Abstract
Most fungal species are commensals and non-pathogenic to plants, humans, or animals. However, several species of the Alternaria, Aspergillus, Trichophyton, and Microsporum genera are common causes of disease, even for immunocompetent individuals. Besides mucosal damage, fungi may contribute to a skin barrier impairment, favoring sensitization and allergy development. A total of 68 allergic dogs were selected from a veterinary dermatology and allergy outpatient consultation for conditions related to both Malassezia overgrowth and other fungal complications. The allergy diagnosis was made through anamnesis and current clinical criteria, with the involved allergenic species being identified by intradermal tests (IDTs) and allergen-specific immunoglobulin E (sIgE) determination in serum. Dermatophagoides farinae, Dactylis glomerata, and Malassezia pachydermatis showed as the higher sensitization species from house dust mites, grass pollen, and fungi, respectively. Significant correlations at p < 0.05 were found between sensitization to Dactylis glomerata and Phleum pratense grass pollens, Dermatophagoides farinae and Dermatophagoides pteronyssinus, Acarus siro, Tyrophagus putrescentiae, and Lepidoglyphus destructor dust/storage mites, and between fungi like Aspergillus mix and Penicillium or Alternaria alternata. A significant correlation was also found between sensitization to the Aspergillus mix and D. farinae, D. pteronyssinus, or A. siro. Rather severe dermatitis was observed when a positive IDT to Malassezia pachydermatis was found, regardless of the detection of circulating sIgE, allowing us to consider the usefulness of both the IDT and the sIgE for a systematic diagnosis of allergy to fungi.
Keywords:
dermatomycosis; allergy; Alternaria; Aspergillus; dermatophytes; fungal allergy; Malassezia; Penicillium 1. Introduction
Most fungi have evolved for approximately 1.5 billion years [1] and belong to saprophytic species that are non-pathogenic to plants, humans, or animals. Nevertheless, a small part may, in fact, become pathogenic, either by producing toxins or infecting or sensitizing other living beings, leading to subsequent allergy development [2]. Species from the Fungi kingdom are ubiquitous, like some from the Alternaria, Aspergillus, Fusarium, Mucor [2], Trichophyton, and Microsporum [3] genera and may be a possible cause of disease to humans and animals, mostly associated with immune-compromised conditions [2].
As organic matter decomposers, fungi secrete enzymes into the surrounding environment, digesting molecules from other organisms like carbohydrates, proteins, and lipids and then absorbing the resulting nutrients (e.g., carbohydrate metabolites) in a heterotrophic way. An eventual enrichment of the surrounding environment in fungal metabolic leftovers occurs due to the airborne spread of spores, hyphae, and their fragments [4].
Different species of fungi have been recognized as sensitizers and are possibly allergenic. Besides respiratory infections [5], several species of the Aspergillus genus have been implicated in bronchopulmonary allergies, sinusitis, and IgE-mediated asthma or hypersensitivity pneumonitis [6,7]. Sensitization to Alternaria has also been reported, mostly in warm climates, to be associated with type I hypersensitivity in both indoor and outdoor environments [8,9]. Species of the Fusarium genus, common contaminants of cereals, may lead to an immune system impairment and sensitization with allergy. Fungi-related respiratory diseases are frequently associated with either atopy [8] or immune impairment [2]. Allergic alveolitis, atopic conjunctivitis, bronchial asthma, and rhinitis are among the most common manifestations [10].
Allergens from Fusarium are known to cross-react with each other [11] and sometimes also with allergens from other species [12]. Curvularia, a genus with over 40 mostly saprophytic species, may lead to sensitization, causing mainly respiratory symptoms in humans [13]. It also cross-reacts with Alternaria alternata and Epicoccum nigrum [14,15]. Curvularia infection and allergy have also been reported in dogs [16,17]. Cladosporium is another ubiquitous genus with reported infection of humans and dogs [18], horses [19], and cats [20,21], as well as allergies in humans [2,22] and dogs [23]. Mucor and Rhizopus, two genera from the Mucorales group, have also been associated with allergy in humans [8] and animals [17,24,25]. Besides Alternaria, Aspergillus, Candida, or Epicoccum, Cladosporium, another mostly outdoor genus belonging to the Ascomycota phylum, also comprises relevant allergy-causing species with several identified allergens [26].
Dermatophytosis, a condition mainly caused by fungi from the Microsporum, Trichophyton, and Epidermophyton genera, is a common condition in immunocompetent human and animal individuals [27,28] (Figure 1). With several allergens already identified from Trichophyton, sensitization and allergy to dermatophytes is a well-known condition. Those allergens may either trigger immediate or delayed hypersensitivity. IgE-mediated asthma has also been reported in fungi-sensitized human patients, while delayed responses seem to provide some protection. Allergens from Trichophyton may play a simultaneous role in fungal pathogenesis and allergenicity, as suggested by their amino-acid sequence. This dual condition may be associated with particular T-cell epitopes, which may play a key role in new peptide vaccine development, with better efficiency regarding Trichophyton infection and allergy [29].
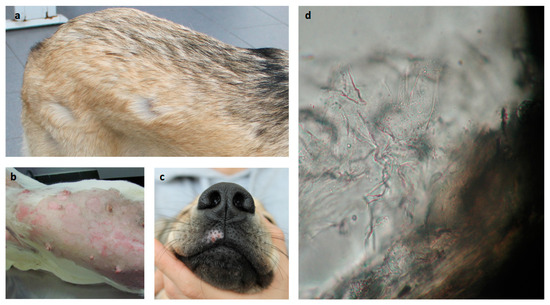
Figure 1.
Common dermatomycosis lesion observations in dogs. (a) Spots of alopecia; (b) pruritic inflammatory spots of alopecia (in previously clipped area); (c) pruritic alopecic and depigmented spot; and (d) surrounding hair debris with refringent spores (400×).
Candida is a genus with over 200 species, most of them commensals in human and animal microbiota and only facultatively pathogenic. A total of 15 species have been isolated from human and animal infections, affecting several organs besides mucosa or skin [30]. Candida is not a frequent cause of infections in animals but may occur associated with atopy [31,32].
Sensitization to fungi reaches roughly 5% of the global human population. However, this rate is higher in the atopic population. Exposure to fungal allergens may vary according to the environment, and contact occurs with intact spores, mycelia, or their fragments. Germinating spores present a higher allergen variety. Thus, the environment in which individuals live will play a crucial role in their contact with fungal particles, with fungal structure-derived particles becoming aerosolized in concentrations of 300 to 500 times greater than the original spores [33]. While certainly high, contact with fungal particles may result in underestimated subclinical sensitization. Moreover, contact with primary sensitization to a small number of fungal species may result in sensitization to a wide variety of species, as sensitization to fungi is considered highly cross-reactive. When a group of 6565 human individuals presenting with sIgE to fungi in at least one test was evaluated with a larger set of fungal species, 1208 showed positive to all [34]. Fungal proteins, sharing homologous structures and similar functions, have shown marked cross-reactivity [35,36].
Fungi commonly comprise a high concentration of airborne allergens, but increased exposure to indoor microbial diversity may play a protective role for atopy [8]. However, studies on the Alternaria genus, one of the most associated with allergy, have shown that even a low level of exposure to fungi for long periods is not necessarily associated with sensitization, except in atopic individuals.
Recurrent airway obstruction (RAO), a common condition in equines, has been associated with frequent exposure to moldy hay, but only non-IgE-mediated mechanisms have been implicated in the pathogenesis. However, sensitization to fungi with clinical deterioration in moldy environments and by challenging with mold extracts was observed. Higher scores in basophil histamine-releasing tests, upon stimulation with fungal allergens in horses with RAO, were also observed when compared to healthy individuals [37]. Regarding the immune response to Aspergillus fumigatus, sIgE and IgG were found in bronchoalveolar lavage (BAL) fluid from RAO-affected horses following in vitro provocation with fungal extracts [38]. Despite showing no difference in sIgE to fungal extracts between healthy and affected horses, sIgE to fungal allergens, such as Alt a 1 and Asp f 7, 8, and 9, were mainly detected in BAL and in serum from RAO-affected patients. Relevant differences in terms of sIgE levels for Asp f7 were also observed between healthy and RAO-affected individuals [39,40].
Despite the lack of identification of the major allergens implicated in sensitization, mold allergomes to horses have already started to be disclosed. Sensitization to fungi in dogs and cats also requires further studies, especially regarding the identification of the implicated allergomes [41].
Skin testing is currently performed with whole-allergen extracts, which may vary in allergen content, compromising inter-brand and even inter-batch reproducibility. After a clinical diagnosis of atopy, the IDT (Figure 2) stands as the veterinary allergy first-line diagnosis method for implicated species. Currently, most suppliers provide allergen extracts in well-defined concentrations for most groups of allergenic species [42], although standardization still needs improvement to ensure better reproducibility [43].
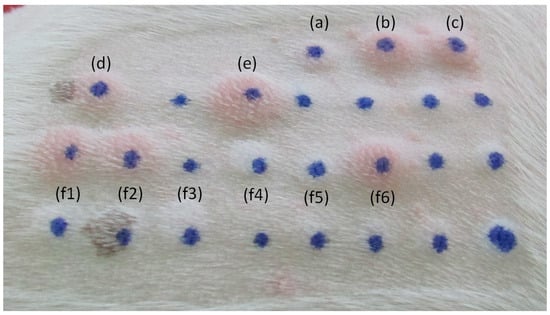
Figure 2.
Common IDT reactions in a 0–4 score range in dogs. (a) Negative control; (b) positive control; (c) Phleum pratense; (d) Dactylis glomerata; (e) Malassezia pachydermatis; and (f1–f6) house dust and storage mites.
Malassezia, a relevant genus from a lipophilic group of yeasts [44], is a common commensal of the dog and cat skin and mucosa, with Malassezia pachydermatis as the most reported species out of 18 [45].
Despite the common commensal equilibrium, different factors such as innate/adaptive immune impairment and secreted virulence factors may allow the Malassezia population to overgrow, leading to severe dermatitis [45], often observed in allergic dogs, with or without sensitization to Malassezia [42] (Figure 3).
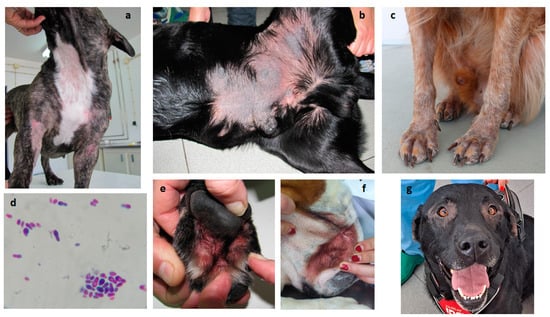
Figure 3.
Pruritic dermatitis in allergic dogs presenting Malassezia overgrowth. (a) Common areas of alopecic acute allergic dermatitis; (b) hyperkeratosis and lichenification in chronic dermatitis; (c) alopecia and early hyperkeratosis; (d) Malassezia overgrowth in skin cytology (400×); (e) chronic interdigital dermatitis; (f) facial intertriginous dermatitis; and (g) periocular alopecia in allergic dermatitis.
The inflammatory skin conditions, mostly associated with an impaired epidermal surface, sebum production, or excessive moisture, may favor Malassezia overgrowth in a faulty context of the complex cutaneous equilibrium. In fact, besides keratinization disorders, several endocrinopathies, metabolic diseases, neoplasia, and atopic dermatitis, food hypersensitivity or flea allergy frequently trigger a set of conditions commonly associated with Malassezia pachydermatis overgrowth, leading to dermatitis [46]. Malassezia overgrowth in dogs usually presents as ceruminous pruritic external otitis or as intertriginous dermatitis. The skin becomes erythematous, evolving to kerato-sebaceous skin thickening [47].
Despite its clinical relevance, not many studies have been published in veterinary medicine regarding Malassezia-derived animal conditions [43]. In 2021, Di Tomaso et al. [48] reported the results of sera evaluation from 45 dogs, in which 11 showed positive for Malassezia. Another study evaluating both IDTs and serum sIgE to Malassezia showed 24% positivity in IDTs despite no positive sera [49]. In 2009, Furiani et al. [50] had already reported 35.5% positive IDTs.
In a previous study with 111 mainly atopic dogs, including 33 from predisposed breeds, mostly with seborrheic disruptive skin barrier, 49.6% showed Malassezia overgrowth with dermatitis [51]. In yet another study with 84 mainly atopic dogs, including 31 from predisposed breeds, 58.3% presented with Malassezia overgrowth and dermatitis [52].
Regarding Malassezia, dog breeds, such as the American Cocker Spaniel, Australian Silky Terrier, Basset Hound, Boxer, Dachshund, English Poodle, Setter, Shih Tzu, and West Highland White Terrier have been appointed as being at higher risk of overgrowth, leading to dermatitis. For cats, the Devon Rex and Sphynx are the two breeds recognized as more predisposed to Malassezia overgrowth [53]. This condition has, however, been mostly diagnosed in individuals suffering from visceral paraneoplastic syndromes [47]. Zoonotic risks are assumed to be low, especially in immunocompetent individuals [45].
Considering an increase in the number of allergic animals, especially in dogs, where clinical deterioration is associated with mycotic complications like the ones related to Malassezia overgrowth and the fact that only a limited number of studies on this subject is available, this research aimed to frame the prevalence of sensitization to the most common fungi in an allergic dog population, by the two main current diagnostic methods, IDTs and sIgE determination in serum.
2. Materials and Methods
2.1. Dog Population
A total of 68 allergic dogs were selected for conditions related to Malassezia overgrowth and/or other fungal complications from the University of Évora Veterinary Hospital (Évora, Portugal) dermatology and allergy outpatient consultation. Primary allergy diagnosis/selection was made through a comprehensive query for anamnestic and clinical criteria, according to Hensel et al. (2015) [43] and Olivry and Mueller (2020) [54]. The owners of all animals presented for consultation at the Veterinary Hospital of the University of Évora were informed and consented to the collection and storage of data, including for research purposes.
2.2. Methods
Dogs were subjected to further diagnostic evaluation through IDT by inoculation of 50 μL of commercial allergen extracts (Diater and Nextmune, Madrid, Spain) from a set of relevant allergenic species, such as Dactylis glomerata and Phleum pratense grass pollens, Dermatophagoides farinae, Dermatophagoides pteronyssinus, Acarus siro, Tyrophagus putrescentiae, and Lepidoglyphus destructor dust/storage mites, and Alternaria alternata, the Aspergillus mix, and Malassezia pachydermatis fungi. Positive (0.01% histamine phosphate solution) and negative (physiological saline solution) commercial controls were also administered (Diater and Nextmune, Madrid, Spain). Dermal wheal and flare reactions were evaluated after 15 min. Reactions were considered positive when the resulting wheals were at least equal to or higher than halfway between the negative and the positive control reaction and then scored increasingly from 0 (negative) to 4 (maximum positive) [43]. All dogs were sedated with commercial medetomidine (Orion Pharma, Espoo, Finland) (roughly 0.03 mg/kg body weight, administered subcutaneously), and their sedation was reversed after the procedure, with the correspondent dose of atipamezole (Orion Pharma, Espoo, Finland) by intramuscular injection. The allergen-sIgE to an environmental panel of allergenic species, including the ones used for IDT, were determined in macELISA (LETI Animal Health Laboratories, Barcelona, Spain), and the results were expressed in ELISA Absorbance Units (EAU). The results above the 150 EAU threshold were considered positive. A total of 30 dogs simultaneously underwent IDT and sIgE determination; 21 of them underwent only sIgE, and 17 underwent only IDT. Differences between intradermal wheal scores and between sIgE EAU were compared using Pearson’s correlation coefficient (www.socscistatistics.com and SPSS, Chicago, IL, USA). Statistical significance was set at p < 0.05.
3. Results
3.1. General Observations
The sIgE and IDT scores varied according to the individual sensitization patterns and skin reactivity, as well as according to each extract potency. sIgE and IDT positive rates are shown in Table 1. The sensitization rate, defined as positive sIgE, showed the following order of magnitude: D. farinae > T. putrescentiae > A. siro > D. pteronyssinus > D. glomerata > P. pratense > L. desctructor > M. pachydermatis > Aspergillus mix. All dog patients with positive IDT to fungal species also showed at least a positive skin test for dust/storage mites or grass pollens. Only three patients with circulating sIgE to a fungal species (M. pachydermatis, in case) did not present with sIgE to dust/storage mites or grass pollens. No circulating sIgE was found to Alternaria alternata in this population. Regarding IDTs, the decreasing positive rate was as follows: D. farinae and L. destructor > A. siro > T. putrescentiae > D. pteronyssinus > A. alternata > Aspergilus mix > M. pachydermatis > D. glomerata. Penicillium was not assessed by IDTs.

Table 1.
Number of positive responses to IDT and sIgE testing for examined allergenic species.
House dust and storage mites were clearly shown to be the most allergenic species for this population, followed by the two grass pollen species regarding circulating sIgE, besides the higher rate of positive IDTs to the fungal species. Three dogs with positive IDTs to a grass pollen mix did not present with sIgE to Dactylis glomerata or Phleum pratense, while nine dogs with sIgE to these species did not simultaneously reveal positive IDTs.
The highest rate of positive sIgE to fungi was found in M. pachydermatis (10 out of 51), followed by Aspergillus mix (6 out of 51) and Penicillium mix (4 out of 51). None of the dogs presented with circulating sIgE to A. alternata, despite 9 out of 47 having shown positive IDT, which is a rate close to that observed regarding other fungal species. None of the dogs revealed simultaneous sensitization to the four fungal species. The highest positivity to fungi was found in M. pachydermatis. Of the 10 dogs with sIgE and 7 with positive IDT, only 1 was positive in both tests. None of the tested dogs showed simultaneous sIgE and positive IDTs to the Aspergillus mix. Malassezia pachydermatis was found to be the only sensitizing fungal species for nine dogs, while the Aspergillus mix was found in five, and Alternaria alternata was found in four. None of the dogs revealed Penicillium as a single fungal sensitizer. A total of 23 dogs revealed reactivity to at least one fungal species/group of species, and 14, most of the fungi-reactive patients, revealed reactive to at least two or three species/group of species.
3.2. Observed Associations
Regarding the sensitization to house dust mites and storage mites, a significant correlation was found between sIgE to D. farinae and D. pteronyssinus (r = 0.587; p < 0.00001), A. siro (r = 0.958; 0.00001), T. putrescentiae (r = 0.967; p < 0.00001) and L. destructor (r = 0.343; p = 0.01). In terms of intradermal reactivity, a significant correlation was also found between D. farinae and D. pteronyssinus (r = 0.384; p = 0.007), A. siro (r = 0.446; p = 0.001), and L. destructor (r = 0.336; p = 0.02).
Regarding grass pollen, a significant correlation was found between D. glomerata and P. pratense, both in terms of sIgE (r = 0.885; p < 0.00001) or intradermal reactivity (r = 0.31; p = 0.027).
For simultaneous sensitization to fungi and house dust or storage mites, a significant correlation was found between the sIgE to Aspergillus mix and to D. farinae (r = 0.283; p = 0.046) or A. siro (r = 0.288; p = 0.042). Sensitization between the Aspergillus mix and the Penicillium mix was also correlated (r = 0.356; p = 0.011), as well as positive IDTs to Alternaria alternata and Aspergillus mix (r = 0.599; p < 0.00001). Correlation between IDTs to the Aspergillus mix and D. pteronyssinus was also observed (0.494; p = 0.0002).
4. Discussion
As ubiquitous forms of life, fungi and their particles are common components of the environment, in contact with mucosal and cutaneous barriers. The depths of penetration will depend on the quality of those barriers. Skin barrier dysfunction is associated with the deep penetration of allergens, which is commonly observed in allergic dogs [55], promoting sensitization [56].
House dust and storage mites are among the most allergenic species for dogs, as was also observed in this study population, with high sensitization and IDT scores. Pollinosis was not found clinically relevant for most patients despite their sensitization to D. glomerata and P. pratense. In fact, many of these dogs presented sensitization to those pollens without suffering from pollinosis.
Regarding fungal species, sensitization was found to be low, despite an expressive rate of positive IDT, associated with a compatible clinical frame, with consistent improvement following directed antifungal treatment and environmental sanitation measures.
Associations found regarding either sIgE or IDT between dust mites and storage mites [57,58,59] and grass pollen species [60,61,62] were already expected due to cross-reaction. Interestingly, the highest significant correlation (r = 0.967; p < 0.00001) observed in terms of sIgE was found between D. farinae and T. putrescentiae, as suggested by ELISA inhibition studies recently performed by Song et al. (2022) [59]. Sometimes, those underlying sensitizations lead to allergy, and sometimes, it does not [42,63,64]. Only three dogs sensitized to grass pollens did not present with sIgE both to D. glomerata and P. pratense, while nine were found without positive IDTs for both, which may reveal a higher sensitization rate without allergy. Pollinosis-associated atopic dermatitis would demand not only circulating sIgE but also the presence of sIgE on skin mast cells, leading to positive IDTs. A similar condition was found regarding M. pachydermtis, where ten dogs presented with sIgE, but only seven showed positive IDT. On the other hand, as observed by Han et al., 2020 [49] regarding M. pachydermatis, in this study, no sIgE to Alternaria alternata was detected in serum, despite a significant number of positive IDTs, which may be due to different levels of preservation of IgE epitopes in the extracts used for those different methods. In addition to an individual predisposition, the associations found between sIgE or IDT to dust and storage mites, and those to fungi (e.g., D. farinae, A. siro, and D. pteronyssinus, regarding the Aspergillus mix) may also be related to (i) living conditions, favoring an environmental prevalence of both groups of allergenic species, and (ii) an increased skin barrier impairment, promoting the sensitization to fungi, in dogs allergic to both dust and storage mites. Other associations, like the simultaneous sensitization to more than one fungal species, were also observed. Cross-sensitization between fungal species [11,12,14,15,65], as well as between fungi and food [66], has also been reported. However, some of the positive correlations found, such as the one between D. pteronyssinus, a less sensitized dust mite for dogs, and the Aspergillus mix, for instance, may decay with sample enlargement.
In this study, the highest sensitization rate to fungi was observed regarding M. pachydermatis, with 16 positive cases (sIgE and IDT). Malassezia pachydermatis was even the single sensitizing fungal species for nine of the patients, which could be explained by the fact that Malassezia overgrowth is much more frequent in atopic dog populations and may result in a higher sensitization rate. In addition to the presence of sIgE in M. pachydermatis, more severe inflammatory skin conditions were found when positive IDT was observed. Moreover, as with other allergen species, and probably even more, due to the proteolytic capacity inherent to mold extracts, with consequent self-disruption, specific diagnosis of allergy to fungi should also undergo both IDT and sIgE, filling the gaps from each method [43,67].
5. Conclusions
Considering the observed results, showing the relevance of sensitization and allergy to fungi in dogs, extensive future studies are needed, aiming to overcome several current limitations associated with (i) different extract origins as well as different methodologies (e.g., none of the dogs presented with circulating sIgE to A. alternata, despite 9 out of 47 having shown positive IDTs, and none of the tested dogs having presented sIgE and positive IDTs to the Aspergillus mix, simultaneously); (ii) the usefulness of a healthy non-allergic population, allowing the identification of possible non-specific reactions, and (iii) the need for a larger sample, providing a more accurate set of correlation results.
Funding
This work is funded by National Funds through FCT—Foundation for Science and Technology under the Project UIDB/05183/2020 and LETI Pharma, S.L.U.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the fact that the owners of all animals presented for consultation at the Veterinary Hospital are aware and consent to the collection and storage of data, including for research purposes. Furthermore, in this study, no intervention was performed on animals besides those strictly needed for their clinical assessment.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are not publicly available due to ethical/deontological secrecy.
Acknowledgments
The author thanks Ana Martins for proofreading the text.
Conflicts of Interest
The author declares no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Funding statement. This change does not affect the scientific content of the article.
References
- Wang, D.Y.; Kumar, S.; Hedges, S.B. Divergence time estimates for the early history of animal phyla and the origin of plants, animals and fungi. Proc. R. Soc. 1999, 266, 163–171. [Google Scholar] [CrossRef] [PubMed]
- De Lucca, A.J. Harmful fungi in both Agriculture and Medicine. Rev. Iberoam Micol. 2007, 24, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Moriello, K.A.; Coyner, K.; Paterson, S.; Mignon, B. Diagnosis and treatment of dermatophytosis in dogs and cats. Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 2017, 28, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Yafetto, L.; Carroll, L.; Cui, Y.; Davis, D.J.; Fischer, M.W.; Henterly, A.C.; Kessler, J.D.; Kilroy, H.A.; Shidler, J.B.; Stolze-Rybczynski, J.L.; et al. The fastest flights in nature: Highspeed spore discharge mechanisms among fungi. PLoS ONE 2008, 3, e3237. [Google Scholar] [CrossRef] [PubMed]
- Akan, M.; Hazirogiu, R.; Ihan, Z.; Sareyyupogiu, B.; Tunca, R. A case of aspergillosis in a broiler breeder flock. Avian Dis. 2002, 46, 497–501. [Google Scholar] [CrossRef]
- Maurya, V.; Gugnami, H.C.; Sarnia, P.U.; Madan, T.; Shah, A. Sensitization to Aspergillus antigens and occurrence of allergic bronchopulmonary aspergillosis in patients with asthma. Chest 2005, 127, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Panjabi, O. Allergic bronchopulmonary aspergillosis: A review of a disease with a worldwide distribution. J. Asthma. 2002, 39, 273–289. [Google Scholar] [CrossRef]
- Barnes, C. Fungi and Atopy. Clin. Rev. Allergy Immunol. 2019, 57, 439–448. [Google Scholar] [CrossRef]
- Čelakovská, J.; Bukač, J.; Ettler, K.; Vaneckova, J.; Ettlerova, K.; Krejsek, J. Sensitisation to outdoor and indoor fungi in atopic dermatitis patients and the relation to the occurrence of food allergy to peanuts and walnuts. Mycoses 2018, 61, 698–703. [Google Scholar] [CrossRef]
- Lacey, J.; Dutkiewicz, J. Bioaerosols and occupational lung disease. J. Aerosol. Sci. 1994, 25, 1371–1404. [Google Scholar] [CrossRef]
- Verma, J.; Singh, B.P.; Sridhara, S.; Gaur, S.N.; Arora, N. Purification and characterization of a cross-reactive 45-kD major allergen of Fusarium solani. Int. Arch. Allergy. Immunol. 2003, 130, 193–199. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, O.E.; McOants, M.L.; Salvaggio, J.E.; Lehrer, S.B. Fusarium solani: Prevalence of skin reactivity and antigenic allergenic analysis. J. Allergy Clin. Immunol. 1986, 77, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Singh, B.P.; Sridhara, S.; Gaur, S.N.; Chaudhary, V.K.; Arora, N. Allergens of Curvularia lunata during cultivation in different media. J. Allergy Clin. Immunol. 1999, 4, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Singh, B.P.; Sridhara, S.; Gaur, S.N.; Kunar, R.; Chaudhary, V.K.; Arora, N. Identification of cross-reactive proteins amongst different Curvularia species. Int. Arch. Allergy Immunol. 2002, 127, 38–46. [Google Scholar] [CrossRef]
- Gupta, R.; Singh, B.P.; Sridhara, S.; Gaur, S.N.; Kumar, R.; Ghaudhary, V.K.; Arora, N. Allergenic cross-reactivity of Curvularia lunata with other airborne fungal species. Allergy 2002, 57, 636–640. [Google Scholar] [CrossRef]
- Strzok, E.; Siepker, C.; Armwood, A.; Howerth, E.; Smith, J.; Banovic, F. Successful treatment of cutaneous Curvularia geniculata, Nocardia niigatensis, and viral papillomatosis in a dog during the therapeutic management of immune-mediated hemolytic anemia. Front. Vet. Sci. 2019, 6, 249. [Google Scholar] [CrossRef]
- Kang, M.; Kim, H.; Jang, H.; Park, H. Sensitization rates of causative allergens for dogs with atopic dermatitis: Detection of canine allergen-specific IgE. J. Vet. Med. Sci. 2014, 15, 545–550. [Google Scholar] [CrossRef]
- Spano, M.; Zuliani, D.; Peano, A.; Bertazzolo, W. Cladosporium cladosporioides-complex infection in a mixed-breed dog. Vet. Clin. Pathol. 2018, 47, 150–153. [Google Scholar] [CrossRef]
- Dauvillier, J.; TerWoort, F.; van Erck-Westergren, E. Fungi in respiratory samples of horses with inflammatory airway disease. J. Vet. Intern. Med. 2019, 33, 968–975. [Google Scholar] [CrossRef]
- Jarrah, S.A.; Zanetti, C.C.; Maruyama, F.H.; Ito, A.T.; Rosa, J.M.A.; Colodel, E.M.; Lima, S.R.; Nakazato, L.; Dutra, V. Cladosporium cladosporioides isolated from a cat with squamous cell carcinoma. Arq. Bras. Med. Vet. Zootec. 2017, 69, 377–380. [Google Scholar] [CrossRef]
- Mariani, C.L.; Platt, S.R.; Scase, T.J.; Howerth, E.W.; Chrisman, C.L.; Clemmons, R.M. Cerebral phaeohyphomycosis caused by Cladosporium spp. in two domestic shorthair cats. J. Am. Anim. Hosp. Assoc. 2002, 38, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, O. Molds and Respiratory Allergy–Part 1. MOJ Immunol. 2015, 2, 00045. [Google Scholar] [CrossRef]
- Meason-Smith, C.; Diesel, A.; Patterson, A.P.; Older, C.E.; Mansell, J.M.; Suchodolski, J.S.; Hoffmann, A.R. What is living on your dog’s skin? Characterization of the canine cutaneous mycobiota and fungal dysbiosis in canine allergic dermatitis. FEMS Microbiol. Ecol. 2015, 91, fiv139. [Google Scholar]
- Cafarchia, C.; Figueredo, L.A.; Otranto, D. Fungal diseases of horses. Vet. Microbiol. 2013, 167, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.Y.; Kang, H.S.; Bhang, D.H.; Kim, M.K.; Hwang, C.Y.; Han, H.R. Allergens causing atopic diseases in canine. J. Vet. Sci. 2002, 3, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Simon-Nobbe, B.; Denk, U.; Pöll, V.; Rid, R.; Breitenbach, M. The spectrum of fungal allergy. Int. Arch. Allergy Immunol. 2008, 145, 58–86. [Google Scholar] [CrossRef]
- Lund, A.; DeBoer, D.J. Immunoprophylaxis of dermatophytosis in animals. Mycopathologia 2008, 166, 407–424. [Google Scholar] [CrossRef]
- Medeiros, F.; Crepaldi, N.; Tognoli, L.; Pereira, R.E.P. Dermatófitos–Revisão de literatura. Rev. Cient Eletrônica. Med. Vet. 2009, 12, 1–5. [Google Scholar]
- Woodfolk, J. Allergy and dermatophytes. Clin. Microbiol. Rev. 2005, 18, 30–43. [Google Scholar] [CrossRef]
- Seyedmousavi, S.; Bosco, S.d.G.; de Hoog, S.; Ebel, F.; Elad, D.; Gomes, R.R.; Jacobsen, I.D.; Jensen, H.E.; Martel, A.; Mignon, B.; et al. Fungal infections in animals: A patchwork of different situations. Med. Mycol. 2018, 56, S165–S187. [Google Scholar] [CrossRef]
- Yurayart, C.; Chindamporn, A.; Suradhat, S.; Tummaruk, P.; Kajiwara, S.; Prapasarakul, N. Comparative analysis of the frequency, distribution and population sizes of yeasts associated with seborrheic dermatitis and healthy skin. Vet. Microbiol. 2011, 148, 356–362. [Google Scholar] [CrossRef]
- Mueller, R.S.; Bettenay, S.V.; Shipstone, M. Cutaneous candidiasis in a dog caused by Candida guilliermondii. Vet. Rec. 2002, 150, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Green, B.J.; Tovey, E.R.; Sercombe, J.K.; Blachere, F.M.; Beezhold, D.H.; Schmechel, D. Airborne fungal fragments and allergenicity. Med. Mycol. 2006, 44 (Suppl. S1), S245–S255. [Google Scholar] [CrossRef] [PubMed]
- Amado, M.; Portnoy, J.M.; Barnes, C. Fungal cross-allergenicity in specific IgE testing. J. Allergy Clin. Immunol. 2014, 133 (Suppl. S2), AB92. [Google Scholar] [CrossRef]
- Williams, P.B.; Barnes, C.S.; Portnoy, J.M. Environmental allergens workgroup. Innate and adaptive immune response to fungal products and allergens. J. Allergy Clin. Immunol. Pract. 2016, 4, 386–395. [Google Scholar] [CrossRef]
- Crameri, R.; Weichel, M.; Fluckiger, S.; Glaser, A.G.; Rhyner, C. Fungal allergies: A yet unsolved problem. Chem. Immunol. Allergy 2006, 91, 121–133. [Google Scholar] [PubMed]
- Dirscherl, P.; Grabner, A.; Buschmann, H. Responsiveness of basophil granulocytes of horses suffering from chronic obstructive pulmonary disease to various allergens. Vet. Immunol. Immunopathol. 1993, 38, 217–227. [Google Scholar] [CrossRef]
- Schmallenbach, K.H.; Rahman, I.; Sasse, H.H.; Dixon, P.M.; Halliwell, R.E.; McGorum, B.C.; Crameri, R.; Miller, H.R. Studies on pulmonary and systemic Aspergillus fumigatus-specific IgE and IgG antibodies in horses affected with chronic obstructive pulmonary disease (COPD). Vet. Immunol. Immunopathol. 1998, 66, 245–256. [Google Scholar] [CrossRef]
- Kunzle, F.; Gerber, V.; Van Der Haegen, A.; Wampfler, B.; Straub, R.; Marti, E. IgE-bearing cells in bronchoalveolar lavage fluid and allergen-specific IgE levels in sera from RAO-affected horses. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2007, 54, 40–47. [Google Scholar] [CrossRef]
- Eder, C.; Crameri, R.; Mayer, C.; Eicher, R.; Straub, R.; Gerber, H.; Lazary, S.; Marti, E. Allergen-specific IgE levels against crude mould and storage mite extracts and recombinant mould allergens in sera from horses affected with chronic bronchitis. Vet. Immunol. Immunopathol. 2000, 73, 241–253. [Google Scholar] [CrossRef]
- Mueller, R.S.; Janda, J.; Jensen-Jarolim, E.; Rhyner, C.; Marti, E. Allergens in veterinary medicine. Allergy 2016, 71, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.; Bento, O.; Inacio, F. Veterinary allergy diagnosis: Past, present and future perspectives. Allergo J. Int. 2016, 25, 20–32. [Google Scholar] [CrossRef]
- Hensel, P.; Santoro, D.; Favrot, C.; Hill, P.; Griffin, C. Canine atopic dermatitis: Detailed guideline for diagnosis and allergen identification. BMC Vet. Res. 2015, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr. Allergy Immunol. 2016, 27 (Suppl. S23), 1–250. [Google Scholar] [CrossRef] [PubMed]
- Bond, R.; Morris, D.O.; Guillot, J.; Bensignor, E.J.; Robson, D.; Mason, K.V.; Kano, R.; Hill, P.B. Biology, diagnosis and treatment of Malassezia dermatitis in dogs and cats. Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 2020, 31, 27.e4. [Google Scholar] [CrossRef]
- Balappanavar, B.R.; Vasanth, M.S. Clinico-diagnostic and therapeutic management of canine malasseziosis. Intas. Polivet. 2013, 14, 353–357. [Google Scholar]
- Guillot, J.; Bond, R. Malassezia yeasts in veterinary dermatology: An updated overview. Front. Cell Infect Microbiol. 2020, 10, 79. [Google Scholar] [CrossRef]
- Di Tommaso, M.; Luciani, A.; Crisi, P.E.; Beschi, M.; Rosi, P.; Rocconi, F.; Miglio, A. Detection of serum allergen-specific IgE in atopic dogs tested in northern Italy: Preliminary study. Animals 2021, 11, 358. [Google Scholar] [CrossRef]
- Han, C.; Chan, W.Y.; Hill, P.B. Prevalence of positive reactions in intradermal and IgE serological allergy tests in dogs from south Australia, and the subsequent outcome of allergen-specific immunotherapy. Aust. Vet. J. 2020, 98, 17–25. [Google Scholar] [CrossRef]
- Furiani, N.; Scarampella, F.; Noli, C.; Ordeix, L. A retrospective study of 486 intradermal tests performed on atopic dogs in Northern Italy. Veterinaria 2009, 23, 41–46. [Google Scholar]
- Cecci, G.M.; Cardoso, M.L.; Bento, O.; Martins, L.M. Allergy approach to a dog population from a veterinary dermatology consultation at the tropical inland city of Londrina, Paraná, Brazil. In Proceedings of the European Academy of Allergy and Clinical Immunology (EAACI) Congress, Lisbon, Portugal, 1–5 June 2019. [Google Scholar]
- Esteves de Campos, I.; Ferreiro Pinto, C.; Munhoz Severino, A.C.; Bento, O.; Antunes, C.; Costa, A.R.; Martins, L.M. Dermatological and allergy approach to a dog population from a veterinary consultation at the tropical coastal city of São Paulo, Brazil. In Proceedings of the European Academy of Allergy and Clinical Immunology (EAACI) Congress, Lisbon, Portugal, 1–5 June 2019. [Google Scholar]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multi–sensitized atopy and T-cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef]
- Olivry, T.; Mueller, R.S. Critically appraised topic on adverse food reactions of companion animals (9): Time to flare of cutaneous signs after a dietary challenge in dogs and cats with food allergies. BMC Vet. Res. 2020, 16, 158. [Google Scholar] [CrossRef] [PubMed]
- Pin, D.; Pendaries, V.; Alassane, K.S.; Froment, C.; Amalric, N.; Cardiergues, M.; Serre, G.; Haftek, M.; Vidémont, E.; Simon, M. Refined immunochemical characterization in healthy dog skin of the epidermal cornification proteins, filaggrin, and corneodesmosin. J. Histochem. Cytochem. 2019, 67, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Paixão, A.; Caldeira, J.; Leocádio, J.; Martins, L. A importância da integridade da barreira cutânea na prevenção da alergia veterinária—The importance of skin barrier integrity for the prevention of veterinary allergy. Rev. Port. Imunoalergologia 2022, 30, 9–20. [Google Scholar] [CrossRef]
- Masuda, K.; Tsujimoto, H.; Fujiwara, S.; Kurata, K.; Hasegawa, A.; Yasueda, H.; Yamashita, K.; DeBoer, D.J.; de Weck, A.L.; Sakaguchi, M. IgE sensitivity and cross-reactivity to crude and purified mite allergens (Der f 1, Der f 2, Der p 1, Der p 2) in atopic dogs sensitive to Dermatophagoides mite allergens. Vet. Immunol. Immunopathol. 1999, 72, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Caldas, E.; Iraola Calvo, V. Mite allergens. Curr. Allergy Asthma Rep. 2005, 5, 402–410. [Google Scholar] [CrossRef]
- Song, H.; Lee, J.; Jeong, K.Y.; Cheon, D.S.; Park, J.W. Comparison of sensitization patterns to dust mite allergens between atopic dermatitis patients and dogs, and non-specific reactivity of canine IgE to the storage mite Tyrophagus putrescentiae. Exp. Appl. Acarol. 2022, 88, 41–55. [Google Scholar] [CrossRef]
- Weber, R.W. Patterns of pollen cross-allergenicity. J. Allergy Clin. Immunol. 2003, 112, 229–239. [Google Scholar] [CrossRef]
- Buckley, L.; Schmidt, V.; McEwan, N.; Nuttall, T. Cross-reaction and co-sensitization among related and unrelated allergens in canine intradermal tests. Vet. Dermatol. 2013, 24, 422–427. [Google Scholar] [CrossRef]
- Li, J.D.; Gu, J.Q.; Xu, Y.Y.; Cui, L.; Li, L.S.; Wang, Z.X.; Yin, J.; Guan, K. Serum IgE profiles in Chinese pollinosis patients with grass pollen sensitisation. World Allergy Organ J. 2022, 11, 100624. [Google Scholar] [CrossRef]
- Martins, L.M.; Marques, A.G.; Pereira, L.M.; Goicoa, A.; Semião-Santos, S.J.; Bento, O.P. House-dust mite allergy: Mapping of Dermatophagoides pteronyssinus allergens for dogs by two-dimensional immunoblotting. Postep. Dermatol. Alergol. 2015, 32, 73–81. [Google Scholar] [CrossRef]
- Martins, L.M.; Marques, A.G.; Pereira, L.M.; Semião-Santos, S.J.; Bento, O.P. Allergy to grass pollen: Mapping of Dactylis glomerata and Phleum pratense allergens for dogs by two-dimensional immunoblotting. Postep. Dermatol. Alergol. 2017, 34, 60–69. [Google Scholar] [CrossRef]
- Bacher, P.; Hohnstein, T.; Beerbaum, E.; Röcker, M.; Blango, M.G.; Kaufmann, S.; Röhmel, J.; Eschenhagen, P.; Grehn, C.; Seidel, K.; et al. Human Anti-fungal Th17 Immunity and Pathology Rely on Cross-Reactivity against Candida albicans. Cell 2019, 176, 1340–1355.e15. [Google Scholar] [CrossRef]
- Xing, H.; Wang, J.; Sun, Y.; Wang, H. Recent Advances in the Allergic Cross-Reactivity between Fungi and Foods. J. Immunol. Res. 2022, 2022, 7583400. [Google Scholar] [CrossRef]
- Lee, K.W.; Blankenship, K.D.; McCurry, Z.M.; McKinney, B.; Ruffner, R.; Esch, R.E.; Drouet, L. Intra and inter-laboratory reproducibility of a monoclonal antibody cocktail-based ELISA for detection of allergen specific IgE in dogs: Proficiency monitoring of macELISA in six laboratories. Vet. Immunol. Immunopathol. 2012, 148, 267–275. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).