Mold in Paradise: A Review of Fungi Found in Libraries
Abstract
1. Introduction
2. Methods for Sampling and Identification of Fungi
2.1. Sampling Methods
2.1.1. Air Sampling
2.1.2. Surface Sampling
2.1.3. Factors Affecting Sampling
2.1.4. Culture-Based and Non-Culture-Based Sampling
2.2. Fungal Identification Techniques
2.2.1. Microscopic Identification
2.2.2. Molecular Identification
2.2.3. Fourier Transform Infrared (FTIR) Spectroscopy Identification
2.2.4. VOCs Identification
2.2.5. Scanning Electron Microscopy (SEM) Identification
2.2.6. Biochemical Identification
3. Mold Prevention in Libraries
4. Literature Survey of Molds Found in Libraries
5. Results of the Literature Survey
6. Discussion
7. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borges, J.L.; Weinberger, E. Selected Non-Fictions; Penguin: London, UK, 2000; p. 575. ISBN 0140290117/9780140290110. [Google Scholar]
- Reed-Scott, J. Preserving Research Collections: A Collaboration between Librarians and Scholars. The Association of Research Libraries, the Modern Language Association, and the American Historical Association on Behalf of the Task Force on the Preservation of the Artifact. 1999. Available online: http://www.arl.org/preserv/prc.html (accessed on 23 June 2023).
- Orlean, S.; Schuster, S. The Library Book; Inner Traditions: Rochester, VT, USA, 2007; p. 336. ISBN 978-1-4767-4018-8. [Google Scholar]
- Polastron, L.X. Books on Fire; Inner Traditions: Rochester, VT, USA, 2007; p. 384. ISBN 9781594771675. [Google Scholar]
- Hammer, J. The Bad-Ass Librarians of Timbuktu; Simon & Schuster: New York, NY, USA, 2017; p. 388. ISBN 9781476777412. [Google Scholar]
- Zyska, B.; Żakowska, Z. Materials Microbiology; Lodz University of Technology: Łódź, Poland, 2005. [Google Scholar]
- Florian, E.M.L. The role of the conidia of fungi in fox spots. Stud. Conserv. 1996, 41, 65–75. [Google Scholar] [CrossRef]
- Ogden, B. Collection Preservation in Library Building Design; Libris Design Project; The U.S. Institute of Museum and Library Services: Washington, DC, USA, 2004; Available online: https://connectingtocollections.org/collection-preservation-in-library-building-design/ (accessed on 1 May 2023).
- Burge, H.A.; Person, D.L.; Groves, T.O.; Strawn, K.E.; Mishra, S.K. Dynamics of airborne fungal populations in a large office building. Curr. Microbiol. 2000, 40, 10–16. [Google Scholar] [CrossRef]
- Burge, H. An update on pollen and fungal spore aerobiology. J. Allergy Clin. Immunol. 2002, 110, 544–552. [Google Scholar] [PubMed]
- Florian, M.; Koestler, R.; Nicholson, K.; Parker, T.; Stanlley, T.; Szczepanowska, H.; Wagner, S. Chapter 12-Mold/fungi. In Paper Conservation Catalog; Bertalan, S., Ed.; American Institute for Conservation/Book and Paper Group: Washington, DC, USA, 1994; pp. 1–39. [Google Scholar]
- Konkol, N.R.; McNamara, C.J.; Hellman, E.; Mitchell, R. Early detection of fungal biomass on library materials. J. Cult. Herit. 2012, 13, 115–119. [Google Scholar] [CrossRef]
- Institute of Medicine (IOM). Damp Indoor Spaces and Health; The National Academies Press: Washington, DC, USA, 2004. [Google Scholar]
- World Health Organization (WHO). Guidelines for Indoor Air Quality: Dampness and Mold; Druckpartner Moser: Rheinbach, Germany, 2009. [Google Scholar]
- Mendell, M.J.; Mirer, A.G.; Cheung, K.; Tong, M.; Douwes, J. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: A review of the epidemiologic evidence. Environ. Health Perspect. 2011, 119, 748–756. [Google Scholar] [PubMed]
- Li, D.W.; Yang, C.S. Fungal contamination as a major contributor to Sick Building Syndrome. Adv. Appl. Microbiol. 2004, 55, 31–112. [Google Scholar] [PubMed]
- Redlich, C.A.; Sparer, J.; Cullen, M.R. Sick-building syndrome. Lancet 1997, 349, 1013–1016. [Google Scholar] [CrossRef]
- Norback, D. An update on sick building syndrome. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Yoshitake, N.; Hiroko, N.; Kohki, T.; Kayo, T.; Masamichi, H.; Tatsuya, H.; Chisato, M. Risk factors for the onset of sick building syndrome: A cross-sectional survey of housing and health in Japan. Build. Environ. 2021, 202, 107976. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. Microbiomes of the Built Environment: A Research Agenda for Indoor Microbiology, Human Health, and Buildings; The National Academies Press: Washington, DC, USA, 2017. [Google Scholar] [CrossRef]
- Rawlinson, S.; Ciric, L.; Cloutman-Green, E. How to carry out microbiological sampling of healthcare environment surfaces? A review of current evidence. J. Hosp. Infect. 2019, 103, 363–374. [Google Scholar]
- Prezant, B.; Weekes, D.M.; Miller, J.D. (Eds.) Recognition, Evaluation, and Control of Indoor Mold; American Industrial Hygiene Association: Fairfax, VA, USA, 2008. [Google Scholar]
- Chin, Y.A.; Heinsohn, P.A. Sampling and Analysis of Indoor Microorganisms; Wiley Interscience: Hoboken, NJ, USA, 2007. [Google Scholar]
- Flannigan, B.; Samson, R.A.; Miller, J.D. Microorganisms in Home and Indoor Work Environments: Diversity, Health Impacts, Investigation and Control; CRC Press: London, UK, 2011. [Google Scholar]
- Verdier, T.; Coutand, M.; Bertron, A.; Roques, C. A review of indoor microbial growth across building materials and sampling and analysis methods. Build. Environ. 2014, 80, 136–149. [Google Scholar] [CrossRef]
- Martin-Sanchez, P.M.; Nunez, M.; Estensmo, E.L.F.; Skrede, I.; Kauserud, H. Comparison of methods to iIdentify and monitor mold damages in buildings. Appl. Sci. 2022, 12, 9372. [Google Scholar] [CrossRef]
- Reponen, T. Sampling for microbial determinations. In Exposure to Microbiological Agents in Indoor and Occupational Environments; Viegas, C., Viegas, S., Gomes, A., Täubel, M., Sabino, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 85–96. [Google Scholar]
- EPA. Collection of Air Samples Potentially Contaminated with Microbiological Agents Using Impingers, Impactors, and Low-Volume Filters; EPA: Cincinnati, OH, USA, 2021. [Google Scholar]
- Rapid Microbiology. Air Samplers for Microbiological Monitoring of Air Quality. Available online: https://www.rapidmicrobiology.com/test-method/air-samplers (accessed on 22 September 2023).
- Ghosh, B.; Lal, H.; Srivastava, A. Review of bioaerosols in indoor environment with special reference to sampling, analysis and control mechanisms. Environ. Int. 2015, 85, 254–272. [Google Scholar] [CrossRef]
- Lin, X.J.; Reponen, T.; Willeke, K.; Wang, Z.; Grinshpun, S.A.; Trunov, M. Survival of airborne microorganisms during swirling aerosol collection. Aerosol. Sci. Technol. 2000, 32, 184–196. [Google Scholar] [CrossRef]
- Chen, Y.C.; Wang, I.J.; Cheng, C.C.; Wu, Y.C.; Bai, C.H.; Yu, K.P. Effect of selected sampling media, flow rate, and time on the sampling efficiency of a liquid impinger packed with glass beads for the collection of airborne viruses. Aerobiologia 2021, 37, 243–252. [Google Scholar] [CrossRef]
- Burge, H.A. Bioaerosols, 1st ed.; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar] [CrossRef]
- Andersen, G.L.; Frisch, A.S.; Kellogg, C.A.; Levetin, E.; Lighthart, B.; Paterno, D. Aeromicrobiology/Air Quality. Encycl. Microbiol. 2009, 11–26. [Google Scholar] [CrossRef]
- Wu, F.Q. Culture-based analytical methods for investigation of indoor fungi. In Sampling and Analysis of Indoor Microorganisms; Yang, C.S., Heinsohn, P.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Kastango, E.S.; Faylor, K. The Importance of Environmental Monitoring, Part II: Surface Testing. 2005, 2, 5. Available online: https://www.pppmag.com/article/100/September_2005/The_Importance_of_Environmental_Monitoring_Part_II_Surface_Testing/ (accessed on 13 September 2023).
- Frankel, M.; Bekö, G.; Timm, M.; Gustavsen, S.; Hansen, E.W.; Madsen, A.M. Seasonal variations of indoor microbial exposures and their relation to temperature, relative humidity, and air exchange rate. Appl. Environ. Microbiol. 2012, 78, 8289–8297. [Google Scholar] [CrossRef] [PubMed]
- Macher, J. (Ed.) Bioaerosol Assessment and Control; America Conference of Government Industrial Hygienists: Cincinnati, OH, USA, 1999. [Google Scholar]
- Buttner, M.P.; Stetzenbach, L.D. Monitoring airborne fungal spores in an experimental indoor environment to evaluate sampling methods and the effects of human activity on air sampling. Appl. Environ. Microhiol. 1993, 59, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Flannigan, B. Air sampling for fungi in indoor environments. J. Aerosol. Sci. 1997, 28, 381–392. [Google Scholar] [CrossRef]
- Zhou, G.; Whong, W.Z.; Ong, T.; Chen, B. Development of a fungus-specific PCR assay for detecting low-level fungi in an indoor environment. Mol. Cell Probes 2000, 14, 339–348. [Google Scholar] [CrossRef]
- Jeewon, R.; Hyde, K.D. Detection and diversity of fungi from environmental samples: Traditional versus molecular approaches. In Advanced Techniques in Soil Microbiology. Soil Biology; Varma, A., Oelmüller, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 11. [Google Scholar] [CrossRef]
- Niemeier, R.T.; Sivasubramani, S.K.; Reponen, T.; Grinshpun, S.A. Assessment of fungal contamination in moldy homes: Comparison of different methods. J. Occup. Environ. Hyg. 2006, 3, 262–273. [Google Scholar] [CrossRef]
- Nazaroff, W.W. Indoor bioaerosol dynamics. Indoor Air 2014, 26, 61–78. [Google Scholar] [CrossRef]
- David Miller, J.; Christopher Young, J. The Use of Ergosterol to Measure Exposure to Fungal Propagules in Indoor Air. Am. Ind. Hyg. Assoc. J. 1997, 58, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Florian, M.L.E. Fungal-problem monitoring for heritage collections: The need for baseline reference levels for fungal structures and beta-glucans. In Art, Biology, and Conservation: Biodeterioration of Works of Art; Metropolitan Museum of Art: New York, NY, USA, 2003; pp. 172–187. [Google Scholar]
- Michaelsen, A.; Pinzari, F.; Ripka, K.; Lubitz, W.; Piñar, G. Application of molecular techniques for identification of fungal communities colonising paper material. Int. Biodeterior. Biodegrad. 2006, 58, 133–141. [Google Scholar] [CrossRef]
- Canhoto, O.; Pinzari, F.; Fanelli, C.; Magan, N. Application of electronic nose technology for the detection of fungal contamination in library paper. Int. Biodeterior. Biodegrad. 2004, 54, 303–309. [Google Scholar] [CrossRef]
- Green, B.J.; Yli-Panula, E.; Tovey, E.R. Halogen Immunoassay, a New Method for the Detection of Sensitization to Fungal Allergens; Comparisons with Conventional Techniques. Allergol. Int. 2006, 55, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, J.; Thangadurai, D. Staining Techniques and Biochemical Methods for the Identification of Fungi. In Laboratory Protocols in Fungal Biology. Fungal Biology; Gupta, V., Tuohy, M., Ayyachamy, M., Turner, K., O’Donovan, A., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Cox, J.; Indugula, R.; Vesper, S.; Zhu, Z.; Jandarov, R.; Reponen, T. Comparison of indoor air sampling and dust collection methods for fungal exposure assessment using quantitative PCR. Environ. Sci. Process. Impacts 2017, 19, 1312–1319. [Google Scholar] [CrossRef]
- Xu, J. Fungal DNA barcoding. Genome 2016, 59, 913–932. [Google Scholar] [CrossRef]
- Amend, A.S.; Seifert, K.A.; Samson, R.; Bruns, T.D. Indoor fungal composition is geographically patterned and more diverse in temperate zones than in the tropics. Proc. Natl. Acad. Sci. USA 2010, 107, 13748–13753. [Google Scholar] [CrossRef] [PubMed]
- Korpelainen, H.; Pietiläinen, M.; Huotari, T. Effective detection of indoor fungi by metabarcoding. Ann. Microbiol. 2016, 66, 495–498. [Google Scholar] [CrossRef]
- Schena, L.; Nigro, F.; Ippolito, A.; Gallitelli, D. Real-time quantitative PCR: A new technology to detect and study phytopathogenic and antagonistic fungi. Eur. J. Plant Pathol. 2004, 110, 893–908. [Google Scholar] [CrossRef]
- Langsiri, N.; Worasilchai, N.; Irinyi, L.; Jenjaroenpun, P.; Wongsurawat, T.; Luangsa-Ard, J.J.; Meyer, W.; Chindamporn, A. Targeted sequencing analysis pipeline for species identification of human pathogenic fungi using long-read nanopore sequencing. IMA Fungus 2023, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, J.M.; Barnes, C.S.; Kennedy, K. Sampling for indoor fungi. J. Allergy Clin. Immunol. 2004, 113, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Dannemiller, K.C.; Lang-Yona, N.; Yamamoto, N.; Rudich, Y.; Peccia, J. Combining real-time PCR and next-generation DNA sequencing to provide quantitative comparisons of fungal aerosol populations. Atmos. Environ. 2014, 84, 113–121. [Google Scholar] [CrossRef]
- Stengel, A.; Stanke, K.M.; Quattrone, A.C.; Herr, J.R. Improving taxonomic delimitation of fungal species in the age of genomics and Phenomics. Front. Microbiol. 2022, 13, 847067. [Google Scholar] [CrossRef]
- Rakotonirainy, M.S.; Bénaud, O.; Vilmont, L.-B. Contribution to the characterization of foxing stains on printed books using infrared spectroscopy and scanning electron microscopy energy dispersive spectrometry. Int. Biodeterior. Biodegrad. 2015, 101, 1–7. [Google Scholar] [CrossRef]
- Lecellier, A.; Gaydou, V.; Mounier, J.; Hermet, A.; Castrec, L.; Barbier, G.; Ablain, W.; Manfait, M.; Toubas, D.; Sockalingum, G.D. Implementation of an FTIR spectral library of 486 filamentous fungi strains for rapid identification of molds. Food Microbiol. 2015, 45, 126–134. [Google Scholar] [CrossRef]
- Barboux, R.; Bousta, F.; Di Martino, P. FTIR Spectroscopy for Identification and Intra-Species Characterization of Serpula lacrymans. Appl. Sci. 2021, 11, 8463. [Google Scholar] [CrossRef]
- Inamdar, A.A.; Morath, S.; Bennett, J.W. Fungal Volatile Organic Compounds: More Than Just a Funky Smell? Annu. Rev. Microbiol. 2020, 74, 101–116. [Google Scholar] [CrossRef]
- Schuchardt, S.; Kruse, H. Quantitative volatile metabolite profiling of common indoor fungi: Relevancy for indoor air analysis. J. Basic Microbiol. 2009, 49, 350–362. [Google Scholar] [CrossRef]
- Cincinelli, A.; Martellini, T.; Amore, A.; Dei, L.; Marrazza, G.; Carretti, E.; Belosi, F.; Ravegnani, F.; Leva, P. Measurement of volatile organic compounds (VOCs) in libraries and archives in Florence (Italy). Sci. Total Environ. 2016, 572, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.T.; Ewlad-Ahmed, A.; Knight, B.; Horie, V.; Mitchell, G.; Robertson, C.J. Measurement of volatile organic compounds emitted in libraries and archives: An inferential indicator of paper decay? Chem. Cent. J. 2012, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Fantuzzi, G.; Aggazzotti, G.; Righi, E.; Cavazzuti, L.; Predieri, G.; Franceschelli, A. Indoor air quality in the university libraries of Modena (Italy). Sci. Total Environ. 1996, 193, 49–56. [Google Scholar] [CrossRef]
- RTI Laboratories. SEM/EDS Analysis. Available online: https://rtilab.com/techniques/sem-eds-analysis/ (accessed on 22 September 2023).
- Williams, S.T.; Veldkamp, C.J. Preparation of fungi for scanning electron microscopy. Trans. Br. Mycol. Soc. 1974, 63, 408–412. [Google Scholar] [CrossRef]
- Orrance, J.S. A justification of air-conditioning in libraries. J. Librariansh. 1975, 7, 199–206. [Google Scholar] [CrossRef]
- Shum, C.; Alipouri, Y.; Zhong, L. Examination of human interaction on indoor environmental quality variables: A case study of libraries at the University of Alberta. Build. Environ. 2022, 207, 108476. [Google Scholar] [CrossRef]
- Preston, L. Mold Prevention and Remediation in a Library Environment. Tex. Libr. J. 2015, 91, 60. [Google Scholar]
- René, T. Preservation of Archives in Tropical Climates: An Annotated Bibliography; International Council on Archives: Paris, France; National Archives of the Netherlands: The Hague, The Netherlands; National Archives of the Republic of Indonesia: Daerah Khusus Ibukota Jakarta, Indonesia, 2001. [Google Scholar]
- Wood, L. Prevention and Treatment of Molds in Library Collections, Especially in Tropical Climates: A RAMP Study; Prepared by Mary Wood Lee [for the] General Information Program and UNISIST; UNESCO: Paris, France, 1988; 56p. [Google Scholar]
- Ren, P.; Jankun, T.M.; Belanger, K.; Bracken, M.B.; Leaderer, B.P. The relation between fungal propagules in indoor air and home characteristics. Allergy 2001, 56, 419–424. [Google Scholar] [CrossRef]
- Bamba, I.; Azuma, M.; Hamada, N.; Kubo, H.; Isoda, N. Case study of airborne fungi according to air temperature and relative humidity in houses with semibasements adjacent to a forested hillside. Biocontrol Sci. 2014, 19, 1–9. [Google Scholar] [CrossRef]
- Brown, D.R. Collection disaster: Mold in the stacks. Coll. Res. Libr. News 2003, 64, 304–306. [Google Scholar] [CrossRef]
- Kalwasińska, A.; Burkowska, A.; Wilk, I. Microbial air contamination in indoor environment of a university library. Ann. Agric. Environ. Med. 2012, 19, 25–29. [Google Scholar] [PubMed]
- Burge, H.P.; Boise, J.R.; Solomon, W.R.; Bandera, E. Fungi in libraries: An aerometric survey. Mycopathologia 1978, 64, 67–72. [Google Scholar] [CrossRef]
- Stryjakowska-Sekulska, M.; Piotraszewska-Pająk, A.; Szyszka, A.; Nowicki, M.; Filipiak, M. Microbiological quality of indoor air in university rooms. Pol. J. Environ. Stud. 2007, 16, 623–632. [Google Scholar]
- Vittal, B.P.R.; Leela Glory, A. Airborne fungus spores of a library in India. Grana 1985, 24, 129–132. [Google Scholar] [CrossRef]
- Ruga, L.; Bonofiglio, T.; Orlandi, F.; Romano, B.; Fornaciari, M. Analysis of the potential fungal biodeteriogen effects in the “Doctorate Library” of the University of Perugia, Italy. Grana 2008, 47, 60–69. [Google Scholar] [CrossRef][Green Version]
- Begum, M.F.; Sarker, S.R.; Mahal, M.F.; Alam, S. Incidence of indoor airborne fungi at the central library of Rajshahi University and their relation to allergy symptoms. Chiang Mai Univ. J. Nat. Sci. 2011, 10, 269–281. [Google Scholar]
- Dalal, L.; Bhowal, M.; Kalbende, S. Incidence of deteriorating fungi in the air inside the college libraries of Wardha city. Sch. Res. Libr. 2011, 3, 479–485. [Google Scholar]
- Ghosh, B.; Himanshu, L.; Rajesh, K.; Naba, H.; Arun, S.; Jain, V.K. Estimation of bioaerosol in indoor environment in the university library of Delhi. Sustain. Environ. Res. 2013, 23, 199–207. [Google Scholar]
- Flores, M.E.; Medina, P.G.; Camacho, S.P.; de Jesús Uribe Beltrán, M.; De la Cruz Otero Mdel, C.; Ramírez, I.O.; Hernández, M.E. Fungal spore concentrations in indoor and outdoor air in university libraries, and their variations in response to changes in meteorological variables. Int. J. Environ. Health Res. 2014, 24, 320–340. [Google Scholar] [CrossRef] [PubMed]
- Kayarkar, A.; Bhajbhuje, M.N. Biodiversity of aeromycoflora from indoor environment of library. Int. J. Life Sci. 2014, 21–24. [Google Scholar]
- Hayleeyesus, S.F.; Manaye, A.M. Microbiological quality of indoor air in university libraries. Asian. Pac. J. Trop. Biomed 2014, 4, S312-7. [Google Scholar] [CrossRef]
- Paramjit, J.; Vaishali, T. Air monitoring of fungal spores inside the B. J. Wadia Library, Pune, India. Intern. J. Curr. Microbiol. Appl. Sci. 2015, 4, 35–40. [Google Scholar]
- Kakde, U.B. A study of fungal bioaerosols in library environment. Online Int. Interdiscip. Res. J. 2016, 6, 42–47. [Google Scholar]
- Valeriani, F.; Cianfanelli, C.; Gianfranceschi, G.; Santucci, S.; Romano Spica, V.; Mucci, N. Monitoring biodiversity in libraries: A pilot study and perspectives for indoor air quality. J. Prev. Med. Hyg. 2017, 58, E238–E251. [Google Scholar]
- Kadaifçiler, D. Indoor air quality of the library at İstanbul University, Turkey. Hacet. J. Biol. Chem. 2017, 45, 43–53. [Google Scholar] [CrossRef]
- Rahmawati, R.; Sembiring, L.; Zakaria, L.; Rahayu, E. The diversity of indoor airborne molds growing in the university libraries in Indonesia. Biodiversitas J. Biol. Divers. 2018, 19, 194–201. [Google Scholar] [CrossRef]
- Giri, S.K. Monitoring of airborne fungi in indoor environments of reading and stock sections of college library. Intern. J. Sci. Res. 2020, 9, 1567–1573. [Google Scholar]
- Pyrri, I.; Tripyla, E.; Zalachori, A.; Chrysopoulou, M.; Parmakelis, A.; Kapsanaki-Gotsi, E. Fungal contaminants of indoor air in the National Library of Greece. Aerobiologia 2020, 36, 387–400. [Google Scholar] [CrossRef]
- da Silva, P.D.; Calumby, R.J.N.; Ribeiro da Silva, L.N.; Omena de Oliveira, J.; Gaia de Sousa, J.R.; Cristina da Silva, D.; Moreira, R.T.F.; Araújo, M.A.D.S. Anemophilous fungi isolated from libraries of educational institutions in the Northeast of Brazil. Rev. Pan-Amazônica Saúde 2021, 12, 1–8. [Google Scholar] [CrossRef]
- Hassan, A.; Zeeshan, M.; Bhatti, M.F. Indoor and outdoor microbiological air quality in naturally and mechanically ventilated university libraries. Atmosph. Pollut. Res. 2021, 12, 101136. [Google Scholar] [CrossRef]
- Camargo Caicedo, Y.; Borja Pérez, H.; Muñoz Fuentes, M.; Vergara-Vásquez, E.; Vélez-Pereira, A.M. Assessment of fungal aerosols in a public library with natural ventilation. Aerobiologia 2023, 39, 37–50. [Google Scholar] [CrossRef]
- Staszowska, A.B. Exposure to bacterial and fungal aerosol in the university library—A case study. J. Ecol. Eng. 2023, 24, 252–258. [Google Scholar] [CrossRef]
- Shamsian, A.; Fata, A.; Mohajeri, M.; Ghazvini, K. Fungal contaminations in historical manuscripts at Astan QudsMuseum Library, Mashhad, Iran. Intern. J. Agric. Biol. 2006, 8, 420422. [Google Scholar]
- Michaelsen, A.; Piñar, G.; Montanari, M.; Pinzari, F. Biodeterioration and restoration of a 16th-century book using a combination of conventional and molecular techniques: A case study. Int. Biodeterior. Biodegrad. 2009, 63, 161–168. [Google Scholar] [CrossRef]
- Reis-Menezes, A.A.; Gambale, W.; Giudice, M.C. A survey of fungal contamination on books in public libraries with mechanical and natural ventilation. Indoor Built. Environ. 2011, 20, 393–399. [Google Scholar] [CrossRef]
- Montanari, M.; Melloni, V.; Pinzari, F.; Innocenti, G. Fungal biodeterioration of historical library materials stored in Compactus movable shelves. Int. Biodeterior. Biodegrad. 2012, 75, 83–88. [Google Scholar] [CrossRef]
- Kraková, L.; Chovanová, K.; Selim, S.A.; Šimonovičová, A.; Puškarová, A.; Maková, A.; Pangallo, D. A multiphasic approach for investigation of the microbial diversity and its biodegradative abilities in historical paper and parchment documents. Int. Biodeterior. Biodegrad. 2012, 70, 117–125. [Google Scholar] [CrossRef]
- El Bergadi, F.; Laachari, F.; Elabed, S.; Mohamed, I.H.; Ibrisouda, S.K. Cellulolytic potential and filter paper activity of fungi isolated from ancient manuscripts from the Medina of Fez. Ann. Microbiol. 2014, 64, 815–822. [Google Scholar] [CrossRef]
- Oetari, A.; Susetyo-Salim, T.; Sjamsuridzal, W.; Suherman, E.A.; Monica, M.; Wongso, R.; Fitri, R.; Nurlaili, D.G.; Ayu, D.C.; Teja, T.P. Occurrence of fungi on deteriorated old dluwang manuscripts from Indonesia. Int. Biodeterior. Biodegrad. 2016, 114, 94–103. [Google Scholar] [CrossRef]
- Alshibly, M.K.; Al Zamily, I.A.; Fazil, N. Survey of fungi found in books on the shelves of the libraries of the University of Qadisiyah–Iraq. IOP Conf. Ser. Mater. Sci. Eng. 2019, 571, 012042. [Google Scholar] [CrossRef]
- Sequeira, O.; Paiva de Carvalho, S.; Mesquita, H.; Portugal, N.; Macedo, M.F. Fungal stains on paper: Is what you see what you get? Conserv. Património 2019, 32, 18–27. [Google Scholar] [CrossRef]
- Abdel-Maksoud, G.; Abdel-Nasser, M.; Sultan, M.H.; Eid, A.M.; Alotaibi, S.H.; Hassan, S.E.; Fouda, A. Fungal biodeterioration of a historical manuscript dating back to the 14th Century: An insight into various fungal strains and their enzymatic cctivities. Life 2022, 12, 1821. [Google Scholar] [CrossRef]
- Fouda, A.; Abdel-Nasser, M.; Khalil, A.M.A.; Hassan, S.E.D.; Abdel-Maksoud, G. Investigate the role of fungal communities associated with a historical manuscript from the 17th century in biodegradation. NPJ Mater. Degrad. 2022, 6, 88. [Google Scholar] [CrossRef]
- Lugauskas, A.; Krikŝtaponis, A. Microscopic fungi found in the libraries of Vilnius and factors affecting their development. Indoor Built Environ. 2004, 13, 169–182. [Google Scholar] [CrossRef]
- Apetrei, I.C.; Drăgănescu, G.E.; Popescu, I.T.; Carp-Cărare, C.; Guguianu, E.; Mihăescu, T.; Ştefanache, A.; Creţu, C.; Patraş, X. Possible cause of allergy for the librarians: Books manipulation and ventilation as sources of fungus spores spreading. Aerobiologia 2009, 25, 159–166. [Google Scholar] [CrossRef]
- Zielinska-Jankiewicz, K.; Kozajda, A.; Piotrowska, M.; Szadkowska-Stanczyk, I. Microbiological contamination with molds in work environment in libraries and archive storage facilities. Ann. Agric. Environ. Med. 2008, 15, 71–78. [Google Scholar]
- Harkawy, A.; Górny, R.L.; Ogierman, L.; Wlazło, A.; Ławniczek-Wałczyk, A.; Niesler, A. Bioaerosol assessment in naturally ventilated historical library building with restricted personnel access. Ann. Agric. Environ. Med. 2011, 18, 323–329. [Google Scholar]
- Karbowska-Berent, J.; Górny, R.F.; Strzelczyk, A.B.; Wlazło, A. Airborne and dust borne microorganisms in selected Polish libraries and archives. Build. Environ. 2011, 46, 1872–1879. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Macedo, M.F.; Jurado, V.; Saiz-Jimenez, C.; Viegas, C.; Brandão, J.; Rosado, L. Mould and yeast identification in archival settings: Preliminary results on the use of traditional methods and molecular biology options in Portuguese archives. Int. Biodeterior. Biodegrad. 2011, 65, 619–627. [Google Scholar] [CrossRef]
- Leite, D.P., Jr.; Yamamoto, A.C.; Amadio, J.V.; Martins, E.R.; do Santos, F.A.; Simões, S.A.; Hahn, R.C. Trichocomaceae: Biodiversity of Aspergillus spp. and Penicillium spp. residing in libraries. J. Infect. Dev. Ctries. 2012, 6, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Pasquarella, C.; Saccani, E.; Sansebastiano, G.E.; Ugolotti, M.; Pasquariello, G.; Albertini, R. Proposal for a biological environmental monitoring approach to be used in libraries and archives. Ann. Agric. Environ. Med. 2012, 19, 209. [Google Scholar]
- Chadeganipour, M.; Ojaghi, R.; Rafiei, H.; Afshar, M.; Hashemi, S. Biodeterioration of library materials: Study of fungi threatening printed materials of libraries in Isfahan University of Medical Sciences in 2011. Jundishapur J. Microbiol. 2013, 6, 127–131. [Google Scholar] [CrossRef]
- Özkan, V.K. Microfungal contaminants on the surface of the books and the atmosphere of the library of Health Services Vocational School in Marmaris, Turkey. Int. J. Microbiol. Mycol. 2014, 2, 1–5. [Google Scholar]
- Skóra, J.; Gutarowska, B.; Pielech-Przybylska, K.; Stępień, Ł.; Pietrzak, K.; Piotrowska, M.; Pietrowski, P. Assessment of microbiological contamination in the work environments of museums, archives and libraries. Aerobiologia 2015, 31, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Oladokun, M.; Osman, S.B.; Samsuddin, N.; Hamzah, H.A.; Salleh, M.N. Influence of indoor micoclimate distribution on mould infestation in a university library. In InCIEC 2014: Proceedings of the International Civil and Infrastructure Engineering Conference 2014; Springer: Singapore, 2015; pp. 1075–1086. [Google Scholar] [CrossRef]
- Kalaskar, P.G.; Zodpe, S.N. Biodeterioration of library resources and possible approaches for their control. Int. J. Appl. Res. 2016, 2, 25–33. [Google Scholar]
- Wahab, S.N.A.; Mohammed, N.I.; Khamidi, M.F.; Ahmad, N.A.; Noor, Z.M.; Ghani, A.A.A.; Ismail, M.R. Sampling and identifying of mould in the library building. In Proceedings of the 4th International Building Control Conference, Kuala Lumpur, Malaysia, 7–8 March 2016; Available online: http://centaur.reading.ac.uk/69812/ (accessed on 15 September 2023).
- Micheluz, A.; Manente, S.; Prigione, V.; Tigini, V.; Pinzari, F.; Cristina Varese, G.; Ravagnan, G. Fungal contamination specifically related to the use of Compacts shelvings: The case study of a Venetian library. In Proceedings of the 11th International Conference on Non-Desctructive Investigations and Microanalysis for the Diagnostics and Conservation of Cultural and Environmental, Madrid, Spain, 11–13 June 2016. [Google Scholar] [CrossRef]
- Kadaifciler, D.G. Bioaerosol assessment in the library of Istanbul University and fungal flora associated with paper deterioration. Aerobiologia 2017, 33, 151–166. [Google Scholar] [CrossRef]
- Osman, M.; Abdel, A.; Yasser, I.; Youssef, A.; Amany, A.; Yuosra, S. Air microbial contamination and factors affecting its occurrence in certain book libraries in Egypt. Egypt. J. Bot. 2017, 57, 93–118. [Google Scholar] [CrossRef]
- Leite, D.J.; Pereira, R.; de Almeida, W.; Alves Simões, S.; Yamamoto, A.; de Souza, J.; Martins, E.; dos Santos, F.; Hahn, R. Indoor air mycological survey and occupational exposure in libraries in Mato Grosso-Central Region—Brazil. Adv. Microbiol. 2018, 8, 324–353. [Google Scholar] [CrossRef][Green Version]
- Okpalanozie, O.E.; Adebusoye, S.A.; Troiano, F.; Cattò, C.; Ilori, M.O.; Cappitelli, M. Assessment of indoor air environment of a Nigerian museum library and its biodeteriorated books using culture-dependent and–Independent techniques. Int. Biodeterior. Biodegrad. 2018, 132, 139–149. [Google Scholar] [CrossRef]
- Landry, K.; Ascension, N.; Armelle, D.; Hortense, G.; François-Xavier, E. Assessment of indoor microbial quality of library’s premise: Case of central library of the university of Yaoundé I. Open J. Prev. Med. 2018, 8, 109–120. [Google Scholar] [CrossRef][Green Version]
- Glevitzky, M.; Aleya, L.; Vică, M.L.; Dumitrel, G.A.; Avram, M.; Tit, D.M.; Popa, M.; Popa, V.C.; Behl, T.; Bungau, S. Assessing the microbiological contamination along with environmental factors of old books in the 1490-founded Bistrița Monastery, Romania. Environ. Sci. Pollut. Res. 2021, 28, 8743–8757. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, baaa062. [Google Scholar] [CrossRef]
- Duchaine, C.A. The importance of combining air sampling and surface analysis when studying problematic houses for mold biodiversity determination. Aerobiologia 2001, 17, 121–125. [Google Scholar] [CrossRef]
- Horner, W.E. Assessment of the indoor environment: Evaluation of mold growth. Immunol. Allergy Clin. N. Am. 2003, 23, 519–531. [Google Scholar] [CrossRef]
- Cabral, J.P. Can we use indoor fungi as bioindicators of indoor air quality? Historical perspectives and open questions. Sci. Total Environ. 2010, 408, 4285–4295. [Google Scholar] [CrossRef]
- Elbert, W.; Taylor, P.E.; Andreae, M.O.; Pöschl, U. Contribution of fungi to primary biogenic aerosols in the atmosphere: Wet and dry discharged spores, carbohydrates, and inorganic ions. Atmos. Chem. Phys. 2007, 7, 4569–4588. [Google Scholar] [CrossRef]
- Oliveira, M.; Amorim, M.I.; Ferreira, E.; Delgado, L.; Abreu, I. Main airborne Ascomycota spores: Characterization by culture, spore morphology, ribosomal DNA sequences and enzymatic analysis. Appl. Microbiol. Biotechnol. 2010, 86, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Gravesen, S.; Nielsen, P.A.; Iversen, R.; Nielsen, K.F. Microfungal contamination of damp buildings--examples of risk constructions and risk materials. Environ. Health Perspect. 1999, 107, 505–508. [Google Scholar] [CrossRef]
- Andersen, B.; Frisvad, J.C.; Søndergaard, I.; Rasmussen, I.S.; Larsen, L.S. Associations between fungal species and water-damaged building materials. Appl. Environ. Microbiol. 2011, 77, 4180–4188. [Google Scholar] [CrossRef] [PubMed]
- Latgé, J.P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef]
- Gammage, R.B.; Berven, B.A. Indoor Air and Human Health, 2nd ed.; CRC Lewis Pubs: Boca Raton, MA, USA, 1996. [Google Scholar]
- CDC. Centers for Disease Control. Testing and Remediation of Dampness and Mold Contamination. NIOSH. 2022. Available online: https://www.cdc.gov/niosh/topics/indoorenv/moldtesting.html (accessed on 15 April 2023).
- Reed, S. Airborne Mould: What should we be measuring & how? Australian Institute of Occupational Hygienists. In Proceedings of the AIOH 29th Annual Conference Proceedings, Brisbane, QLD, Australia, 3–7 December 2011. [Google Scholar]
- Yu, C.; Crump, D. Standards for evaluating indoor air. Indoor Built Environ. 2011, 20, 389–392. [Google Scholar] [CrossRef]
- Saffell, J.; Nehr, S. Improving indoor air quality through standardization. Standards 2023, 3, 240–267. [Google Scholar] [CrossRef]
- ISO. Indoor Air—Part 6: Determination of Volatile Organic Compounds in Indoor and Test Chamber Air by Active Sampling on Tenax TA Sorbent, Thermal Desorption and Gas Chromatography Using MS or MS-FID; ISO: Geneva, Switzerland, 2011. [Google Scholar]
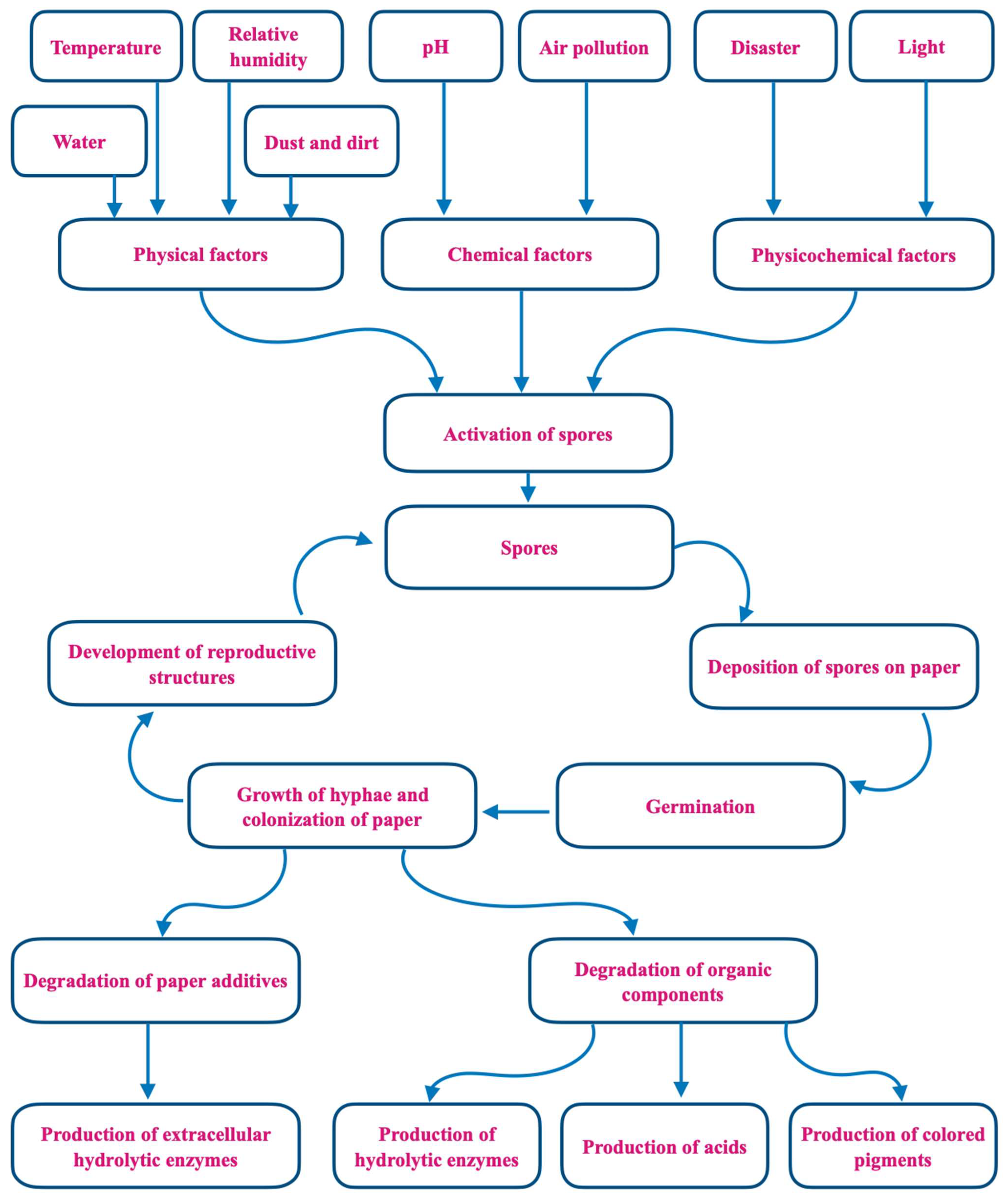

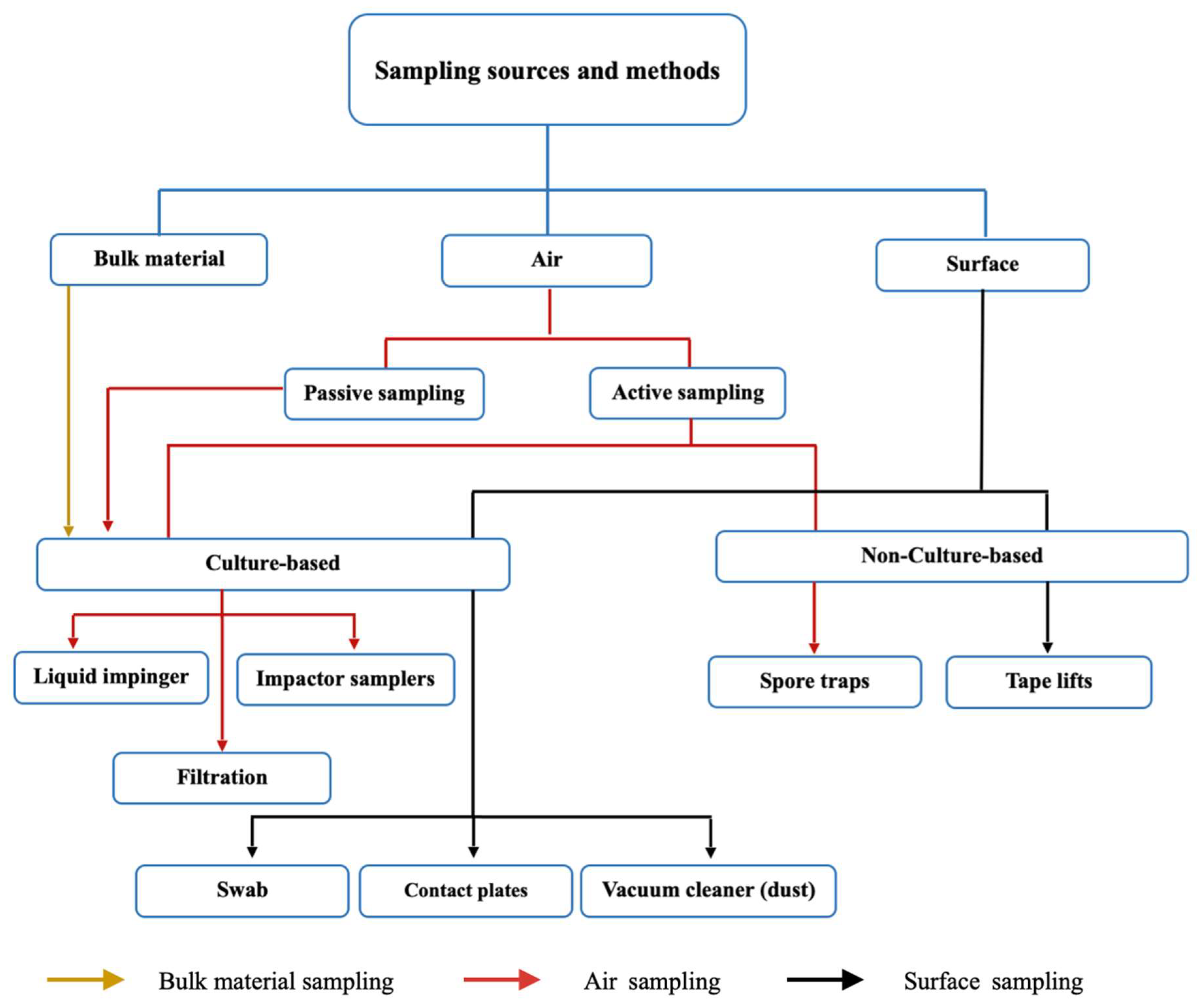
| Geographic Location of Library/Collection Method | Culture Media a/Identification Method b | Main Taxa of Fungi Detected c | Reference |
|---|---|---|---|
| USA Andersen volumetric viable particle samplers | MEA and FPA | Aspergillus fumigatus, Aspergillus niger, and Cladosporium herbarum | [79] |
| POLAND Settles plates | CZA, YEA, and SDA | Cladosporium herbarum, Penicillium chrysogenum, and Aspergillus niger | [80] |
| INDIA Rotorod sampler and settle plates | PDA and SDA | Rotorod sampling: Aspergillus spp., Cladosporium, and Nigrospora Plate sampling: Aspergillus spp., Curvularia, and Penicillium | [81] |
| ITALY Settles plates; personal volumetric air sampler spore trap | CDA and SDA | Alternaria sp., Chaetomium sp., and Cladosporium sp. | [82] |
| BANGLADESH Settle plates | Hemolytic activity test using blood agar | Alternaria, Aspergillus, and Curvularia | [83] |
| INDIA Settle plates | CZA and PDA | Aspergillus niger, Aspergillus fumigatus, and Curvularia | [84] |
| INDIA Buck Bioculture pump | PDA, EMA, and blood agar | Rhizopus oryzae, Aspergillus nidulans, and Aspergillus flavus | [85] |
| MEXICO Microbio MB2®Aerosol Sampler | PDA PCR (ITS1–5.8S–ITS2) | Aspergillus niger, Aspergillus tamarii, and Aspergillus oryzae | [86] |
| INDIA Settle plates | PDA | Aspergillus flavus, Cladosporium cladosporioides, and Mucor pusillus | [87] |
| ETHIOPIA Settle plate | SDA | Cladosporium sp., Alternaria sp., and Penicillium sp. | [88] |
| INDIA Rotorod air sampler | Spore counts | Aspergillus, Cladosporium, and Alternaria | [89] |
| INDIA Settle plates | SDA and PDA | Aspergillus niger, Aspergillus flavus, and Aspergillus fumigatus | [90] |
| ITALY Active Surface Air System (plate impact active sampler) | SDA PCR and NGS sequencing | Penicillium rivolii, Penicillium viticola, and Cladosporium uredinicola | [91] |
| TURKEY Portable volumetric microbiological air sampler | DG18, MEA, CDA, CY20S, CYA, and PDA | Aspergillus flavus, Penicillium chrysogenum, and Alternaria alternata | [92] |
| INDONESIA Settle plates | DG 18 for sampling; MEA, DG 18, or CY20S for identification | Aspergillus, Cladosporium, and Penicillium | [93] |
| INDIA Air sampler: Hi-Air sampler | Two media strips:PS-640 and PS-290 | Curvularia lunata, Curvularia geniculata, and Curvularia tetramera | [94] |
| GREECE Single-stage Portable Burkard sampler | MEA PCR (ITS1-5.8S-ITS2, b-tubulin and calmodulin) | Cladosporium, Penicillium, and Aspergillus | [95] |
| BRAZIL Settle plates | Collection SDA, PDA, and SDA + chloramphenicol for isolation | Penicillium sp., Cladosporium sp., and Alternaria sp. | [96] |
| PAKISTAN Air sampler (Gilian5000) | PDA PCR (ITS1 and ITS4) | Cladosporium asperulatum, Penicillium oxalicum, and Aspergillus niger | [97] |
| COLOMBIA Two-stage cascade impactor | SDA | Aspergillus, Curvularia, and Cladosporium | [98] |
| POLAND Settle plates, six-stage Andersen cascade sampler | SDA | Cladosporium, Penicillium, and Aspergillus spp. | [99] |
| Geographic Location of Library/Collection Method | Culture Media a/Identification Method b | Main Taxa of Fungi Detected c | Reference |
|---|---|---|---|
| IRAN Sterile swabs and scalpels | SDA + Chloramphenicol | Aspergillus sp. and Penicillium sp. | [100] |
| ITALY Sterile cotton swabs and needles; 3 M adhesive tape | Agar and broth culture MEA, CYA, and CZA broth | Cladosporium cladosporioides, Penicillium pinophilum, and Aspergillus versicolor | [101] |
| BRAZIL Sterile swabs | SDA for collection; SDA and PDA for isolation; CMA, CZA, and PDA for identification | Cladosporium, Penicillium, and Aspergillus | [102] |
| ITALY Sterile swabs and nitrocellulose membranes; removable transparent adhesive tape | MEA, CY20S, and DG18 PCR (ITS); total DNA | Cladosporium, Penicillium spp., and Aspergillus spp. | [103] |
| SLOVAKIA Adhesive tape and swabs | DRBC, SDA, and MEA 28S rDNA sequencing (NL1 and NL4) | Aspergillus fumigatus, Pen-icillium funiculosum, and Mucor spinosus | [104] |
| MOROCCO Sterile swabs, scalpel | MEA, LB agar, YPG antibiotics agar, and screening on CMC PCR (ITS1, 5.8S, and ITS2) | Penicillium chrysogenum, Aspergillus niger, and Mucor racemosus | [105] |
| INDONESIA Adhesive tape and sterile swabs | PCA, PDA ITS (ITS5-ITS4) | Aspergillus awamori, Penicillium citrinum, and Pseudocercospora chiangmaiensis | [106] |
| IRAQ Sterile swabs | SDA | Aspergillus spp., Penicillium spp., and Rhizopus spp. | [107] |
| PORTUGAL adhesive tape (2.25 mm2); scalpel, tweezers, and cotton swabs | Microscopic examination of stains; PDA and MEA PCR (ITS4 and ITS1F) | Chaetomium globosum, Penicillium chrysogenum, and Myxotrichum deflexum | [108] |
| EGYPT Sterile swabs | CYB, CYA PCR (ITS1-ITS2) | Penicillium citrinum, Aspergillus ustus, and Penicillium chrysogenum | [109] |
| EGYPT Sterile swabs, collection of deteriorated fragments | CYB, CYA PCR (ITS1-ITS3) | Aspergillus niger, Penicillium chrysogenum, and Aspergillus quadrilineatus | [110] |
| Geographic Location of Library/Collection Method | Culture Media a/Identification Method b | Main Taxa of Fungi Detected c | Reference |
|---|---|---|---|
| LITHUANIA Slit-to-agar single-stage impactor (Krotov 8180; settle plates; sterile swabs and adhesive tape) | MEA and SDA for sampling; CZA, CMA, and MEA for identification | Penicillium expansum, Aspergillus flavus, and Mucor spp. | [111] |
| ROMANIA Settle plates and sterile swabs | PDA and MEA for isolation; CDA, MEA, PDA, CGA, and SDA for identification | Aspergillus spp., Penicillium spp., and Cladosporium spp. | [112] |
| POLAND Impaction method; press counting plate with MEA against artifact surface | MEA for sampling; CZA for identification | Cladosporium and Penicillium | [113] |
| POLAND Six-stage Andersen Impactor; GSP and Button aerosol samplers; sterile swabs | MEA | Aspergillus niger, Penicillium verrucosum, and Candida famata | [114] |
| POLAND Six-stage Graseby–Andersen impactor | MEA | Penicillium spp., Trichothecium laxicephalum, and Alternaria tenuis | [115] |
| PORTUGAL M Air Tester, sterile swabs | MEA, DG18, and MA 28S and ITS | Penicillium spp. and Cladosporium spp. | [116] |
| BRAZIL Settle plate and sterile swabs | SDA for isolation; MEA and CZA for identification | Aspergillus spp. and Penicillium spp. | [117] |
| ITALY DUO SAS 360 sampler; vacuum cleaner Settle plates; Hirst spore trap; volumetric trap; nitrocellulose filters | SDA | Cladosporium spp., Fusarium spp., and Ustilago spp. | [118] |
| IRAN Settle plates; sterile swabs | SDA, yeast colonies tested with germ tube and chlamydoconidia production | Cladosporium sp., Penicillium sp., and As-pergillus sp. | [119] |
| TURKEY Settle plates; sterile swabs | RbCA, PDA, MEA, CDA | Aspergillus niger, Penicillium, and Cladosporium herbarum | [120] |
| POLAND Air sampler MAS-110 Ec; six-stage Andersen sampler; swab method using saline solution | TSA, DG18 and MEA for air samples; SDA for surface samples; CYA and YES for identification PCR (ITS1/2) | Aspergillus puulaauensis, Cladosporium cladosporioides, and Penicillium crustosum | [121] |
| MALAYSIA Settle plate and sterile swabs | PDA and SDA | Aspergillus sp., Onychocola sp., and Microsporum sp. | [122] |
| INDIA Settle plates; cotton swabs; sections of deteriorated paper | PDA; analysis of cellulase activity | Aspergillus spp. and Fusarium | [123] |
| MALAYSIA Coriolis air sampler, settle plates, sterile swabs | MEA | Aspergillus sp., Penicillium sp., and Stachybotrys sp. | [124] |
| ITALY Sterile swabs, adhesive tape, Sampl’Air Lite sampler | MEA | Eurotium halophilicum and Aspergillus creber. | [125] |
| TURKEY Portable volumetric microbiological air sampler; sterile swabs | DG18, MEA, PDA MF were inoculated into CDA, CY20S, CYA, MEA, PDA, and 25% glycerol nitrate agar | Aspergillus versicolor, Cladosporium sphaerospermum, and Penicillium dierckxii | [126] |
| EGYPT Andersen two-stage air sampler | Rose Bengal streptomycin agar for sampling; SDA, MEA, and CDA for identification Analysis of fungal enzymatic activity | Aspergillus niger, Aspergillus fumigatus, and Penicillium | [127] |
| BRAZIL Andersen air sampler; settle plates; sterile swabs | SDA for isolation; CYA 25, CYA37, CY20S, MEA CLA, BLA, and PDA for identification | Aspergillus niger, Cryptococcus, and Cladosporium cladosporioides | [128] |
| NIGERIA Settle plates; nitrocellulose membranes | MEA; focus on cellulolytic isolates PCR (NS5 and ITS4) | Aspergillus niger and Penicillium georgiense | [129] |
| CAMEROON Settle plate and sterile swabs | SDA | Aspergillus spp., Penicillium spp., and Curvularia spp. | [130] |
| ROMANIA Destructive and nondestructive methods; Settle plates; Air sampler | DG 18 | Penicillium spp., Cladosporium spp., and Fusarium spp. | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Jaddaoui, I.; Ghazal, H.; Bennett, J.W. Mold in Paradise: A Review of Fungi Found in Libraries. J. Fungi 2023, 9, 1061. https://doi.org/10.3390/jof9111061
El Jaddaoui I, Ghazal H, Bennett JW. Mold in Paradise: A Review of Fungi Found in Libraries. Journal of Fungi. 2023; 9(11):1061. https://doi.org/10.3390/jof9111061
Chicago/Turabian StyleEl Jaddaoui, Islam, Hassan Ghazal, and Joan W. Bennett. 2023. "Mold in Paradise: A Review of Fungi Found in Libraries" Journal of Fungi 9, no. 11: 1061. https://doi.org/10.3390/jof9111061
APA StyleEl Jaddaoui, I., Ghazal, H., & Bennett, J. W. (2023). Mold in Paradise: A Review of Fungi Found in Libraries. Journal of Fungi, 9(11), 1061. https://doi.org/10.3390/jof9111061







