Problems Encountered Using Fungal Extracts as Test Solutions for Fungal Allergy Diagnosis
Abstract
1. Introduction
1.1. Prevalence of Fungal Sensitization
1.2. Allergenic Fungal Species
1.3. Fungal Allergens
2. Diagnosis of Fungal Allergy
2.1. Variability of Commercial Fungal Extracts
2.2. Fungal Extracts–Imperfect but Not Yet Obsolete
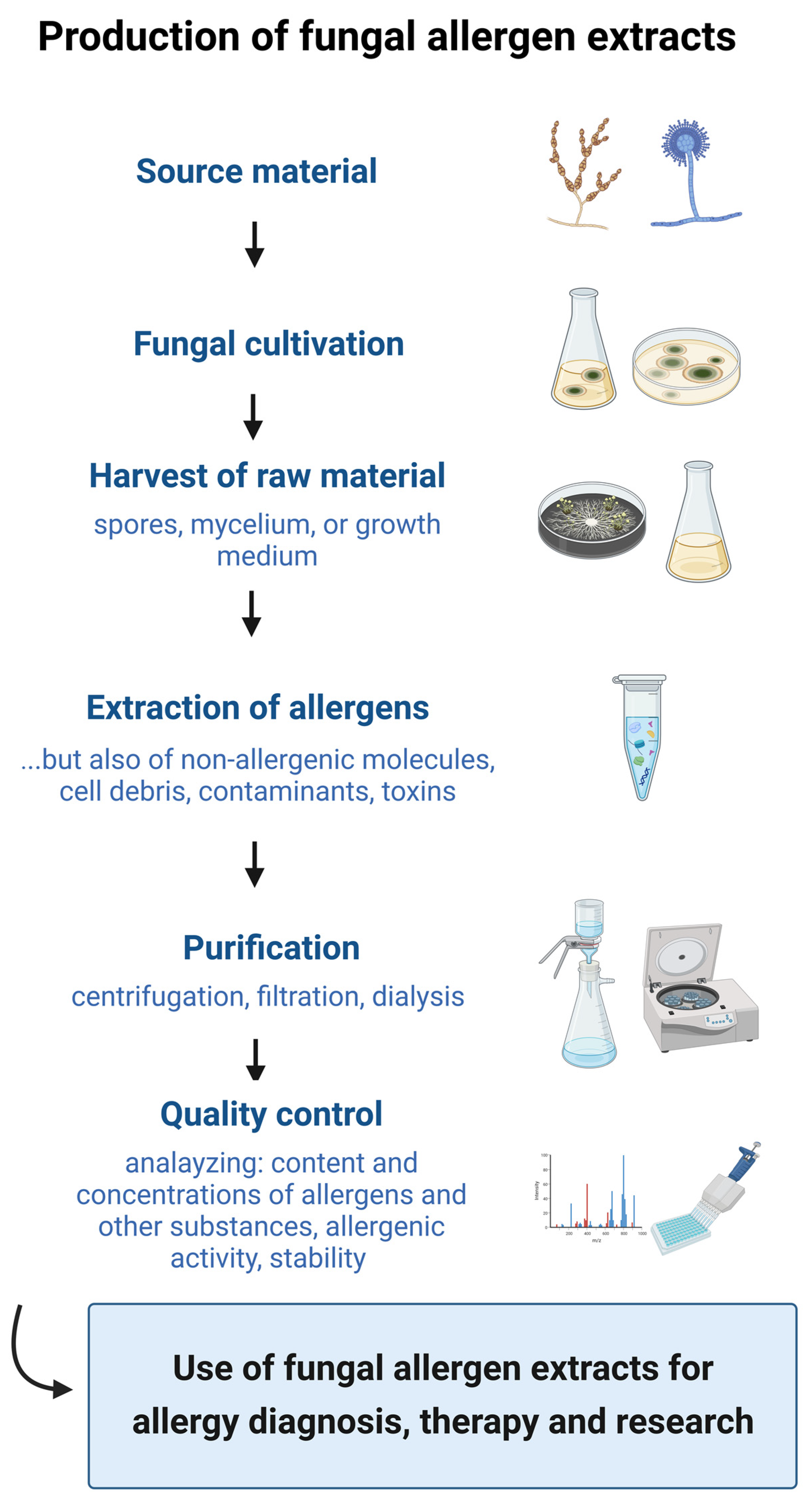
3. Preparation of Fungal Allergen Extracts
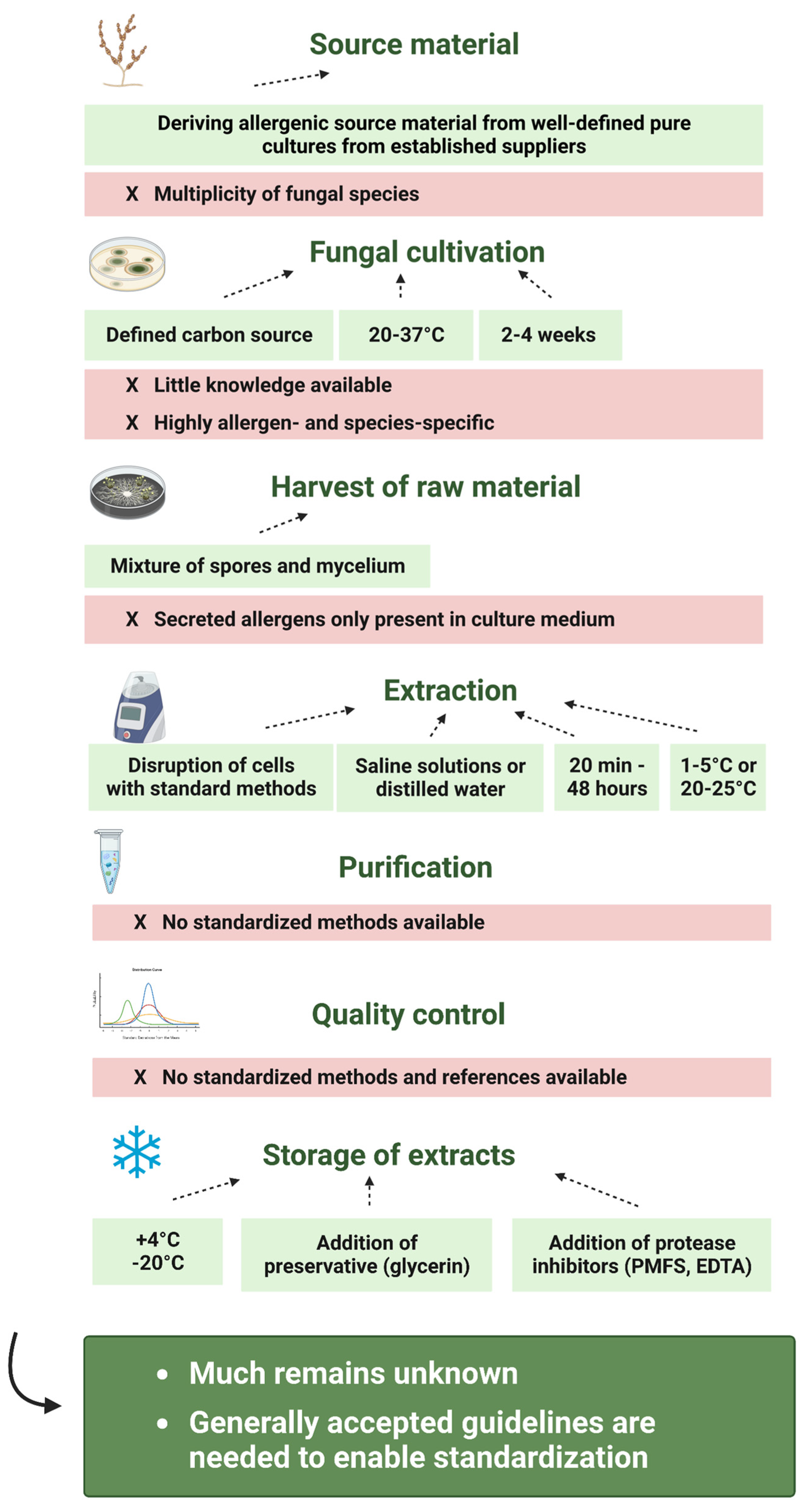
3.1. Source Material and Strain Variability
3.2. Fungal Cultivation
3.2.1. Cultivation Medium
3.2.2. Cultivation Temperature
3.2.3. Exposure to Light
3.2.4. Growth Time
3.3. Harvest of Fungal Material
3.3.1. Spores, Mycelia or Growth Medium?
3.3.2. Harvest
3.4. Extraction
3.4.1. Disruption Method
3.4.2. Buffer
3.4.3. Time
3.4.4. Temperature
3.5. Purification
3.6. Quality Control
3.7. Stability and Storage
4. Standardization of Fungal Allergen Extracts
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- von Pirquet, C. Allergie. Münchener Med. Wochenschr. 1906, 120, 1457–1458. [Google Scholar]
- Floyer, J. Violent asthma after visiting a wine cellar. In A Treatise on Asthma, 3rd ed.; Innys and Parker: London, UK, 1726. [Google Scholar]
- Twaroch, T.E.; Curin, M.; Valent, R.; Swoboda, I. Mold allergens in respiratory allergy: From structure to therapy. Allergy Asthma Immunol. Res. 2015, 7, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Crameri, R.; Weichel, M.; Flückiger, S.; Glaser, G.; Rhyner, C. Fungal allergies: A yet unsolved problem. Chem. Immunol. Allergy 2006, 91, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threat posed by the fungal kingdom to humans, wildlife, and agriculture. Am. Soc. Microbiol. 2020, 11, e00449-20. [Google Scholar] [CrossRef] [PubMed]
- Licorish, K.; Novey, H.; Kozak, P.; Fairshter, R.; Wilson, A. Role of and spores in the pathogenesis of asthma. J. Allergy Clin. Immunol. 1985, 76, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Green, B.J.; Sercombe, J.K.; Tovey, E.R. Fungal fragments and undocumented conidia function as new aeroallergen sources. J. Allergy Clin. Immunol. 2005, 115, 1043–1048. [Google Scholar] [CrossRef]
- Aukrust, L.; Borch, S.; Einarsson, R. Mold allergy—Spores and mycelium as allergen sources. Allergy 1985, 40, 43–48. [Google Scholar] [CrossRef]
- Arruda, L.; Mann, B.; Chapman, M. Selective expression of a major allergen and cytotoxin, Asp f I, in Aspergillus fumigatus. Implications for the immunopathogenesis of Aspergillus-related diseases. J. Immunol. 1992, 149, 3354–3359. [Google Scholar] [CrossRef]
- Portnoy, J.; Pacheco, F.; Ballam, Y.; Barnes, C. The effect of time and extraction buffers on residual protein and allergen content of extracts derived from four strains of Alternaria. J. Allergy Clin. Immunol. 1993, 91, 930–938. [Google Scholar] [CrossRef]
- Nissen, D.; Petersen, L.J.; Esch, R.; Svejgaard, E.; Skov, P.S.; Poulsen, L.K.; Nolte, H. IgE-Sensitization to Cellular and Culture Filtrates of Fungal Extracts in Patients with Atopic Dermatitis. Ann. Allergy Asthma Immunol. 1998, 81, 247–255. [Google Scholar] [CrossRef]
- Esch, R.E.; Codina, R. Fungal raw materials used to produce allergen extracts. Ann. Allergy Asthma Immunol. 2017, 118, 399–405. [Google Scholar] [CrossRef]
- Paris, S.; Fitting, C.; Ramirez, E.; Latgé, J.P.; David, B. Comparison of different extraction methods of Alternaria allergens. J. Allergy Clin. Immunol. 1990, 85, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Stark, P.C.; Burge, H.A.; Ryan, L.M.; Milton, D.K.; Gold, D.R. Fungal levels in the home and lower respiratory tract illnesses in the first year of life. Am. J. Respir. Crit. Care Med. 2003, 168, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Reijula, K.; Leino, M.; Mussalo-Rauhamaa, H.; Nikulin, M.; Alenius, H.; Mikkola, J.; Elg, P.; Kari, O.; Mäkinen-Kiljunen, S.; Haahtela, T. IgE-mediated allergy to fungal allergens in Finland with special reference to Alternaria alternata and Cladosporium herbarum. Ann. Allergy Asthma Immunol. 2003, 91, 280–287. [Google Scholar] [CrossRef]
- Anees-Hill, S.; Douglas, P.; Pashley, C.H.; Hansell, A.; Marczylo, E.L. A systematic review of outdoor airborne fungal spore seasonality across Europe and the implications for health. Sci. Total Environ. 2022, 818, 151716. [Google Scholar] [CrossRef]
- Ahluwalia, S.K.; Matsui, E.C. Indoor Environmental Interventions for Furry Pet allergens, Pest Allergens, and Mold: Looking to the Future. J. Allergy Clin. Immunol. Pr. 2018, 6, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Pomés, A.; Chapman, M.D.; Wünschmann, S. Indoor Allergens and Allergic Respiratory Disease. Curr. Allergy Asthma Rep. 2016, 16, 43. [Google Scholar] [CrossRef]
- Knutsen, A.P.; Bush, R.K.; Demain, J.G.; Denning, D.W.; Dixit, A.; Fairs, A.; Greenberger, P.A.; Kariuki, B.; Kita, H.; Kurup, V.P.; et al. Fungi and allergic lower respiratory tract diseases. J. Allergy Clin. Immunol. 2012, 129, 280–291. [Google Scholar] [CrossRef]
- Fukutomi, Y.; Taniguchi, M. Sensitization to fungal allergens: Resolved and unresolved issues. Allergol. Int. 2015, 64, 321–331. [Google Scholar] [CrossRef]
- Mari, A.; Schneider, P.; Wally, V.; Breitenbach, M.; Simon-Nobbe, B. Sensitization to fungi: Epidemiology, comparative skin tests, and IgE reactivity of fungal extracts. Clin. Exp. Allergy 2003, 33, 1429–1438. [Google Scholar] [CrossRef]
- Simon-Nobbe, B.; Denk, U.; Pöll, V.; Rid, R.; Breitenbach, M. The spectrum of fungal allergy. Int. Arch. Allergy Immunol. 2008, 145, 58–86. [Google Scholar] [CrossRef] [PubMed]
- Crameri, R.; Garbani, M.; Rhyner, C.; Huitema, C. Fungi: The neglected allergenic sources. Allergy 2014, 69, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Corey, J.P.; Kaiseruddin, S.; Gungor, A. Prevalence of mold-specific immunoglobulins in a Midwestern allergy practice. Otolaryngol. Head Neck Surg. 1997, 117, 516–520. [Google Scholar] [CrossRef]
- Fernández-Soto, R.; Navarrete-Rodríguez, E.M.; Del-Rio-Navarro, B.E.; Sienra-Monge, J.J.L.; Meneses-Sánchez, N.A.; Saucedo-Ramírez, O.J. Fungal Allergy: Pattern of sensitization over the past 11 years. Allergol. Immunopathol. 2018, 46, 557–564. [Google Scholar] [CrossRef]
- Rodríguez-Orozco, A.; Moreno-Chimal, K.; Méndez-López, T.; Gómez-Alonso, C. Prevalencia comparada de sensibilización a géneros de hongos alergénicos en pacientes con alergias respiratorias provenientes de Michoacán y Altos de Jalisco-León, Gto., años 2004–2006 vs 2007–2009. Rev. Mex. Micol. 2010, 32, 1–9. [Google Scholar]
- Forkel, S.; Beutner, C.; Schröder, S.S.; Bader, O.; Gupta, S.; Fuchs, T.; Schön, M.P.; Geier, J.; Buhl, T. Sensitization against Fungi in Patients with Airway Allergies over 20 Years in Germany. Int. Arch. Allergy Immunol. 2021, 182, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C. Fungi and Atopy. Clin. Rev. Allergy Immunol. 2019, 57, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, P.-J.; Chinn, S.; Janson, C.; Kogevinas, M.; Burney, P.; Jarvis, D. Geographical variation in the prevalence of positive skin tests to environmental aeroallergens in the European Community Respiratory Health Survey I. Allergy 2007, 62, 301–309. [Google Scholar] [CrossRef]
- D’Amato, G.; Chatzigeorgiou, G.; Corsico, R.; Gioulekas, D.; Jäger, L.; Jäger, S.; Kontou-Fili, K.; Kouridakis, S.; Liccardi, G.; Meriggi, A.; et al. Evaluation of the prevalence of skin prick test positivity to Alternaria and Cladosporium in patients with suspected respiratory allergy. Allergy 1997, 52, 711–716. [Google Scholar] [CrossRef]
- McLaughlin, D.J.; Hibbett, D.S.; Lutzoni, F.; Spatafora, J.W.; Vilgalys, R. The search for the fungal tree of life. Trends Microbiol. 2009, 17, 488–497. [Google Scholar] [CrossRef]
- Williams, P.B.; Barnes, C.S.; Portnoy, J.M. Innate and adaptive immune response to fungal products and allergens. J. Allergy Clin. Immunol. Pract. 2016, 4, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Bush, R.K.; Portnoy, J.M. The role and abatement of fungal allergens in allergic diseases. J. Allergy Clin. Immunol. 2001, 107 (Suppl. 3), S430–S440. [Google Scholar] [CrossRef]
- Soeria-Atmadja, D.; Önell, A.; Borga, A. IgE sensitization to fungi mirrors fungal phylogenetic systematics. J. Allergy Clin. Immunol. 2010, 125, 1379–1386. [Google Scholar] [CrossRef]
- WHO/IUIS Allergen Nomenclature. Allergen Nomenclature. Available online: http://allergen.org/ (accessed on 1 September 2023).
- Cramer, R.A.; Rivera, A.; Hohl, T.M. Immune responses against Aspergillus fumigatus: What have we learned? Curr. Opin. Infect. Dis. 2011, 24, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Crameri, R. Structural aspects of fungal allergens. Semin. Immunopathol. 2015, 37, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, D.; Hu, F.; Pacheco, F.; Landuyt, J.; Barnes, C.; Portnoy, J. Northern Blot Identification of mRNA Containing Sequence for Protein Allergen, Alt a 1, in Eight Strains of Alternaria alternata. Ann. Allergy Asthma Immunol. 1998, 80, 471–475. [Google Scholar] [CrossRef]
- Teifoori, F.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M.; Martinez, J. Gene profiling and expression of major allergen Alt a 1 in Alternaria alternata and related members of the Pleosporaceae family. Rev. Iberoam. Micol. 2019, 36, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Saenz-De-Santamaria, M.; Postigo, I.; Gutierrez-Rodriguez, A.; Cardona, G.; Guisantes, J.A.; Asturias, J.; Martinez, J. The major allergen of Alternaria alternata (Alt a 1) is expressed in other members of the Pleosporaceae family. Mycoses 2006, 49, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, S.; Sandler, P.; Raith, M.; Pascal, M.; Munoz-Cano, R.M.; Bartolome, C.S.; Nöbauer, K.; Quirce, S.; Razzazi-Fazeli, E.; Focke-Tejkl, M.; et al. Identification of Ulocladium chartarum as an important indoor allergen source. Allergy 2021, 76, 3202–3206. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach, M.; Simon, B.; Probst, G.; Oberkofler, H.; Ferreira, F.; Briza, P.; Achatz, G.; Unger, A.; Ebner, C.; Kraft, D.; et al. Enolases are highy conserved fungal allergens. Int. Arch. Allergy Immunol. 1997, 113, 114–117. [Google Scholar] [CrossRef]
- Unger, A.; Stöger, P.; Simon-Nobbe, B.; Susani, M.; Crameri, R.; Ebner, C.; Hintner, H.; Breitenbach, M. Clinical testing of recombinant allergens of the mold Alternaria alternata. Int. Arch. Allergy Immunol. 1999, 118, 220–221. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Gupta, R.; Jhingran, A.; Singh, B.P.; Sridhara, S.; Gaur, S.N.; Arora, N. Cloning, recombinant expression and activity studies of a major allergen ‘enolase’ from the fungus Curvularia lunata. J. Clin. Immunol. 2006, 26, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.J.; Holland, J.P. Isolation and identification of yeast messenger ribonucleic acids coding for enolase, glyceraldehyde-3-phosphate dehydrogenase, and phosphoglycerate kinase. Biochemistry 1978, 17, 4900–4907. [Google Scholar] [CrossRef]
- Pfeiffer, S.; Raith, M.; Pascal, M.; Munoz-Cano, R.M.; Bartolome, C.S.; Nöbauer, K.; Quirce, S.; Razzazi-Fazeli, E.; Focke-Tejkl, M.; Sterflinger, K.; et al. The emerging pathogen Paecilomyces variotii—A novel and important fungal allergen source. Allergy 2022, 77, 1045–1048. [Google Scholar] [CrossRef] [PubMed]
- Bush, R.K. Fungal Extracts in Clinical Practice. Allergy Asthma Proc. 1993, 14, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Løwenstein, H.; Aukrust, L.; Gravesen, S. Cladosporium herbarum Extract Characterized by Means of Quantitative Immunoelectrophoretic Methods with Special Attention to Immediate Type Allergy. Int. Arch. Allergy Immunol. 1977, 55, 1–12. [Google Scholar] [CrossRef]
- Yunginger, J.W.; Roberts, G.D.; Gleich, G.J. Studies on Alternaria allergens. J. Allergy Clin. Immunol. 1976, 57, 293–301. [Google Scholar] [CrossRef]
- Vijay, H.M.; Huang, H.; Young, N.M.; Bernstein, I.L. Studies on Alternaria Allergens. Int. Arch. Allergy Immunol. 1984, 74, 256–261. [Google Scholar] [CrossRef]
- Esch, R.E. Manufacturing and standardizing fungal allergen products. J. Allergy Clin. Immunol. 2004, 113, 210–215. [Google Scholar] [CrossRef]
- Kespohl, S.; Maryska, S.; Zahradnik, E.; Sander, I.; Brüning, T.; Raulf-Heimsoth, M. Biochemical and immunological analysis of mould skin prick test solution: Current status of standardization. Clin. Exp. Allergy 2013, 43, 1286–1296. [Google Scholar] [CrossRef]
- Twaroch, T.; Curin, M.; Sterflinger, K.; Focke-Tejkl, M.; Swoboda, I.; Valenta, R. Specific Antibodies for the Detection of Alternaria Allergens and the Identification of Cross-Reactive Antigens in Other Fungi. Int. Arch. Allergy Immunol. 2016, 170, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Wurth, M.A.; Hadadianpour, A.; Horvath, D.J.; Daniel, J.; Bogdan, O.; Goleniewska, K.; Pomés, A.; Hamilton, R.G.; Peebles, R.S.; Smith, S.A. Human IgE mAbs define variability in commercial Aspergillus extract allergen composition. JCI Insight 2018, 3, e123387. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.F.; Postigo, I.; Tomaz, C.T.; Martínez, J. Alternaria alternata allergens: Markers of exposure, phylogeny and risk of fungi-induced respiratory allergy. Environ. Int. 2016, 89–90, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Malling, H.-J.; Agrell, B.; Croner, S.; Dreborg, S.; Foucard, T.; Kjellman, M.; Koivikko, A.; Roth, A.; Weeke, B. Diagnosis and Immunotherapy of Mould Allergy. Allergy 1985, 40, 108–114. [Google Scholar] [CrossRef]
- Piechura, J.E.; Huang, C.J.; Cohen, S.H.; Kidd, J.M.; Kurup, V.P.; Calvanico, N.J. Antigens of Aspergillus fumigatus. II. Electrophoretic and clinical studies. Immunology 1983, 49, 657–665. [Google Scholar]
- Vailes, L.; Sridhara, S.; Cromwell, O.; Weber, B.; Breitenbach, M.; Chapman, M. Quantitation of the major fungal allergens, Alt a 1 and Asp f 1, in commercial allergenic products. J. Allergy Clin. Immunol. 2001, 107, 641–646. [Google Scholar] [CrossRef]
- Tarlo, S.M.; Fradkin, A.; Tobin, R.S. Skin testing with extracts of fungal species derived from the homes of allergy clinic patients in Toronto, Canada. Clin. Exp. Allergy 1988, 18, 45–52. [Google Scholar] [CrossRef]
- Asturias, J.A.; Ibarrola, I.; Ferrer, A.; Andreu, C.; López-Pascual, E.; Quiralte, J.; Florido, F.; Martínez, A. Diagnosis of Alternaria alternata sensitization with natural and recombinant Alt a 1 allergens. J. Allergy Clin. Immunol. 2005, 115, 1210–1217. [Google Scholar] [CrossRef]
- Lorenz, A.R.; Lüttkopf, D.; Seitz, R.; Vieths, S. The Regulatory System in Europe with Special Emphasis on Allergen Products. Int. Arch. Allergy Immunol. 2008, 147, 263–275. [Google Scholar] [CrossRef]
- Larenas-Linnemann, D.; Baxi, S.; Phipatanakul, W.; Portnoy, J.M.; Environmental Allergens Workgroup. Clinical Evaluation and Management of Patients with Suspected Fungus Sensitivity. J. Allergy Clin. Immunol. Pract. 2016, 4, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Grendelmeier, P.; Crameri, R. Recombinant allergens for skin testing. Int. Arch. Allergy Immunol. 2001, 125, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Carnés, J.; Iraola, V.; Gallego, M.; Leonor, J.R. Control Process for Manufacturing and Standardization of Allergenic Molecules. Curr. Allergy Asthma Rep. 2015, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.Y.; Hong, C.-S.; Lee, J.-S.; Park, J.-W. Optimization of Allergen Standardization. Yonsei Med. J. 2011, 52, 393. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marquardt, H. Spontaneous Mutations in Fungi. Humangenetik 1972, 16, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.; Gutiérrez, A.; Postigo, I.; Cardona, G.; Guisantes, J. Variability of Alt a 1 expression by different strains of Alternaria alternata. J. Investig. Allergol. Clin. Immunol. 2006, 16, 279–282. [Google Scholar] [PubMed]
- Steringer, I.; Aukrust, L.; Einarsson, R. Variability of Antigenicity/Allergenicity in Different Strains of Alternaria alternata. Int. Arch. Allergy Immunol. 1987, 84, 190–197. [Google Scholar] [CrossRef]
- Schumacher, M.; Jeffery, S. Variability of Biochemical and immunological characteristics of culture filtrates from seven isolates. J. Allergy Clin. Immunol. 1976, 58, 263–277. [Google Scholar] [CrossRef]
- Vijay, H.M.; Young, N.M.; Jackson, G.E.D.; White, G.P.; Bernstein, I.L. Studies on Alternaria Allergens. Int. Arch. Allergy Immunol. 1985, 78, 37–42. [Google Scholar] [CrossRef]
- Kendrick, B. The Fifth Kingdom; Mycologue Publications: Toronto, ON, Canada, 1985. [Google Scholar]
- Burge, H.A. Fungus allergens. Clin. Rev. Allergy 1985, 3, 319–329. [Google Scholar] [CrossRef]
- Vijay, H.M.; Burton, M.; Young, N.M.; Copeland, D.F.; Corlett, M. Allergenic components of isolates of Cladosporium herbarum. Grana 1991, 30, 161–165. [Google Scholar] [CrossRef]
- Helm, R.; Squillace, D.; Aukrust, L.; Borch, S.; Baer, H.; Bush, R.; Løwenstein, H.; Znamirowski, R.; Nitchuk, W.; Yunginger, J. Production of an International Reference Standard Alternaria Extract. Int. Arch. Allergy Immunol. 1987, 82, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Bisht, V.; Kukreja, N.; Singh, B.; Arora, N.; Sridhara, S. Current status of fungal allergens. Indian. J. Allergy Asthma Immunol. 2003, 17, 9–19. [Google Scholar]
- Esch, R.E. Allergen Source Materials and Quality Control of Allergenic Extracts. Methods 1997, 13, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Reed, C. Variability of antigenicity of Aspergillus fumigatus. J. Allergy Clin. Immunol. 1978, 61, 227–229. [Google Scholar] [CrossRef]
- Gupta, R.; Singh, B.P.; Sridhara, S.; Gaur, S.N.; Chaudhary, V.K.; Arora, N. Allergens of Curvularia lunata during cultivation in different media. J. Allergy Clin. Immunol. 1999, 104, 857–862. [Google Scholar] [CrossRef]
- Fraczek, M.G.; Rashid, R.; Denson, M.; Denning, D.W.; Bowyer, P. Aspergillus fumigatus allergen expression is coordinately regulated in response to hydrogen peroxide and cyclic AMP. Clin. Mol. Allergy 2010, 8, 15. [Google Scholar] [CrossRef]
- Thurston, J.R.; Richard, J.L.; McMillen, S. Cultural and serological comparison of ten strains of Aspergillus fumigatus Fresenius. Mycopathol. Mycol. Appl. 1973, 51, 327–335. [Google Scholar] [CrossRef]
- Bisht, V.; Singh, B.P.; Arora, N.; Sridhara, S. Allergens of Epicoccum nigrum grown in different media for quality source material. Allergy 2000, 55, 274–280. [Google Scholar] [CrossRef]
- van der Heide, S.; Kauffman, H.F.; de Vries, K. Cultivation of Fungi in Synthetic and Semi-Synthetic Liquid Medium. Allergy 1985, 40, 586–591. [Google Scholar] [CrossRef]
- Pfeiffer, S.; Focke-Tejkl, M.; Sterflinger, K.; Swoboda, I. Optimizing cultivation conditions for the highest expression of fungal allergens. Ann. Allergy Asthma Immunol. 2023, 130, 479–484.e3. [Google Scholar] [CrossRef]
- Ibarrola, I.; Suárez-Cervera, M.; Arilla, M.C.; Martínez, A.; Monteseirín, J.; Conde, J.; Asturias, J.A. Production profile of the major allergen Alt a 1 in Alternaria alternata cultures. Ann. Allergy Asthma Immunol. 2004, 93, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Little, S.A.; Longbottom, J.L.; Warner, J.O. Optimized preparation of Aspergillus fumigatus extracts for allergy diagnosis. Clin. Exp. Allergy 1993, 23, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, D.K.; Burrell, R. Further environmental factors affecting the antigenicity of Trichophyton rubrum. Mycopathol. Mycol. Appl. 1970, 42, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, H.F.; de Vries, K. Antibodies against Aspergillus fumigatus. Int. Arch. Allergy Immunol. 1980, 62, 252–264. [Google Scholar] [CrossRef]
- Machida, M.; Chang, Y.C.; Manabe, M.; Yasukawa, M.; Kunihiro, S.; Jigami, Y. Molecular cloning of a cDNA encoding enolase from the filamentous fungus, Aspergillus oryzae. Curr. Genet. 1996, 30, 423–431. [Google Scholar] [CrossRef]
- McAlister, L.; Holland, M.J. Targeted deletion of a yeast enolase structural gene. Identification and isolation of yeast enolase isozymes. J. Biol. Chem. 1982, 257, 7181–7188. [Google Scholar] [CrossRef]
- Hubballi, M.; Nakkeeran, S.; Raguchander, T.; Anand, T.; Samiyappan, R. Effect of Environmental Conditions on Growth of Alternaria alternata causing Leaf Blight of Noni. World J. Agric. Sci. 2010, 6, 171–177. [Google Scholar]
- Mitakakis, T.Z.; O’meara, T.J.; Tovey, E.R. The effect of sunlight on allergen release from spores of the fungus Alternaria. Grana 2003, 42, 43–46. [Google Scholar] [CrossRef]
- Rotem, J. The Role of Solar Radiation, Especially Ultraviolet, in the Mortality of Fungal Spores. Phytopathology 1985, 75, 510. [Google Scholar] [CrossRef]
- Fargues, J.; Goettel, M.S.; Smits, N.; Ouedraogo, A.; Vidal, C.; Lacey, L.A.; Lomer, C.J.; Rougier, M. Variability in susceptibility to simulated sunlight of conidia among isolates of entomopathogenic Hyphomycetes. Mycopathologia 1996, 135, 171–181. [Google Scholar] [CrossRef]
- Parnell, M.; Burt, P.J.A.; Wilson, K. The influence of exposure to ultraviolet radiation in simulated sunlight on ascospores causing Black Sigatoka disease of banana and plantain. Int. J. Biometeorol. 1998, 42, 22–27. [Google Scholar] [CrossRef]
- Hauck, P.R.; Williamson, S. The Manufacture of Allergenic Extracts in North America. Clin. Rev. Allergy Immunol. 2001, 21, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, H.F.; van der Heode, S.; van der Laan, S.; Hovenga, H.; Beaumont, F.; de Vries, K. Standardization of allergenic extracts of Aspergillus fumigatus. Int. Arch. Allergy Immunol. 1985, 76, 168–173. [Google Scholar] [CrossRef]
- Kroutil, L. Allergen production by Alternaria alternata strain 46582 is dependent upon culturing time. J. Allergy Clin. Immunol. 1984, 73, 113. [Google Scholar]
- Lang-Yona, N.; Levin, Y.; Dannemiller, K.C.; Yarden, O.; Peccia, J.; Rudich, Y. Changes in atmospheric CO2 influence the allergenicity of Aspergillus fumigatus. Glob. Chang. Biol. 2013, 19, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, H.; van der Heide, S.; Beaumont, F.; Demonchy, J.; de Vries, K. The allergenic and antigenic properties of spore extracts of: A comparative study of spore extracts with mycelium and culture filtrate extracts. J. Allergy Clin. Immunol. 1984, 73, 567–573. [Google Scholar] [CrossRef]
- Vijay, S.M.; Burton, M.; Young, N.M.; Corlette, M.; Bernstein, I.L. Comparative studies of allergens from mycelia and culture media of four new strains of Alternaria tenuis. Grana 1989, 28, 53–61. [Google Scholar] [CrossRef]
- Dreborg, S.; Einarsson, R. The major allergen content of allergenic preparations reflects their biological activity. Allergy 1992, 47, 418–423. [Google Scholar] [CrossRef]
- Longbottom, J.; Austwick, P. Fungal antigens. In Handbook of Experimental Immunology Vol I Immunochemistry, 4th ed.; Weir, D., Herzenberg, L., Blackwell, C., Eds.; Balckwell Scientific Publications: Oxford, UK, 1987; pp. 7.1–7.11. [Google Scholar]
- Mitakakis, T.; Barnes, C.; Tovey, E. Spore germination increases allergen release from Alternaria. J. Allergy Clin. Immunol. 2001, 107, 388–390. [Google Scholar] [CrossRef]
- Green, B.; Mitakakis, T.; Tovey, E. Allergen detection from 11 fungal species before and after germination. Immunology 2003, 111, 285–289. [Google Scholar] [CrossRef]
- Zahradnik, E.; Kespohl, S.; Sander, I.; Schies, U.; Khosravie-Hohn, J.; Lorenz, W.; Engelhart, S.; Kolk, A.; Schneider, G.; Brüning, T.; et al. A new immunoassay to quantify fungal antigens from the indoor mould Aspergillus versicolor. Environ. Sci. Process Impacts 2013, 15, 1162. [Google Scholar] [CrossRef] [PubMed]
- Curran, I.; Burton, M.; Muradia, G.; Zhang, L.; Vijay, H. Influence of culture conditions on extraction time period of Alternaria alternata allergens. J. Allergy Clin. Immunol. 1993, 91, 273. [Google Scholar]
- Larsen, J.N.; Dreborg, S. Standardization of Allergen Extracts; Humana: New York, NY, USA, 2019; pp. 63–76. [Google Scholar] [CrossRef]
- Weissman, D.N.; Halmepuro, L.; Salvaggio, J.E.; Lehrer, S.B. Antigenic/Allergenic Analysis of Basidiomycete Cap, Mycelia, and Spore Extracts. Int. Arch. Allergy Immunol. 1987, 84, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.; Gangal, S.V. Fusarium solani: Immunochemical Characterization of Allergens. Int. Arch. Allergy Immunol. 1994, 104, 175–183. [Google Scholar] [CrossRef]
- Krishnaswamy, A.; Barnes, N.; Lotlikar, N.P.; Damare, S.R. An Improved Method for Protein Extraction from Minuscule Quantities of Fungal Biomass. Indian J. Microbiol. 2019, 59, 100–104. [Google Scholar] [CrossRef]
- Bush, R.K.; Yunginger, J.W. Standardization of fungal allergens. Clin. Rev. Allergy 1987, 5, 3–21. [Google Scholar] [CrossRef]
- Ariaee, N.; Sankian, M.; Varasteh, A.; Moghadam, M.; Jabbari, F. Introducing a Stabilizer Formulation for Allergenic Mold Extracts. Rep. Biochem. Mol. Biol. 2020, 9, 106–114. [Google Scholar] [CrossRef]
- Bouziane, H.; Latgé, J.P.; Mecheri, S.; Fitting, C.; Prévost, M.C. Release of Allergens from Cladosporium Conidia. Int. Arch. Allergy Immunol. 1989, 88, 261–266. [Google Scholar] [CrossRef]
- Wongtim, S.; Lehrer, S.B.; Salvaggio, J.E.; Horner, W.E. Protease Activity in Cockroach and Basidiomycete Allergen Extracts. Allergy Asthma Proc. 1993, 14, 263–268. [Google Scholar] [CrossRef]
- Daigle, B.J.; Rekkerth, D.J. Practical Recommendations for Mixing Allergy Immunotherapy Extracts. Allergy Rhinol. 2015, 6, ar.2015.6.0111. [Google Scholar] [CrossRef]
- Balenga, N.A.; Klichinsky, M.; Xie, Z.; Chan, E.C.; Zhao, M.; Jude, J.; Laviolette, M.; Panettieri, R.A., Jr.; Druey, K.M. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat. Commun. 2015, 6, 6763. [Google Scholar] [CrossRef] [PubMed]
- Rijckaert, G.; Broers, J.L.V. Time Dependent Release of Allergens from Some Xerophilic Fungi. Allergy 1980, 35, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Grier, T.J.; Hazelhurst, D.M.; Duncan, E.A.; West, T.K.; Esch, R.E. Major allergen measurements: Sources of variability, validation, quality assurance, and utility for laboratories, manufacturers, and clinics. Allergy Asthma Proc. 2002, 23, 125–131. [Google Scholar]
- Wallenbeck, I.; Aukrust, L.; Einarsson, R. Antigenic Variability of Different Strains of Aspergillus fumigatus. Int. Arch. Allergy Immunol. 1984, 73, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Karaulov, A.; Niederberger, V.; Zhernov, Y.; Elisyutina, O.; Campana, R.; Focke-Tejkl, M.; Curin, M.; Namazova-Baranova, L.; Wang, J.-Y.; et al. Allergen Extracts for In Vivo Diagnosis and Treatment of Allergy: Is There a Future? J. Allergy Clin. Immunol. Pr. 2018, 6, 1845–1855.e2. [Google Scholar] [CrossRef] [PubMed]
- Vijay, H.M.; Young, N.M.; Curran, I.H.A.; Copeland, D.F.; Bernstein, I.L. A major antigen of Alternaria alternata with potential for safe and effective immunotherapy. J. Allergy Clin. Immunol. 1993, 91, 826–828. [Google Scholar] [CrossRef]
- Yunginger, J.W. Allergens: Recent Advances. Pediatr. Clin. N. Am. 1988, 35, 981–993. [Google Scholar] [CrossRef]
- Breitenbach, M.; Simon-Nobbe, B. The allergens of Cladosporium herbarum and Alternaria alternata. In Fungal Allergy and Pathogenicitiy; Karger: Basel, Switzerland, 2002; pp. 48–72. [Google Scholar]
- Aas, K.; Leegaard, J.; Aukrust, L.; Grimmer, Ø. Immediate Type Hypersensitivity to Common Moulds Comparison of Different Diagnostic Materials. Allergy 1980, 35, 443–451. [Google Scholar] [CrossRef]
- Portnoy, J.; Chapman, J.; Burge, H.; Muilenberg, M.; Solomon, W. Epicoccum allergy: Skin reaction patterns and spore/mycelium disparities recognized by IgG and IgE ELISA inhibition. Ann. Allergy 1987, 59, 39–43. [Google Scholar]
- Bonertz, A.; Roberts, G.; Slater, J.E.; Bridgewater, J.; Rabin, R.L.; Hoefnagel, M.; Timon, M.; Pini, C.; Pfaar, O.; Sheikh, A.; et al. Allergen manufacturing and quality aspects for allergen immunotherapy in Europe and the United States: An analysis from the EAACI AIT Guidelines Project. Allergy 2018, 73, 816–826. [Google Scholar] [CrossRef]
- Zimmer, J.; Vieths, S.; Kaul, S. Standardization and Regulation of Allergen Products in the European Union. Curr. Allergy Asthma Rep. 2016, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, J.; Bridgewater, J.; Ferreira, F.; van Ree, R.; Rabin, R.L.; Vieths, S. The History, Present and Future of Allergen Standardization in the United States and Europe. Front. Immunol. 2021, 12, 725831. [Google Scholar] [CrossRef] [PubMed]
- van Kampen, V.; de Blay, F.; Folletti, I.; Kobierski, P.; Moscato, G.; Olivieri, M.; Quirce, S.; Sastre, J.; Walusiak-Skorupa, J.; Raulf-Heimsoth, M. EAACI position paper: Skin prick testing in the diagnosis of occupational type I allergies. Allergy 2013, 68, 580–584. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfeiffer, S.; Swoboda, I. Problems Encountered Using Fungal Extracts as Test Solutions for Fungal Allergy Diagnosis. J. Fungi 2023, 9, 957. https://doi.org/10.3390/jof9100957
Pfeiffer S, Swoboda I. Problems Encountered Using Fungal Extracts as Test Solutions for Fungal Allergy Diagnosis. Journal of Fungi. 2023; 9(10):957. https://doi.org/10.3390/jof9100957
Chicago/Turabian StylePfeiffer, Sandra, and Ines Swoboda. 2023. "Problems Encountered Using Fungal Extracts as Test Solutions for Fungal Allergy Diagnosis" Journal of Fungi 9, no. 10: 957. https://doi.org/10.3390/jof9100957
APA StylePfeiffer, S., & Swoboda, I. (2023). Problems Encountered Using Fungal Extracts as Test Solutions for Fungal Allergy Diagnosis. Journal of Fungi, 9(10), 957. https://doi.org/10.3390/jof9100957






