adhesion Molecule. I. Taxonomy, Fermentation, Isolation and Biological Activities. J. Antibiot. 1995, 48, 1435–1439. [Google Scholar] [CrossRef]
- Takamatsu, S.; Hiraoka, H.; Kim, Y.-P.; Hayashi, M.; Natori, M.; Komiyama, K.; Omura, S. Macrosphelides C and D, Novel Inhibitors of Cell Adhesion. J. Antibiot. 1997, 50, 878–880. [Google Scholar] [CrossRef]
- Lam, Y.K.T.; Hensens, O.D.; Ransom, R.; Giacobbe, R.A.; Polishook, J.; Zink, D. L-755,807, A new non-peptide bradykinin binding inhibitor from an endophytic Microsphaeropsis sp. Tetrahedron 1996, 52, 1481–1486. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, B.G.; Brauers, G.; Guan, H.-S.; Proksch, P.; Ebel, R. Microsphaerones A and B, two novel γ-pyrone derivatives from the sponge-derived fungus Microsphaeropsis sp. J. Nat. Prod. 2002, 65, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Rinehart, K.L.; Jares-Erijman, E.A. Ophidiacerebrosides: Cytotoxic glycosphingolipids containing a novel sphingosine from a sea star. J. Org. Chem. 1994, 59, 144–147. [Google Scholar] [CrossRef]
- Funabashi, Y.; Horiguchi, T.; Iinuma, S.; Tanida, S.; Harada, S. TAN-1496-A, TAN-1496-C and TAN-1496-E, diketopiperazine antibiotics with inhibitory activity against mammalian DNA topoisomerase-I. J. Antibiot. 1994, 47, 1202–1218. [Google Scholar] [CrossRef]
- Yu, C.M.; Jonathan, M.C.; Jeffrey, L.C.; Ayer, S.W. An unusual fatty acid and its glyceride from the marine fungus Microsphaeropis olivacea. Can. J. Chem. 1996, 74, 730–735. [Google Scholar] [CrossRef]
- Hussain, H.; Root, N.; Jabeen, F.; Al-Harrasi, A.; Ahmad, M.; Mabood, F.; Hassan, Z.; Shah, A.; Green, I.R.; Schulz, B.; et al. Microsphaerol and Seimatorone: Two New Compounds Isolated from the Endophytic Fungi, Microsphaeropsis sp. and Seimatosporium sp. Chem. Biodivers. 2015, 12, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Harms, V.; Kirschning, A.; Dickschat, J.S. Nature-driven approaches to non-natural terpene analogues. Nat. Prod. Rep. 2020, 37, 1080–1097. [Google Scholar] [CrossRef]
- Jagatap, V.R.; Ahmad, I.; Patel, H.M. Recent updates in natural terpenoids as potential anti-mycobacterial agents. Indian J. Tuberc. 2022, 69, 282–304. [Google Scholar] [CrossRef] [PubMed]
- Belakhov, V.V.; Garabadzhiu, A.V.; Chistyakova, T.B. Polyene macrolide antibotic derivatives: Preparation, overcoming drug resistance, and prospects for use in medical practice (Review). Pharm. Chem. J. 2019, 52, 890–901. [Google Scholar] [CrossRef]
- Wang, C.; Du, W.; Lu, H.; Lan, J.; Liang, K.; Cao, S. A Review: Halogenated Compounds from Marine Actinomycetes. Molecules 2021, 26, 2754. [Google Scholar] [CrossRef] [PubMed]
- Yayehrad, A.T.; Wondie, G.B.; Marew, T. Different Nanotechnology Approaches for Ciprofloxacin Delivery Against Multidrug-Resistant Microbes. Infect. Drug Resist. 2022, 15, 413–426. [Google Scholar] [CrossRef]
- Sharma, D.; Patel, R.P.; Zaidi, S.T.R.; Sarker, M.R.; Lean, Q.Y.; Ming, L.C. Interplay of the Quality of Ciprofloxacin and Antibiotic Resistance in Developing Countries. Front. Pharmacol. 2017, 8, 546. [Google Scholar] [CrossRef]
- Shariati, A.; Arshadi, M.; Khosrojerdi, M.A.; Abedinzadeh, M.; Ganjalishahi, M.; Maleki, A.; Heidary, M.; Khoshnood, S. The resistance mechanisms of bacteria against ciprofloxacin and new approaches for enhancing the efficacy of this antibiotic. Front. Public Health 2022, 10, 1025633. [Google Scholar] [CrossRef]
- Keusgen, M.; Yu, C.-M.; Curtis, J.M.; Brewer, D.; Ayer, S.W. A cerebroside from the marine fungus Microsphaeropsis olivacea (Bonord.) Höhn. Biochem. Syst. Ecol. 1996, 24, 465–468. [Google Scholar] [CrossRef]
- Salerno, S.; Da Settimo, F.; Taliani, S.; Simorini, F.; La Motta, C.; Fornaciari, G.; Marini, A.M. Recent Advances in the Development of Dual Topoisomerase I and II Inhibitors as Anticancer Drugs. Curr. Med. Chem. 2010, 17, 4270–4290. [Google Scholar] [CrossRef]
- Duraivelan, K.; Samanta, D. Emerging roles of the nectin family of cell adhesion molecules in tumour-associated pathways. Biochim. Biophys. Acta BBA Rev. Cancer 2021, 1876, 188589. [Google Scholar] [CrossRef]
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef]
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell adhesion in cancer: Beyond the migration of single cells. J. Biol. Chem. 2020, 295, 2495–2505. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H. The Roles of Junctional Adhesion Molecules (JAMs) in Cell Migration. Front. Cell Dev. Biol. 2022, 10, 843671. [Google Scholar] [CrossRef]
- Takamatsu, S.; Kim, Y.P.; Hayashi, M.; Hiraoka, H.; Natori, M.; Komiyama, K.; Omura, S. Macrosphelide, a novel inhibitor of cell-cell adhesion molecule.2. Physicochemical properties and structural elucidation. J. Antibiot. 1996, 49, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Zink, D.L.; Liesch, J.M.; Ball, R.G.; Goetz, M.A.; Bolessa, E.A.; Giacobbe, R.A.; Silverman, K.C.; Bills, G.F.; Pelaez, F.; et al. Preussomerins and Deoxypreussomerins: Novel Inhibitors of Ras Farnesyl-Protein Transferase. J. Org. Chem. 1994, 59, 6296–6302. [Google Scholar] [CrossRef]
- Schueffler, A.; Anke, T. Fungal natural products in research and development. Nat. Prod. Rep. 2014, 31, 1425–1448. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism—From biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.B.; Bethe, B.; Hofs, R.; Zeeck, A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. ChemBioChem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Peng, X.-Y.; Wu, J.-T.; Shao, C.-L.; Li, Z.-Y.; Chen, M.; Wang, C.-Y. Co-culture: Stimulate the metabolic potential and explore the molecular diversity of natural products from microorganisms. Mar. Life Sci. Technol. 2021, 3, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Pfannenstiel, B.T.; Keller, N.P. On top of biosynthetic gene clusters: How epigenetic machinery influences secondary metabolism in fungi. Biotechnol. Adv. 2019, 37, 107345. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jin, S.; Lu, Q.; Chen, Y. Advances and Challenges in CRISPR/Cas-Based Fungal Genome Engineering for Secondary Metabolite Production: A Review. J. Fungi 2023, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Bai, J.; Yan, D.; Bao, X.; Li, W.; Liu, B.; Zhang, D.; Qi, X.; Yu, D.; Hu, Y. Genome mining combined metabolic shunting and OSMAC strategy of an endophytic fungus leads to the production of diverse natural products. Acta Pharm. Sin. B 2021, 11, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zeng, H.-C.; Zou, Y. Divergent Biosynthesis of Fungal Dioxafenestrane Sesquiterpenes by the Cooperation of Distinctive Baeyer–Villiger Monooxygenases and α-Ketoglutarate-Dependent Dioxygenases. ACS Catal. 2021, 11, 948–957. [Google Scholar] [CrossRef]
- Wu, M.; Gong, D.-C.; Yang, Q.; Zhang, M.-Q.; Mei, Y.-Z.; Dai, C.-C. Activation of Naringenin and Kaempferol through Pathway Refactoring in the Endophyte Phomopsis Liquidambaris. ACS Synth. Biol. 2021, 10, 2030–2039. [Google Scholar] [CrossRef]
- Ben Slama, H.; Bouket, A.C.; Alenezi, F.N.; Pourhassan, Z.; Golińska, P.; Oszako, T.; Belbahri, L. Potentials of Endophytic Fungi in the Biosynthesis of Versatile Secondary Metabolites and Enzymes. Forests 2021, 12, 1784. [Google Scholar] [CrossRef]
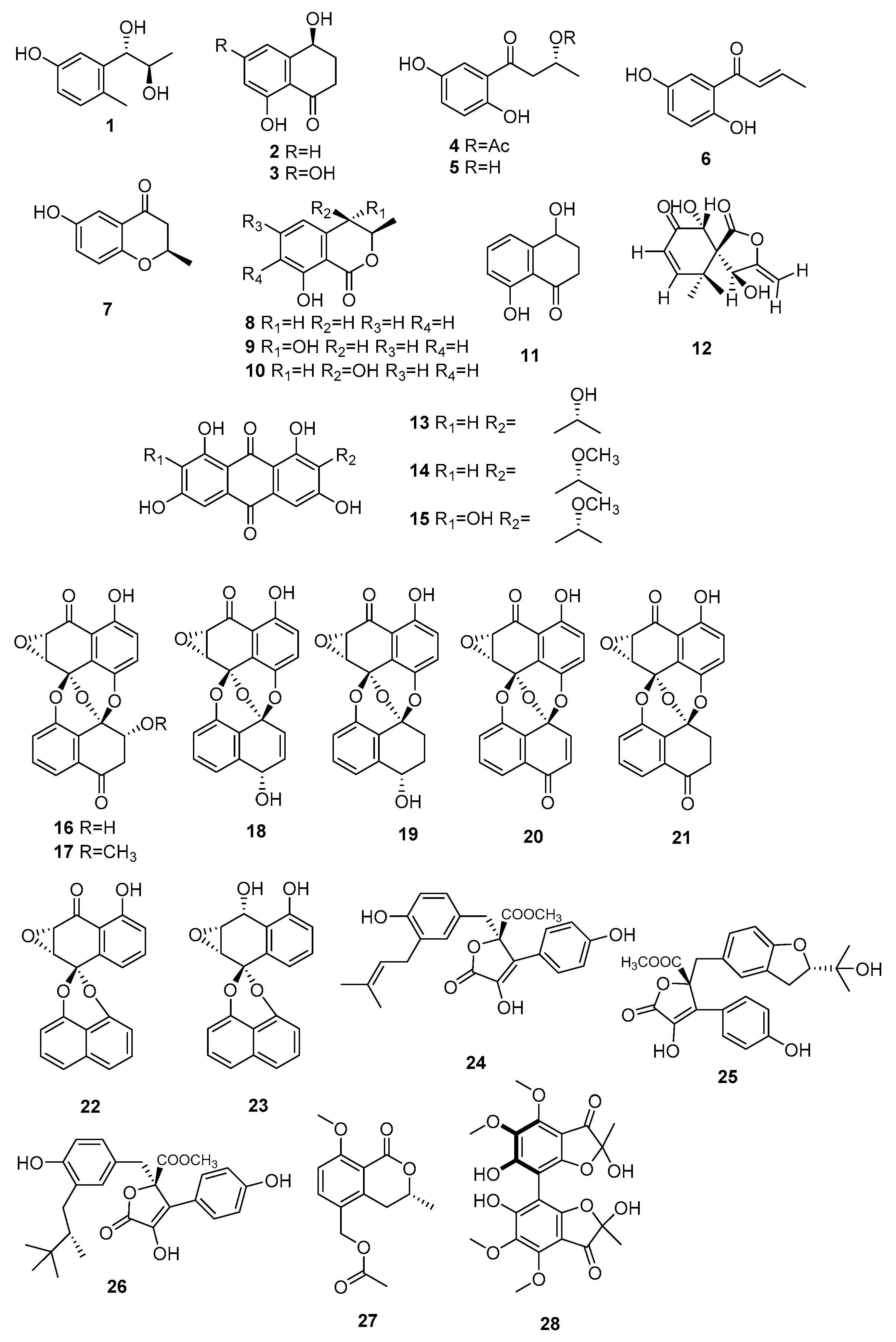
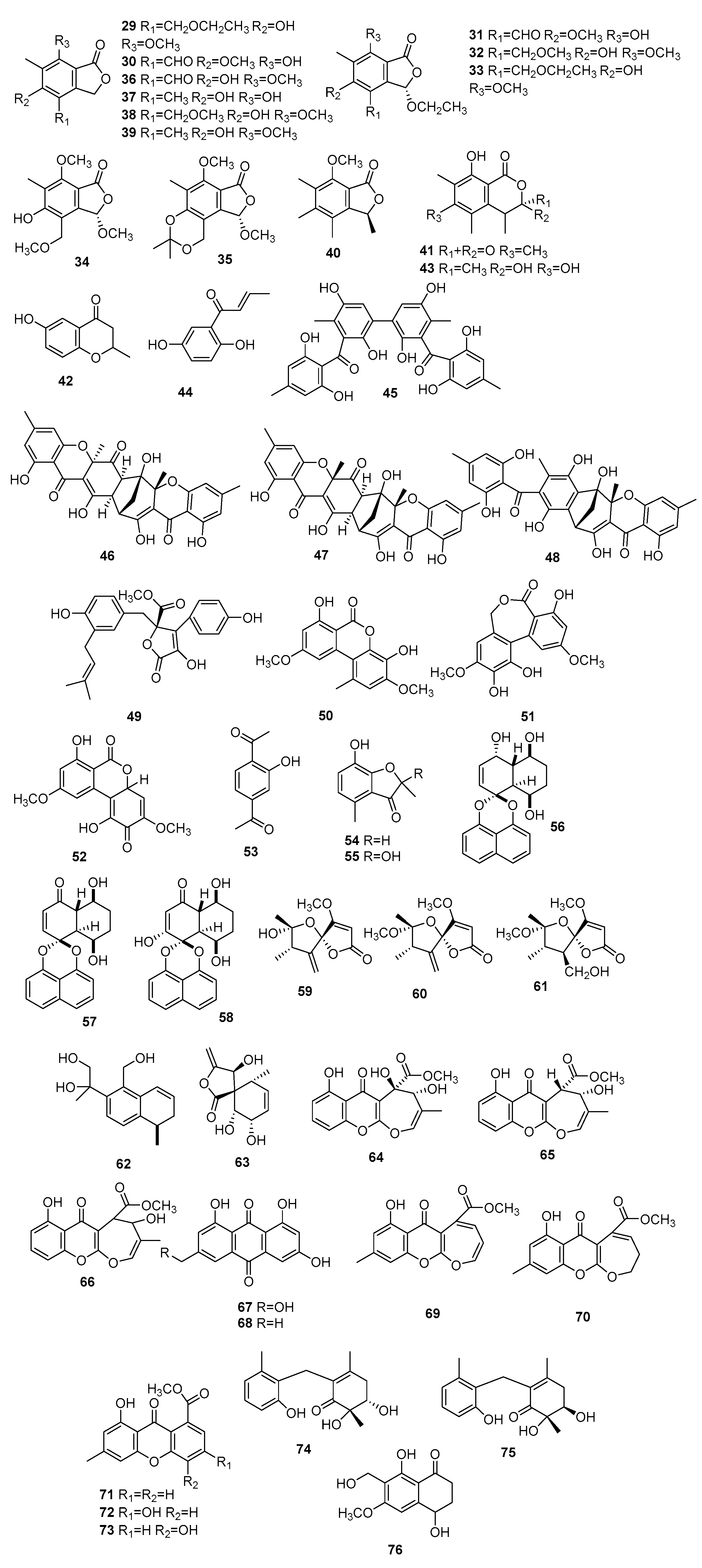
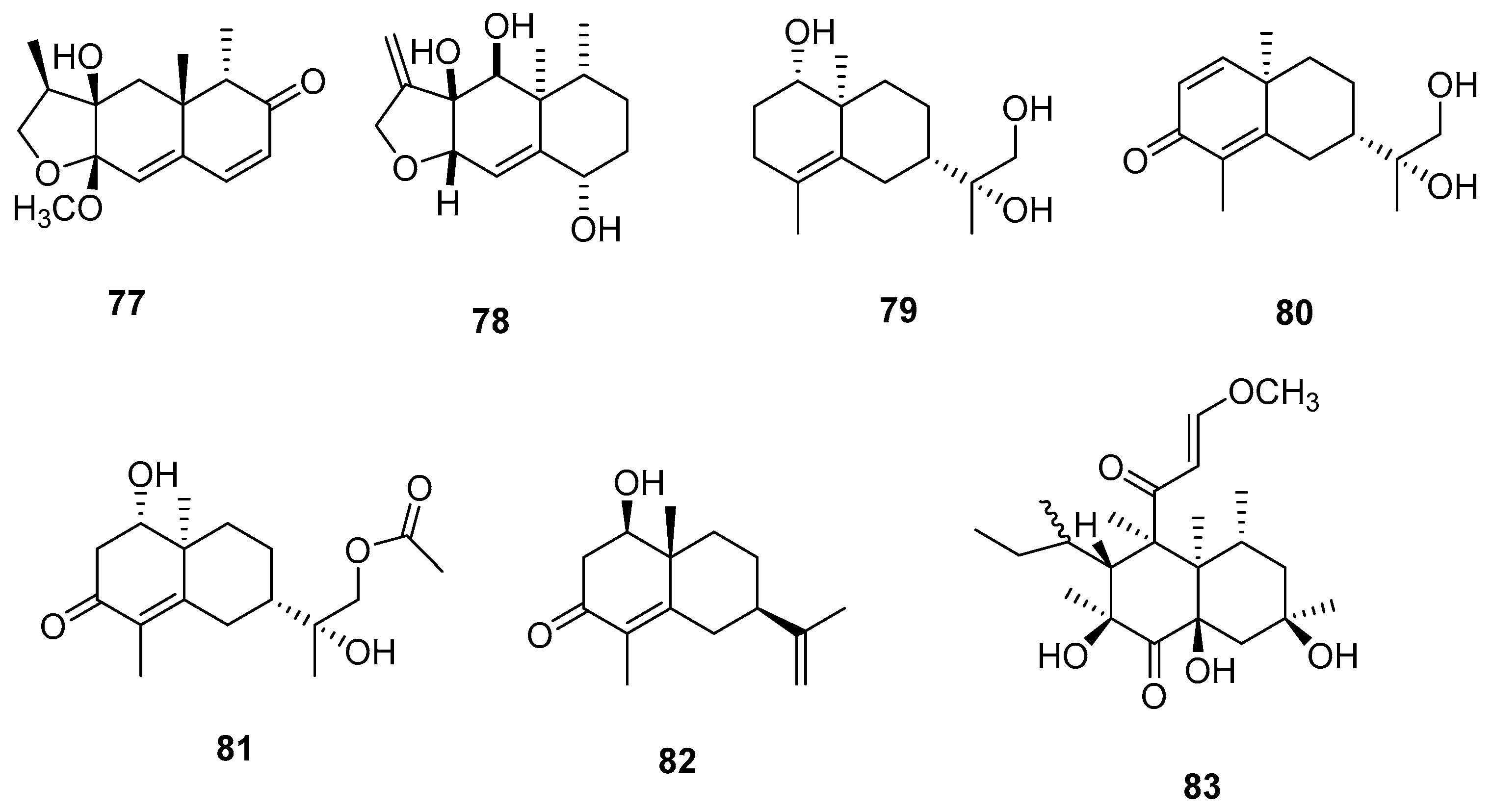

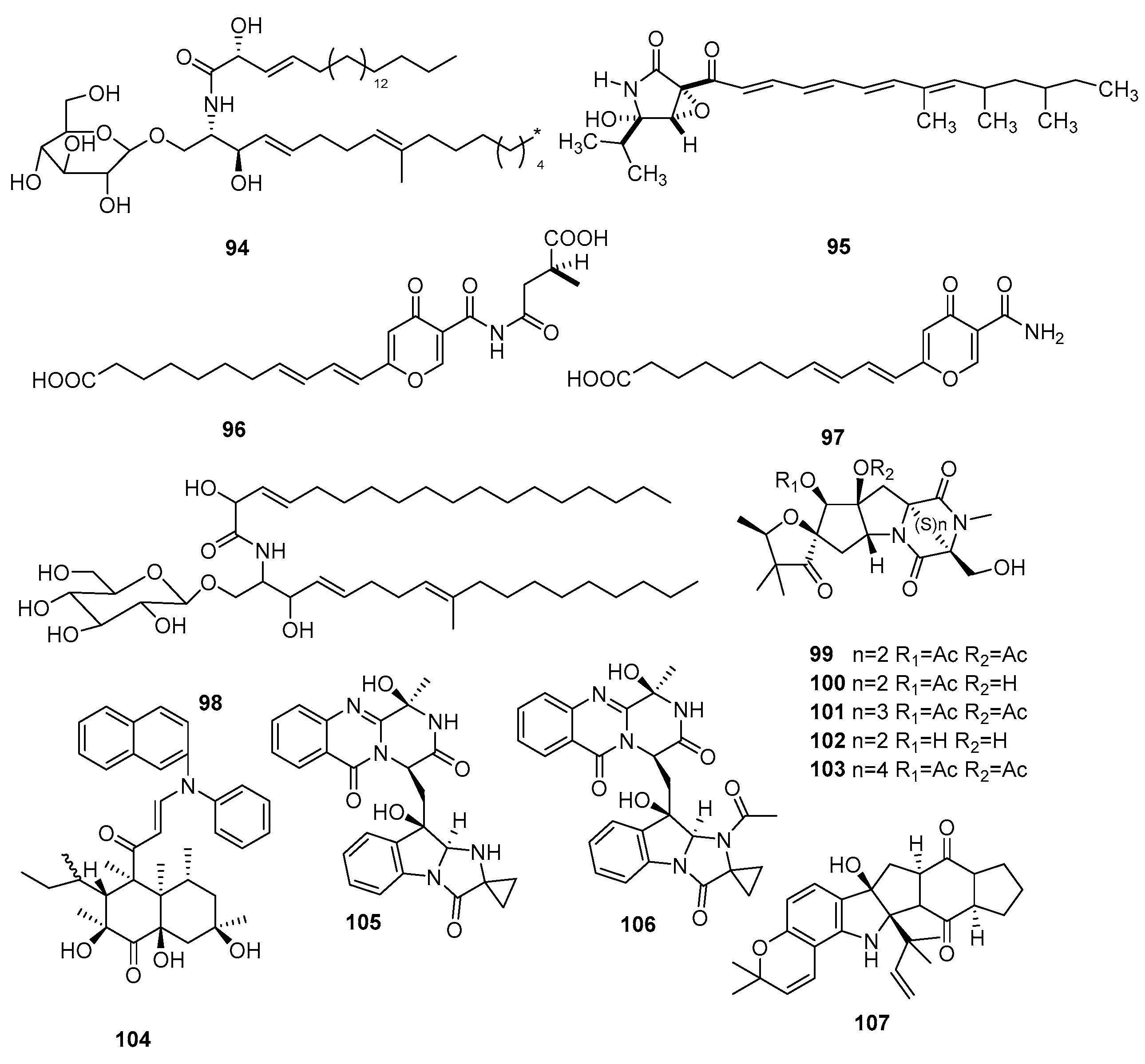

| No. | Chemical Name | Source | Bioactivities/Inactivity | Ref. |
|---|---|---|---|---|
| 1 | Erythro-2-methyl-5-hydroxyphenylpropane-7,8-diol | M. arundinis PH 30472 | Antifungal activity against Alternaria. tenuissima | [7] |
| 2 | (4S)-4,8-dihydroxy-3,4-dihydro-1(2H)-naphthaln-1-one | Inactive | ||
| 3 | (4S)-4,6,8-trihydroxy-3,4-dihydro-1(2H)-naphthalen-1-one | Inactive | ||
| 4 | (R)-1′-(2,5-dihydroxyphenyl)-1′-oxobutan-3′-ylacetate | M. arundinis | Inactive | [8] |
| 5 | (R)-1-(2,5-dihydroxyphenyl)-3-hydroxybutanone | Anti-inflammatory activity and cytotoxicity activity against LM3 | ||
| 6 | 1-(2,5-dihydroxyphenyl)-2-buten-1-one | Anti-inflammatory activity and cytotoxicity activity against LM3 | ||
| 7 | (R)-6-hydroxy-2-methyl-4-chromanone | Inactive | ||
| 8 | (R)-mellein | Microsphaeropsis sp. H5-50 | Antifungal activity against Eurotium repens | [9] |
| 9 | (3R,4S)-hydroxymellein | Antifungal activity against Ustilago violacea | ||
| 10 | (3R,4R)-hydroxymellein | Antifungal activity against E. repens and U. violacea | ||
| 11 | 4,8-dihydroxy-3,4-dihydro-2H-naphthalen-1-one | Antifungal activity against E. repens and U. violacea | ||
| 12 | 6-ep-i 5′-hydroxymycosporulone | Microsphaeropsis sp. FO-5050 | Cytotoxicity activity against HL-60 | [10] |
| 13–15 | 1,3,6,8-tetrahydroxyanthraquinone congenners | Microsphaeropsis sp. KMPB W-22 | Inhibited protein kinases activity (PKC, CDK 4, and EGF-R) | [11] |
| 16 | 3′-O-demethylpreussomerin I | Microsphaeropsis sp. BCC 3050 | Antibacterial activity and antiplasmodial activity against plasmodium falciparum and cytotoxicity activity against KB, BC-1, and vero cells | [12] |
| 17 | Preussomerin I | - | ||
| 18 | Preussomerin E | - | ||
| 19 | Preussomerin F | - | ||
| 20 | Preussomerin G | - | ||
| 21 | Preussomerin H | - | ||
| 22 | Deoxypreussomerin A | Antibacterial activity and antiplasmodial activity against p. falciparum | ||
| 23 | Bipendensin | Inactive | ||
| 24 | Butyrolactones I | Microsphaeropsis sp. CF09-11 | Inactive | [13] |
| 25 | Butyrolactones IV | Inactive | ||
| 26 | Aspernolide D | Inactive | ||
| 27 | Arundinone A | M. arundinis E12-2112 | Inactive | [14] |
| 28 | Arundinone B | Cytotoxicity activity against T24 and A549 | ||
| 29 | Microsphaerophthalides A | M. arundinis PSU-G18 | Antifungal activity against Microsporum gypseum SH-MU-4, and Cryptococcus neoformans | [15] |
| 30 | Microsphaerophthalides B | Inactive | ||
| 31 | Microsphaerophthalides C | Inactive | ||
| 32 | Microsphaerophthalides D | Inactive | ||
| 33 | Microsphaerophthalides E | Antifungal activity against Microsporum gypseum SH-MU-4 and Cryptococcus neoformans | ||
| 34 | Microsphaerophthalides F | Inactive | ||
| 35 | Microsphaerophthalides G | Inactive | ||
| 36 | Deoxycyclopaldic acid | Inactive | ||
| 37 | 5,7-dihydroxy-4,6- dimethyl-1(3H)-isobenzofuranone | Inactive | ||
| 38 | 5-hydroxy-7-methoxy-4-(methoxymethyl)-6-methylisobenzofuran-1(3H)-one | Inactive | ||
| 39 | 5-hydroxy-7-methoxy-4,6-dimethylphthalide | Inactive | ||
| 40 | 7-methoxy-3,4,5,6-tetramethylphthalide | Inactive | ||
| 41 | Sclerin | Inactive | ||
| 42 | 6-hydroxy-2-methyl-4-chromanone | Inactive | ||
| 43 | Sclerotinin A | Inactive | ||
| 44 | 1-(2,5-dihydroxyphenyl)-2-buten-1-one | Antifungal activity against M. gypseum SH-MU-4, antiplasmodial activity against P. falciparum, and radical scavenging potency | ||
| 45 | Microsphaerins A | Microsphaeropsis sp. F2076 | Antibacterial activity against MR31SA | [16] |
| 46 | Microsphaerins B | - | ||
| 47 | Microsphaerins C | Microsphaeropsis sp. F2078 | - | |
| 48 | Microsphaerins D | - | ||
| 49 | Butyrolactone I | Microsphaeropsis olivacea | Inactive | [17] |
| 50 | Graphislactone A | Activity against acetylcholinesterase (AChE) | ||
| 51 | Ulocladol | Inactive | ||
| 52 | Botrallin | Activity against AChE | ||
| 53 | 2,5-diacetylphenol | Inactive | ||
| 54 | 7-hydroxy-2,4-dimethyl-3(2H)-benzofuranone | Inactive | ||
| 55 | Enalin | Inactive | ||
| 56 | Palmarumycin M1 | Microsphaeropsis sp. 7291 | Antibacterial activity against Bacillus megaterium, and antialgal activity against Chlorella fusca | [18] |
| 57 | Decaspirone | Antibacterial activity against B. megaterium and Microbotryum violaceum, and antialgal activity against C. fusca | ||
| 58 | Palmarumycin M2 | Antibacterial activity against B. megaterium | ||
| 59 | Papyracillic acid A | Inactive | ||
| 60 | Papyracillic acid B | Inactive | ||
| 61 | Papyracillic acid C | Inactive | ||
| 62 | Microsphaeropsins B | Inactive | ||
| 63 | Massarigenin A | Microsphaeropsis sp. | Inactive | [19] |
| 64 | Microsphaeropsones A | Microsphaeropsis sp. 8875 | Antibacterial activity against Escherichia coli and B. megaterium, and antialgae activity against C. fusca | [20] |
| 65 | Microsphaeropsones B | Antibacterial activity and antialgae activity against C. fusca | ||
| 66 | Microsphaeropsones C | Antibacterial activity against E. coli and B. megaterium, and antialgae activity against C. fusca | ||
| 67 | Citreorosein | Antibacterial activity against E. coli, B. megaterium, and M. violaceum, and antialgae activity against C. fusca | ||
| 68 | Emodin | Antibacterial activity and antialgae activity against C. fusca | ||
| 69 | Fusidienol A | Microsphaeropsis sp. 7177 | Antibacterial activity against E. coli, B. megaterium, and M. violaceum, and antialgae activity against C. fusca | |
| 70 | 3,4-dihydrofusidienol A | Antibacterial activity and antialgae activity against C. fusca | ||
| 71 | 8-hydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylic acid methyl ester | Antibacterial activity against E. coli and B. megaterium, and antialgae activity against C. fusca | ||
| 72 | Methyl 3,8-dihydroxy-6-methyl-9-oxo-9H-xanth- ene-1-carboxylate | Antibacterial activity and antialgae activity against C. fusca | ||
| 73 | Microxanthone | Antibacterial activity and antialgae activity against C. fusca | ||
| 74 | Microketides A | Microsphaeropsis sp. RA10-14 | Antibacterial activity against pseudomonas aeruginosa, Nocardia brasiliensis, B. anthraci, and Kocuria rhizophila, and antiphytoplankton activity | [21] |
| 75 | Microketides B | - | ||
| 76 | 10-Norparvulenone | Microsphaeropsis sp. F0-5050 | Anti-influenza virus against A/PR/8/34 | [22] |
| 77 | Microsphaeropsisin | Microsphaeropsis sp. H5-50 | Antifungal activity against E. repens and U. violacea | [9] |
| 78 | Microsphaeropsins A | Microsphaeropsis sp. 7291 | Antialgal activity against C. fusca | [18] |
| 79 | Arundinols A | M. arundinis E12-2112 | Inactive | [14] |
| 80 | Arundinols B | Inactive | ||
| 81 | Arundinols C | Inactive | ||
| 82 | 1β-hydroxy-α-cyperone | Antibacterial activity against Staphylococcus aureus | ||
| 83 | Betaenone derivative | Microsphaeropsis sp. KMPB W-22 | Inhibited protein kinases activity (PKC, CDK 4, and EGF-R) | [11] |
| 84 | Modiolide D | M. arundinis | Inactive | [23] |
| 85 | Modiolide E | Inactive | ||
| 86 | Modiolide A | Inactive | ||
| 87 | Macrosphelide A | Microsphaeropsis sp. FO-5050 | Cytotoxicity activity against HL-60 | [24] |
| 88 | Macrosphelide B | - | ||
| 89 | Macrosphelide C | - | [25] | |
| 90 | Macrosphelide D | - | ||
| 91 | Macrosphelide J | - | [10] | |
| 92 | Macrosphelide K | - | ||
| 93 | Modiolide | M. arundinis PSU-G18 | Inactive | [15] |
| 94 | Chrysogeside D | M. arundinis PH 30472 | Antifungal activity against A. tenuissima | [7] |
| 95 | L-755, 807 | Microsphaeropsis sp. MF6057 | Cell adhesion inhibition activity | [26] |
| 96 | Microsphaerones A | Microsphaeropsis sp. KMPB W-22 | Insecticidal activity against Spodoptera littoralis and Artemia salina | [27] |
| 97 | Microsphaerones B | - | ||
| 98 | N-2”-hydroxy-3’Eoctedecenoyl-1-O-β-D-glucopy-ranosyl-9-methyl-4E,8E-sphingadiene | M. olivacea F010 | Cytotoxic activity against L1210 | [28] |
| 99 | TAN-1496 A | Microsphaeropsis sp. FL-16144 | Cytotoxicity activity against P815 murine mastcytoma and A59 human lung carcinoma | [29] |
| 100 | TAN-1496 B | - | ||
| 101 | TAN-1496 C | - | ||
| 102 | TAN-1496 D | - | ||
| 103 | TAN-1496 E | - | ||
| 104 | Betaenone derivative | Microsphaeropsis sp. KMPB W-22 | Inactive | [11] |
| 105 | Fumiquinazolines L | Microsphaeropsis sp. CF09-11 | Inactive | [13] |
| 106 | Fumiquinazolines N | Inactive | ||
| 107 | Notoamide D | Inactive | ||
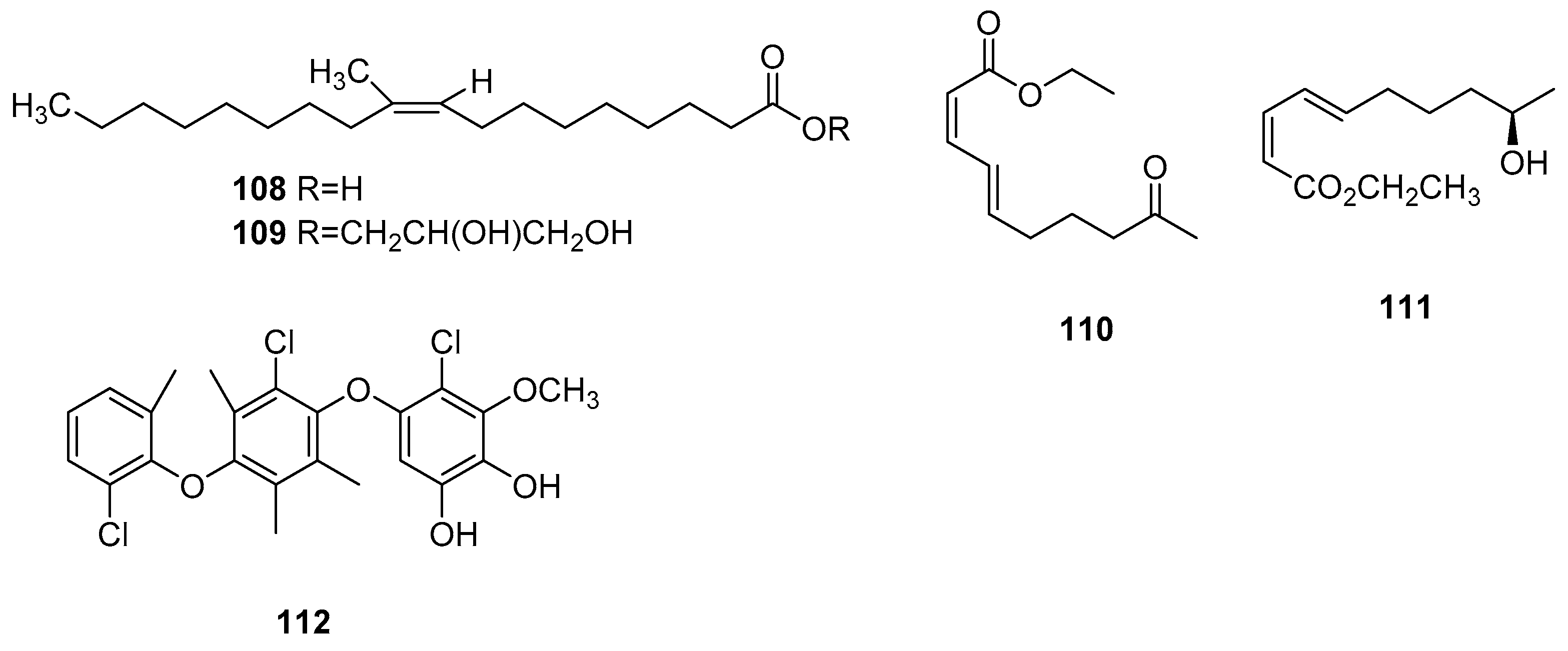
| 108 | 10-methyl-9Z-octadecenoic acid | Microsphaeropsis olivacea | Inactive | [30] |
| 109 | Glyceride | Inactive | ||
| 110 | Microsphaerodiolin | M. arundinis PSU-G18 | Inactive | [15] |
| 111 | Modiolin | Inactive | ||
| 112 | Microsphaerol | Microsphaeropsis sp. 7820 | Antibacterial activity against E. coli and B. megaterium | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, G.; Zhang, Z.; Niu, X.; Zhu, D. Secondary Metabolites from Fungi Microsphaeropsis spp.: Chemistry and Bioactivities. J. Fungi 2023, 9, 1093. https://doi.org/10.3390/jof9111093
Song G, Zhang Z, Niu X, Zhu D. Secondary Metabolites from Fungi Microsphaeropsis spp.: Chemistry and Bioactivities. Journal of Fungi. 2023; 9(11):1093. https://doi.org/10.3390/jof9111093
Chicago/Turabian StyleSong, Guodong, Zhibin Zhang, Xuenan Niu, and Du Zhu. 2023. "Secondary Metabolites from Fungi Microsphaeropsis spp.: Chemistry and Bioactivities" Journal of Fungi 9, no. 11: 1093. https://doi.org/10.3390/jof9111093
APA StyleSong, G., Zhang, Z., Niu, X., & Zhu, D. (2023). Secondary Metabolites from Fungi Microsphaeropsis spp.: Chemistry and Bioactivities. Journal of Fungi, 9(11), 1093. https://doi.org/10.3390/jof9111093





