Hyphae of Rhizopus arrhizus and Lichtheimia corymbifera Are More Virulent and Resistant to Antifungal Agents Than Sporangiospores In Vitro and in Galleria mellonella
Abstract
1. Introduction
2. Materials and Methods
2.1. Organisms and Growth Conditions
2.2. Optimization of Media for the Production of Sporangiospores and Generation of Hyphae of R. arrhizus and L. corymbifera
2.3. In Vitro Antifungal Susceptibility Testing
2.4. Galleria mellonella Infection and Treatments
2.5. Statistical Analysis
3. Results
3.1. Comparative Virulence of R. arrhizus and L. corymbifera Hyphae and Sporangiospores Infections in G. mellonella
3.2. Efficacy of Liposomal amphotericin B against Hyphae and Sporangiospores of R. arrhizus and L. corymbifera in G. mellonella
3.3. In Vitro Susceptibility of Antifungal Agents against R. arrhizus and L. corymbifera
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrikkos, G.; Tsioutis, C. Recent Advances in the Pathogenesis of Mucormycoses. Clin. Ther. 2018, 40, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Pushparaj, K.; Kuchi Bhotla, H.; Arumugam, V.A.; Pappusamy, M.; Easwaran, M.; Liu, W.-C.; Issara, U.; Rengasamy, K.R.R.; Meyyazhagan, A.; Balasubramanian, B. Mucormycosis (Black Fungus) Ensuing COVID-19 and Comorbidity Meets—Magnifying Global Pandemic Grieve and Catastrophe Begins. Sci. Total Environ. 2022, 805, 150355. [Google Scholar] [CrossRef]
- Rodriguez, R.C.J.; Ganesan, A.; Shaikh, F.; Carson, M.L.; Bradley, W.; Warkentien, T.E.; Tribble, D.R. Combat-Related Invasive Fungal Wound Infections. Mil. Med. 2022, 187, 34–41. [Google Scholar] [CrossRef]
- Tribble, D.R.; Ganesan, A.; Rodriguez, C.J. Combat Trauma-Related Invasive Fungal Wound Infections. Curr. Fungal Infect. Rep. 2020, 14, 186–196. [Google Scholar] [CrossRef]
- Ganesan, A.; Wells, J.; Shaikh, F.; Peterson, P.; Bradley, W.; Carson, M.L.; Petfield, J.L.; Klassen-Fischer, M.; Akers, K.S.; Downing, K.; et al. Molecular Detection of Filamentous Fungi in Formalin-Fixed Paraffin-Embedded Specimens in Invasive Fungal Wound Infections Is Feasible with High Specificity. J. Clin. Microbiol. 2019, 58, e01259-19. [Google Scholar] [CrossRef]
- Lloyd, B.; Weintrob, A.C.; Rodriguez, C.; Dunne, J.R.; Weisbrod, A.B.; Hinkle, M.; Warkentien, T.; Murray, C.K.; Oh, J.; Millar, E.V. Effect of Early Screening for Invasive Fungal Infections in U.S. Service Members with Explosive Blast Injuries. Surg. Infect. 2014, 15, 619–626. [Google Scholar] [CrossRef]
- Radowsky, J.S.; Malone, D.L. Soft Tissue Infection. In Managing Dismounted Complex Blast Injuries in Military & Civilian Settings: Guidelines and Principles; Galante, J.M., Martin, M.J., Rodriguez, C.J., Gordon, W.T., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 181–195. ISBN 978-3-319-74672-2. [Google Scholar]
- Tribble, D.R.; Conger, N.G.; Fraser, S.; Gleeson, T.D.; Wilkins, K.; Antonille, T.; Weintrob, A.; Ganesan, A.; Gaskins, L.J.; Li, P.; et al. Infection-Associated Clinical Outcomes in Hospitalized Medical Evacuees Following Traumatic Injury-Trauma Infectious Disease Outcome Study (TIDOS). J. Trauma 2011, 71, S33–S42. [Google Scholar] [CrossRef]
- Tribble, D.R.; Rodriguez, C.J. Combat-Related Invasive Fungal Wound Infections. Curr. Fungal Infect. Rep. 2014, 8, 277–286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Warkentien, T.; Rodriguez, C.; Lloyd, B.; Wells, J.; Weintrob, A.; Dunne, J.R.; Ganesan, A.; Li, P.; Bradley, W.; Gaskins, L.J.; et al. Invasive Mold Infections Following Combat-Related Injuries. Clin. Infect. Dis. 2012, 55, 1441–1449. [Google Scholar] [CrossRef]
- Weintrob, A.C.; Weisbrod, A.B.; Dunne, J.R.; Rodriguez, C.J.; Malone, D.; Lloyd, B.A.; Warkentien, T.E.; Wells, J.; Murray, C.K.; Bradley, W.; et al. Combat Trauma-Associated Invasive Fungal Wound Infections: Epidemiology and Clinical Classification. Epidemiol. Infect. 2015, 143, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Singh, R. The Emerging Epidemiology of Mould Infections in Developing Countries. Curr. Opin. Infect. Dis. 2011, 24, 521–526. [Google Scholar] [CrossRef]
- Hajjej, Z.; Walid, S.; Jedidi, K.; Latifa, M.; Nawel, B.; Boutheina, J.; Hedi, G.; Labbene, I.; Ferjani, M. Cutaneous Mucormycosis Caused by Saksenaea vasiformis following Combat-Related Injuries: First Report in Tunisia and Literature Review. EJPMR 2021, 8, 7. [Google Scholar]
- Skiada, A.; Drogari-Apiranthitou, M.; Pavleas, I.; Daikou, E.; Petrikkos, G. Global Cutaneous Mucormycosis: A Systematic Review. J. Fungi 2022, 8, 194. [Google Scholar] [CrossRef]
- Walsh, T.J.; Hospenthal, D.R.; Petraitis, V.; Kontoyiannis, D.P. Necrotizing Mucormycosis of Wounds Following Combat Injuries, Natural Disasters, Burns, and Other Trauma. J. Fungi 2019, 5, 57. [Google Scholar] [CrossRef]
- Carod-Artal, F.J. Fungal Infections of the Central Nervous System. CNS Infect. A Clin. Approach 2018, 129–156. [Google Scholar] [CrossRef]
- Gomes, M.Z.; Lewis, R.E.; Kontoyiannis, D.P. Mucormycosis Caused by Unusual Mucormycetes, Non-Rhizopus, -Mucor, and -Lichtheimia Species. Clin. Microbiol. Rev. 2011, 24, 411–445. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Jin, L.; Fu, H.; Shen, Y.; Lu, G.; Mei, H.; Cao, X.; Wang, H.; Liu, W. Interleukin-22 Mediates Early Host Defense against Rhizomucor pusilluscan Pathogens. PLoS ONE 2013, 8, e65065. [Google Scholar] [CrossRef]
- Jensen, H.E. Murine Subcutaneous Granulomatous Zygomycosis Induced by Absidia corymbifera. Mycoses 1992, 35, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhou, Y.-B.; Li, D.-M. Atypical Hyperplasia in Mice Caused by Mucor irregularis and Rhizopus oryzae Isolated from Lethal Midline Granuloma. Mycostema 2019, 38, 1314–1322. [Google Scholar] [CrossRef]
- Lewis, R.E.; Ben-Ami, R.; Best, L.; Albert, N.; Walsh, T.J.; Kontoyiannis, D.P. Tacrolimus Enhances the Potency of Posaconazole Against Rhizopus oryzae In Vitro and in an Experimental Model of Mucormycosis. J. Infect. Dis. 2013, 207, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, W.H.; Bauer, H. Activation of Quiescent Mucormycotic Granulomata in Rabbits by Induction of Acute Alloxan Diabetes. J. Exp. Med. 1958, 108, 171–178. [Google Scholar] [CrossRef]
- Sheldon, W.H.; Bauer, H. The Development of the Acute Inflammatory Response to Experimental Cutaneous Mucormycosis in Normal and Diabetic Rabbits. J. Exp. Med. 1959, 110, 845–852. [Google Scholar] [CrossRef]
- Sheldon, W.H.; Bauer, H. Tissue Mast Cells and Acute Inflammation in Experimental Cutaneous Mucormycosis of Normal, 48/80-Treated, and Diabetic Rats. J. Exp. Med. 1960, 112, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, S.; Wan, Z.; Li, R.; Yu, J. In Vivo and In Vitro Impairments in T Helper Cell and Neutrophil Responses against Mucor irregularis in Card9 Knockout Mice. Infect. Immun. 2021, 89, e00040-21. [Google Scholar] [CrossRef]
- Ellis, J.J.; Ajello, L. An Unusual Source for Apophysomyces elegans and a Method for Stimulating Sporulation of Saksenaea vasiformis. Mycologia 1982, 74, 144–145. [Google Scholar] [CrossRef]
- Padhye, A.A.; Ajello, L. Simple Method of Inducing Sporulation by Apophysomyces elegans and Saksenaea vasiformis. J. Clin. Microbiol. 1988, 26, 1861–1863. [Google Scholar] [CrossRef]
- Curtis, A.; Binder, U.; Kavanagh, K. Galleria mellonella Larvae as a Model for Investigating Fungal—Host Interactions. Front. Fungal Biol. 2022, 3, 893494. [Google Scholar] [CrossRef]
- Maurer, E.; Hörtnagl, C.; Lackner, M.; Grässle, D.; Naschberger, V.; Moser, P.; Segal, E.; Semis, M.; Lass-Flörl, C.; Binder, U. Galleria mellonella as a Model System to Study Virulence Potential of Mucormycetes and Evaluation of Antifungal Treatment. Med. Mycol. 2019, 57, 351–362. [Google Scholar] [CrossRef]
- Jemel, S.; Guillot, J.; Kallel, K.; Botterel, F.; Dannaoui, E. Galleria mellonella for the Evaluation of Antifungal Efficacy against Medically Important Fungi, a Narrative Review. Microorganisms 2020, 8, 390. [Google Scholar] [CrossRef]
- Walther, G.; Wagner, L.; Kurzai, O. Outbreaks of Mucorales and the Species Involved. Mycopathologia 2020, 185, 765–781. [Google Scholar] [CrossRef]
- Vaezi, A.; Fakhim, H.; Ilkit, M.; Faeli, L.; Fakhar, M.; Alinejad, V.; Wiederhold, N.P.; Badali, H. Rapid and Low-Cost Culture-Based Method for Diagnosis of Mucormycosis Using a Mouse Model. Front. Microbiol. 2020, 11, 440. [Google Scholar] [CrossRef]
- Kraibooj, K.; Park, H.-R.; Dahse, H.-M.; Skerka, C.; Voigt, K.; Figge, M.T. Virulent Strain of Lichtheimia corymbifera Shows Increased Phagocytosis by Macrophages as Revealed by Automated Microscopy Image Analysis. Mycoses 2014, 57, 56–66. [Google Scholar] [CrossRef]
- Montaño, D.E.; Hartung, S.; Wich, M.; Ali, R.; Jungnickel, B.; von Lilienfeld-Toal, M.; Voigt, K. The TLR-NF-KB Axis Contributes to the Monocytic Inflammatory Response against a Virulent Strain of Lichtheimia corymbifera, a Causative Agent of Invasive Mucormycosis. Front. Immunol. 2022, 13, 882921. [Google Scholar] [CrossRef]
- Schulze, B.; Rambach, G.; Schwartze, V.U.; Voigt, K.; Schubert, K.; Speth, C.; Jacobsen, I.D. Ketoacidosis Alone Does Not Predispose to Mucormycosis by Lichtheimia in a Murine Pulmonary Infection Model. Virulence 2017, 8, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Schwartze, V.U.; Hoffmann, K.; Nyilasi, I.; Papp, T.; Vágvölgyi, C.; de Hoog, S.; Voigt, K.; Jacobsen, I.D. Lichtheimia Species Exhibit Differences in Virulence Potential. PLoS ONE 2012, 7, e40908. [Google Scholar] [CrossRef] [PubMed]
- Baijal, U. A Physiological Study of Saksenaea vasiformis Saksena. Mycopathol. Mycol. Appl. 1967, 33, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A.; Fothergill, A.; Ghannoum, M.; Manavathu, E.; Ostrosky-Zeichner, L.; Pfaller, M.; Rinaldi, M.; Schell, W.; Walsh, T. Quality Control and Reference Guidelines for CLSI Broth Microdilution Susceptibility Method (M38-A Document) for Amphotericin B, Itraconazole, Posaconazole, and Voriconazole. J. Clin. Microbiol. 2005, 43, 5243–5246. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.E.; Albert, N.D.; Liao, G.; Hou, J.; Prince, R.A.; Kontoyiannis, D.P. Comparative Pharmacodynamics of Amphotericin B Lipid Complex and Liposomal Amphotericin B in a Murine Model of Pulmonary Mucormycosis. Antimicrob. Agents Chemother. 2010, 54, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Powers-Fletcher, M.V.; Kendall, B.A.; Griffin, A.T.; Hanson, K.E. Filamentous Fungi. Microbiol. Spectr. 2016, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Ziaee, A.; Zia, M.; Bayat, M.; Hashemi, J. Occurrence of Important Mucormycosis Agents in the Soil of Populous Areas of Isfahan and Their Pathogenicity in Immunocompromised Patients. J. Pure Appl. Microbiol. 2016, 10, 81–88. [Google Scholar]
- Singh, R.; Shivaprakash, M.R.; Chakrabarti, A. Biofilm Formation by Zygomycetes: Quantification, Structure and Matrix Composition. Microbiology 2011, 157, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Harding, M.W.; Marques, L.L.; Howard, R.J.; Olson, M.E. Biofilm Morphologies of Plant Pathogenic Fungi. Am. J. Plant Sci. Biotechnol. 2010, 4, 43–47. [Google Scholar]
- Villa, F.; Cappitelli, F.; Cortesi, P.; Kunova, A. Fungal Biofilms: Targets for the Development of Novel Strategies in Plant Disease Management. Front. Microbiol. 2017, 8, 654. [Google Scholar] [CrossRef] [PubMed]
- Peiqian, L.; Xiaoming, P.; Huifang, S.; Jingxin, Z.; Ning, H.; Birun, L. Biofilm Formation by Fusarium oxysporum f. sp. Cucumerinum and Susceptibility to Environmental Stress. FEMS Microbiol. Lett. 2014, 350, 138–145. [Google Scholar] [CrossRef]
- Ganesan, A.; Tribble, D. Tissue-Based Molecular Diagnostics Evaluation in Combat-Related Invasive Fungal Wound Infections; Uniformed Services University of the Health Sciences (USU): Bethesda, MD, USA, 2018; p. 0075. [Google Scholar]
- Bain, J.M.; Alonso, M.F.; Childers, D.S.; Walls, C.A.; Mackenzie, K.; Pradhan, A.; Lewis, L.E.; Louw, J.; Avelar, G.M.; Larcombe, D.E.; et al. Immune Cells Fold and Damage Fungal Hyphae. Proc. Natl. Acad. Sci. USA 2021, 118, e2020484118. [Google Scholar] [CrossRef]
- Gil-Lamaignere, C.; Simitsopoulou, M.; Roilides, E.; Maloukou, A.; Winn, R.M.; Walsh, T.J. Interferon-γ and Granulocyte-Macrophage Colony-Stimulating Factor Augment the Activity of Polymorphonuclear Leukocytes against Medically Important Zygomycetes. J. Infect. Dis. 2005, 191, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Roilides, E.; Kontoyiannis, D.P.; Walsh, T.J. Host Defenses against Zygomycetes. Clin. Infect. Dis. 2012, 54 (Suppl. S1), S61–S66. [Google Scholar] [CrossRef]
- Romani, L. Immunity to Fungal Infections. Nat. Rev. Immunol. 2004, 4, 11–24. [Google Scholar] [CrossRef]
- Swamydas, M.; Break, T.J.; Lionakis, M.S. Mononuclear Phagocyte-Mediated Antifungal Immunity: The Role of Chemotactic Receptors and Ligands. Cell. Mol. Life Sci. 2015, 72, 2157–2175. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil Extracellular Traps Capture and Kill Candida albicans Yeast and Hyphal Forms. Cell. Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef]
- FQ Smith, D.; Casadevall, A. Fungal Immunity and Pathogenesis in Mammals versus the Invertebrate Model Organism Galleria mellonella. Pathog. Dis. 2021, 79, ftab013. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.C.; de Barros, P.P.; Fugisaki, L.R.d.O.; Rossoni, R.D.; Ribeiro, F.d.C.; de Menezes, R.T.; Junqueira, J.C.; Scorzoni, L. Recent Advances in the Use of Galleria mellonella Model to Study Immune Responses against Human Pathogens. J. Fungi 2018, 4, 128. [Google Scholar] [CrossRef]
- Roilides, E.; Simitsopoulou, M. Immune Responses to Mucorales Growth Forms: Do We Know Everything? Virulence 2017, 8, 1489–1491. [Google Scholar] [CrossRef]
- Antachopoulos, C.; Demchok, J.P.; Roilides, E.; Walsh, T.J. Fungal Biomass Is a Key Factor Affecting Polymorphonuclear Leucocyte-Induced Hyphal Damage of Filamentous Fungi. Mycoses 2010, 53, 321–328. [Google Scholar] [CrossRef]
- Antachopoulos, C.; Meletiadis, J.; Roilides, E.; Sein, T.; Sutton, D.A.; Wickes, B.L.; Rinaldi, M.G.; Merz, W.G.; Shea, Y.R.; Walsh, T.J. Relationship between Metabolism and Biomass of Medically Important Zygomycetes. Med. Mycol. 2006, 44, 429–438. [Google Scholar] [CrossRef][Green Version]
- Spreghini, E.; Orlando, F.; Giannini, D.; Barchiesi, F. In Vitro and in Vivo Activities of Posaconazole against Zygomycetes with Various Degrees of Susceptibility. J. Antimicrob. Chemother. 2010, 65, 2158–2163. [Google Scholar] [CrossRef]
- Labuda, R.; Bernreiter, A.; Hochenauer, D.; Schüller, C.; Kubátová, A.; Strauss, J.; Wagner, M. Saksenaea dorisiae sp. nov., a New Opportunistic Pathogenic Fungus from Europe. Int. J. Microbiol. 2019, 2019, 6253829. [Google Scholar] [CrossRef] [PubMed]
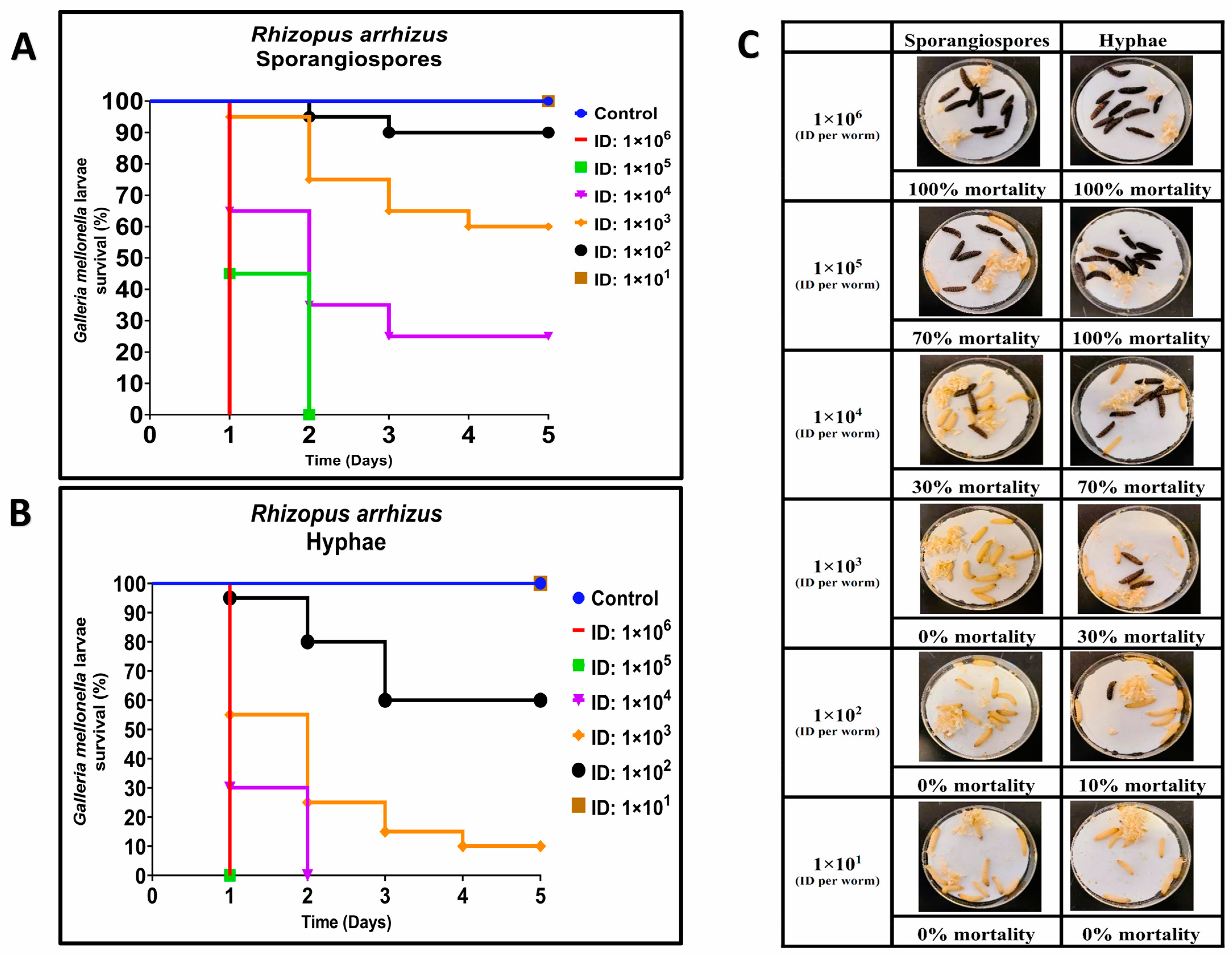
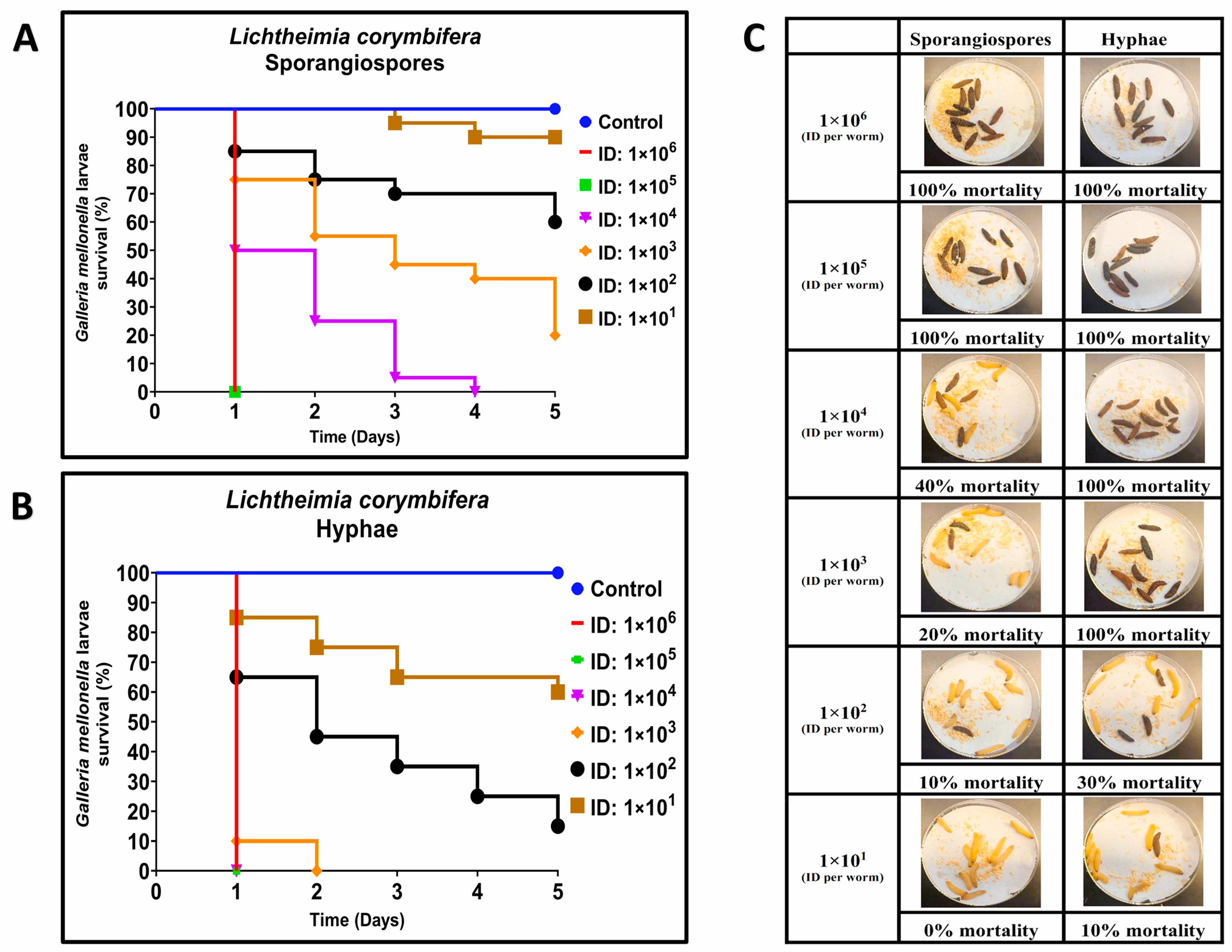
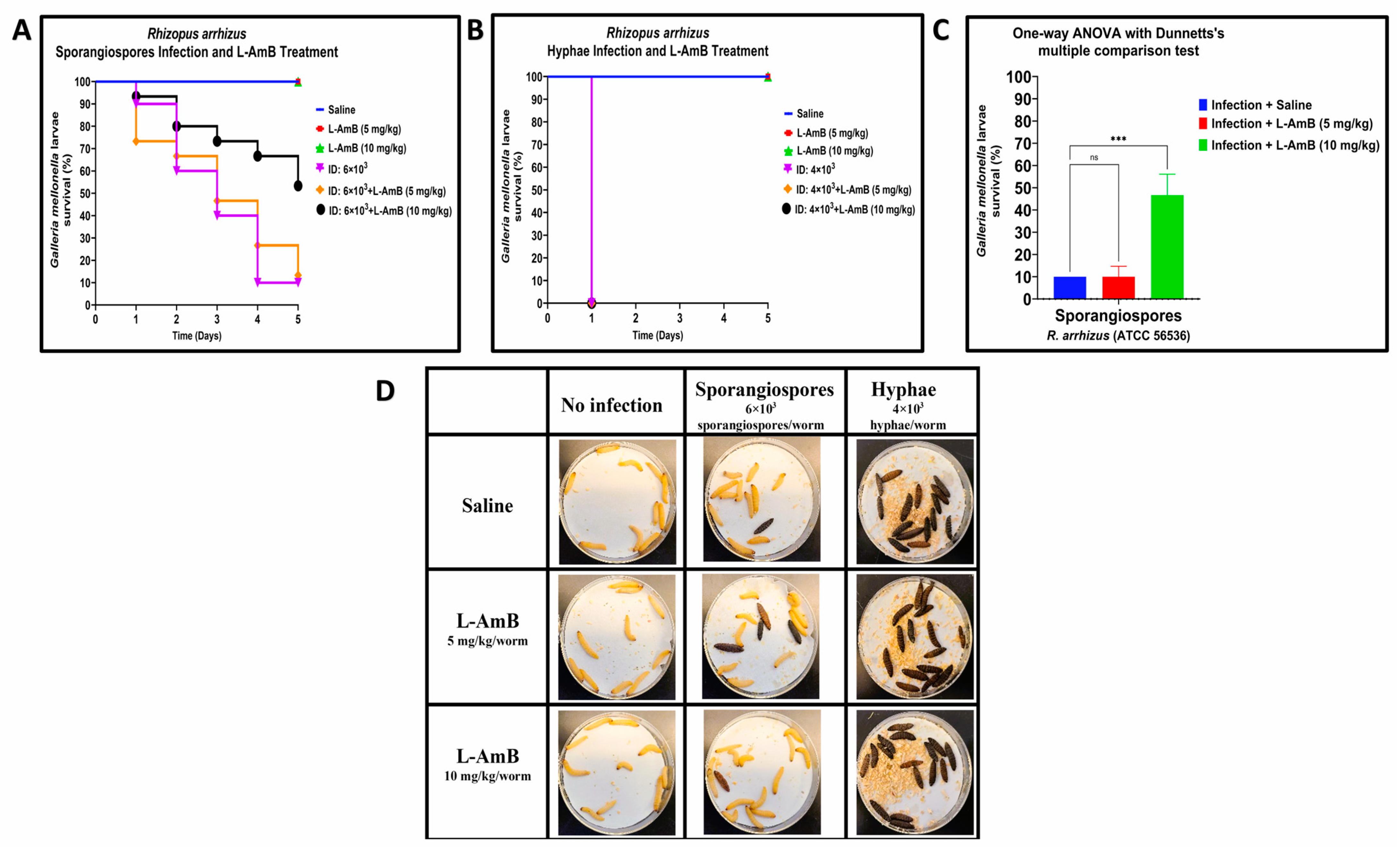
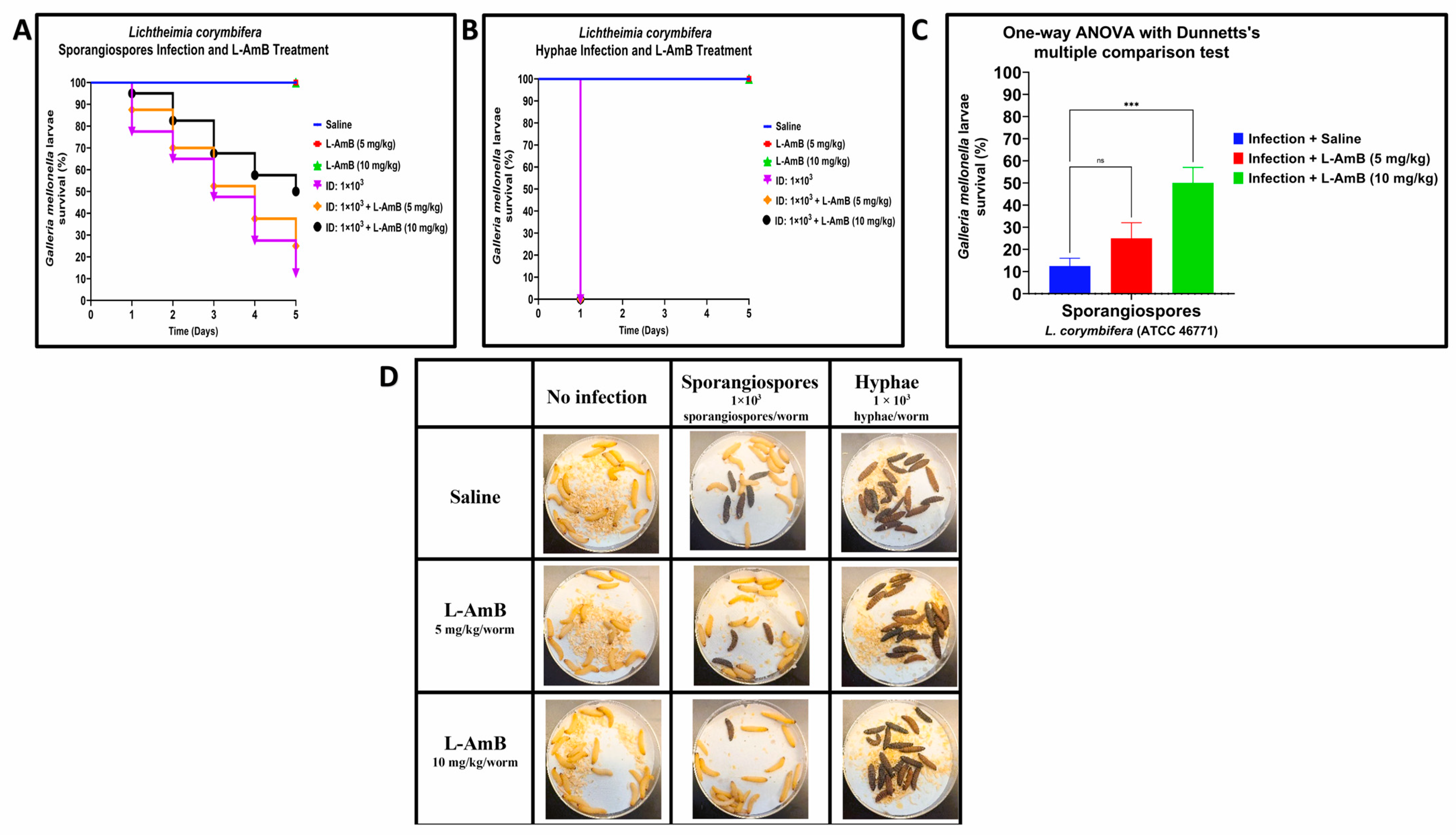
| Strain | LD50 | |
|---|---|---|
| Sporangiospores | Hyphae | |
| R. arrhizus (ATCC 56536) | 1.93 × 103 ± 7.38 × 102 * | 2.02 × 102 ± 5.40 × 101 * |
| L. corymbifera (ATCC 46771) | 6.21 × 102 ± 2.57 × 102 ** | 3.78 × 101 ± 1.59 × 101 ** |
| Minimum Inhibitory Concentration (MIC) | Posaconazole (µg/mL) | Amphotericin B (µg/mL) | L-Amphotericin B (µg/mL) | ||||
|---|---|---|---|---|---|---|---|
| 24 (h) | 48 (h) | 24 (h) | 48 (h) | 24 (h) | 48 (h) | ||
| Rhizopus arrhizus (ATCC 56536) | Sporangiospores | 2 | 2 | 2 | 2 | 2 | 2 |
| Hyphae | 4 | 8 | 4 | 8 | 8 | >16 | |
| Lichtheimia corymbifera (ATCC 46771) | Sporangiospores | 4 | 4 | 2 | 2 | 4 | 4 |
| Hyphae | 8 | >16 | 8 | >16 | 8 | >16 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samdavid Thanapaul, R.J.R.; Roberds, A.; Rios, K.E.; Walsh, T.J.; Bobrov, A.G. Hyphae of Rhizopus arrhizus and Lichtheimia corymbifera Are More Virulent and Resistant to Antifungal Agents Than Sporangiospores In Vitro and in Galleria mellonella. J. Fungi 2023, 9, 958. https://doi.org/10.3390/jof9100958
Samdavid Thanapaul RJR, Roberds A, Rios KE, Walsh TJ, Bobrov AG. Hyphae of Rhizopus arrhizus and Lichtheimia corymbifera Are More Virulent and Resistant to Antifungal Agents Than Sporangiospores In Vitro and in Galleria mellonella. Journal of Fungi. 2023; 9(10):958. https://doi.org/10.3390/jof9100958
Chicago/Turabian StyleSamdavid Thanapaul, Rex Jeya Rajkumar, Ashleigh Roberds, Kariana E. Rios, Thomas J. Walsh, and Alexander G. Bobrov. 2023. "Hyphae of Rhizopus arrhizus and Lichtheimia corymbifera Are More Virulent and Resistant to Antifungal Agents Than Sporangiospores In Vitro and in Galleria mellonella" Journal of Fungi 9, no. 10: 958. https://doi.org/10.3390/jof9100958
APA StyleSamdavid Thanapaul, R. J. R., Roberds, A., Rios, K. E., Walsh, T. J., & Bobrov, A. G. (2023). Hyphae of Rhizopus arrhizus and Lichtheimia corymbifera Are More Virulent and Resistant to Antifungal Agents Than Sporangiospores In Vitro and in Galleria mellonella. Journal of Fungi, 9(10), 958. https://doi.org/10.3390/jof9100958






