Emergomycosis, an Emerging Thermally Dimorphic Fungal Infection: A Systematic Review
Abstract
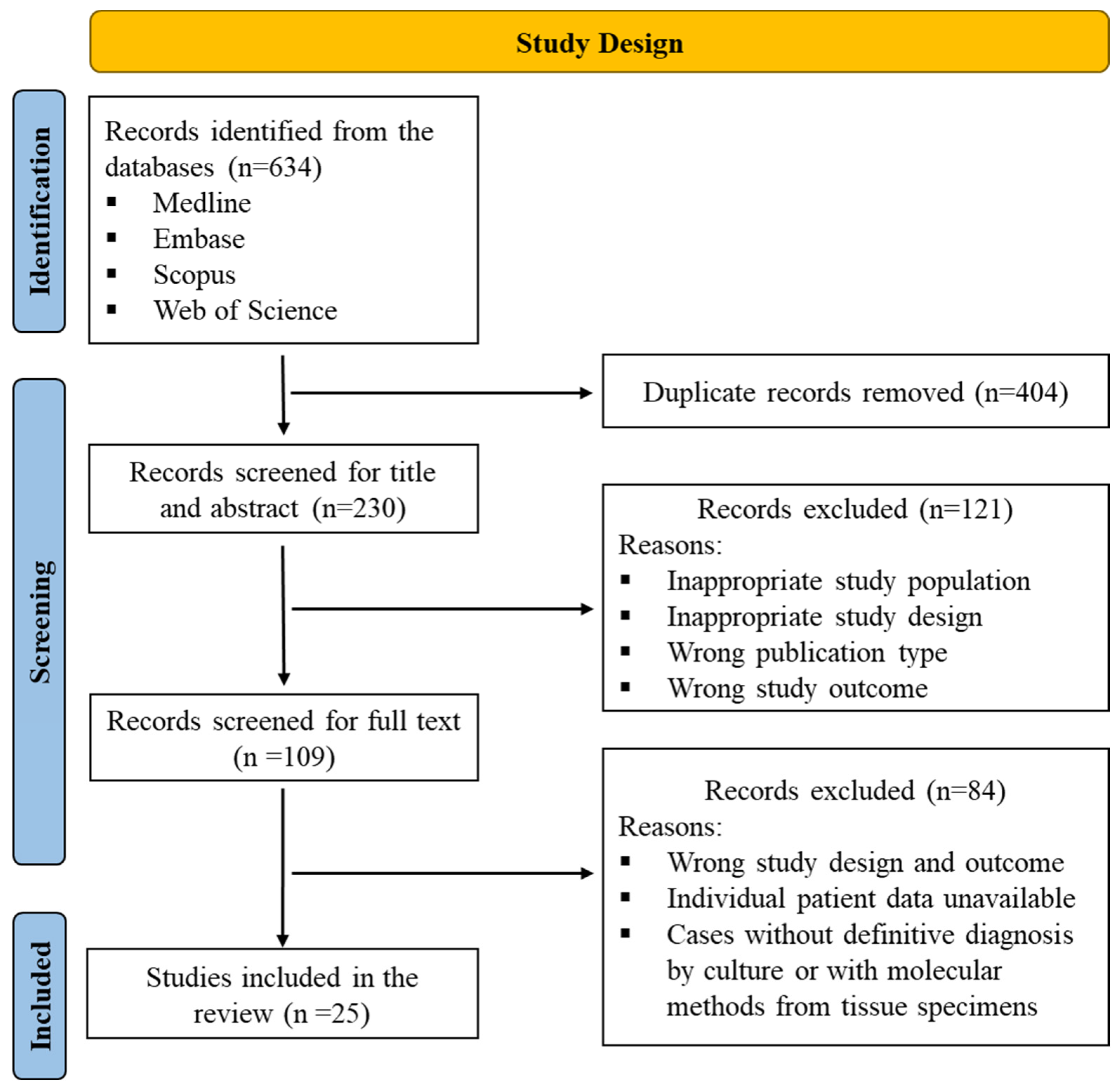
:1. Introduction
2. Methods
2.1. Study Design, Selection, and Data Extraction
2.2. Risk of Bias Assessment
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, Y.; Dukik, K.; Muñoz, J.F.; Sigler, L.; Schwartz, I.S.; Govender, N.P.; Kenyon, C.; Feng, P.; van den Ende, B.G.; Stielow, J.B.; et al. Phylogeny, ecology and taxonomy of systemic pathogens and their relatives in Ajellomycetaceae (Onygenales): Blastomyces, Emergomyces, Emmonsia, Emmonsiellopsis. Fungal Divers. 2018, 90, 245–291. [Google Scholar] [CrossRef]
- Dukik, K.; Muñoz, J.F.; Jiang, Y.; Feng, P.; Sigler, L.; Stielow, J.B.; Freeke, J.; Jamalian, A.; Gerrits van den Ende, B.; McEwen, J.G.; et al. Novel taxa of thermally dimorphic systemic pathogens in the Ajellomycetaceae (Onygenales). Mycoses 2017, 60, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tsui, C.K.M.; Ahmed, S.A.; Hagen, F.; Shang, Z.; Gerrits van den Ende, A.H.G.; Verweij, P.E.; Lu, H.; de Hoog, G.S. Intraspecific Diversity and Taxonomy of Emmonsia crescens. Mycopathologia 2020, 185, 613–627. [Google Scholar] [CrossRef]
- Samaddar, A.; Sharma, A. Emergomycosis, an Emerging Systemic Mycosis in Immunocompromised Patients: Current Trends and Future Prospects. Front. Med. 2021, 8, 670731. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, I.S.; Govender, N.P.; Sigler, L.; Jiang, Y.; Maphanga, T.G.; Toplis, B.; Botha, A.; Dukik, K.; Hoving, J.C.; Muñoz, J.F.; et al. Emergomyces: The global rise of new dimorphic fungal pathogens. PLoS Pathog. 2019, 15, e1007977. [Google Scholar] [CrossRef]
- Mapengo, R.E.; Maphanga, T.G.; Grayson, W.; Govender, N.P. Endemic mycoses in South Africa, 2010-2020: A decade-long description of laboratory-diagnosed cases and prospects for the future. PLoS Negl. Trop. Dis. 2022, 16, e0010737. [Google Scholar] [CrossRef]
- Ibe, C.; Mnyambwa, N.P.; Mfinanga, S.G. Emergomycosis in Africa: Time to Pay Attention to This Emerging Deadly Fungal Infection. Int. J. Gen. Med. 2023, 16, 2313–2322. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Lerm, B.; Hoving, J.C.; Kenyon, C.; Horsnell, W.G.; Basson, W.J.; Otieno-Odhiambo, P.; Govender, N.P.; Colebunders, R.; Botha, A. Emergomyces africanus in Soil, South Africa. Emerg. Infect. Dis. 2018, 24, 377–380. [Google Scholar] [CrossRef]
- Peterson, S.W.; Sigler, L. Molecular genetic variation in Emmonsia crescens and Emmonsia parva, etiologic agents of adiaspiromycosis, and their phylogenetic relationship to Blastomyces dermatitidis (Ajellomyces dermatitidis) and other systemic fungal pathogens. J. Clin. Microbiol. 1998, 36, 2918–2925. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Govender, N.P.; Corcoran, C.; Dlamini, S.; Prozesky, H.; Burton, R.; Mendelson, M.; Taljaard, J.; Lehloenya, R.; Calligaro, G.; et al. Clinical Characteristics, Diagnosis, Management, and Outcomes of Disseminated Emmonsiosis: A Retrospective Case Series. Clin. Infect. Dis. 2015, 61, 1004–1012. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.; Rutjes, A.W.S.; Reitsma, J.B.; Bossuyt, P.M.M.; Kleijnen, J. The development of QUADAS: A tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med. Res. Methodol. 2003, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Wellinghausen, N.; Kern, W.V.; Haase, G.; Rozdzinski, E.; Kern, P.; Marre, R.; Essig, A.; Hetzel, J.; Hetzel, M. Chronic granulomatous lung infection caused by the dimorphic fungus Emmonsia sp. Int. J. Med. Microbiol. 2003, 293, 441–445. [Google Scholar] [CrossRef]
- Dot, J.-M.; Debourgogne, A.; Champigneulle, J.; Salles, Y.; Brizion, M.; Puyhardy, J.M.; Collomb, J.; Plénat, F.; Machouart, M. Molecular diagnosis of disseminated adiaspiromycosis due to Emmonsia crescens. J. Clin. Microbiol. 2009, 47, 1269–1273. [Google Scholar] [CrossRef]
- Pelegrín, I.; Ayats, J.; Xiol, X.; Cuenca-Estrella, M.; Jucglà, A.; Boluda, S.; Fernàndez-Sabé, N.; Rafecas, A.; Gudiol, F.; Cabellos, C. Disseminated adiaspiromycosis: Case report of a liver transplant patient with human immunodeficiency infection, and literature review. Transpl. Infect. Dis. 2011, 13, 507–514. [Google Scholar] [CrossRef]
- Pelegrín, I.; Alastruey-Izquierdo, A.; Ayats, J.; Cuenca-Estrella, M.; Cabellos, C. A second look at Emmonsia infection can make the difference. Transpl. Infect. Dis. 2014, 16, 519–520. [Google Scholar] [CrossRef]
- Kenyon, C.; Bonorchis, K.; Corcoran, C.; Meintjes, G.; Locketz, M.; Lehloenya, R.; Vismer, H.F.; Naicker, P.; Prozesky, H.; van Wyk, M.; et al. A Dimorphic Fungus Causing Disseminated Infection in South Africa. N. Engl. J. Med. 2013, 369, 1416–1424. [Google Scholar] [CrossRef]
- Fielli, M.; Ceccato, A.; Capece, P.; Posse, G.; Monteverde, A.; Gonzalez, A. Human Adiaspiromycosis: A Case from Argentina. Chest 2013, 144, 220A. [Google Scholar] [CrossRef]
- van Hougenhouck-Tulleken, W.G.; Papavarnavas, N.S.; Nel, J.S.; Blackburn, L.Y.; Govender, N.P.; Spencer, D.C.; Lippincott, C.K. HIV-associated disseminated emmonsiosis, Johannesburg, South Africa. Emerg. Infect. Dis. 2014, 20, 2164–2166. [Google Scholar] [CrossRef]
- Heys, I.; Taljaard, J.; Orth, H. An Emmonsia species causing disseminated infection in South Africa. N. Engl. J. Med. 2014, 370, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Yin, S.; Zhu, G.; Li, M.; Wu, B.; Xie, Y.; Ma, H.; Zhang, J.; Cheng, C.; de Hoog, G.S.; et al. Disseminated infection caused by Emmonsia pasteuriana in a renal transplant recipient. J. Dermatol. 2015, 42, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Lochan, H.; Naicker, P.; Maphanga, T.; Ryan, A.; Pillay, K.; Govender, N.P.; Eley, B. A case of emmonsiosis in an HIV-infected child. South. Afr. J. HIV Med. 2015, 16, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Mutyaba, A.; Sonderup, M.W.; Locketz, M.; Okpechi, I.; Spearman, C.W.; Tooke, A. Disseminated Emmonsia in an HIV-HBV co-infected man. IDCases 2015, 2, 35–36. [Google Scholar] [CrossRef]
- Tang, X.H.; Zhou, H.; Zhang, X.Q.; De Han, J.; Gao, Q. Cutaneous Disseminated Emmonsiosis Due to Emmonsia pasteuriana in a Patient with Cytomegalovirus Enteritis. JAMA Dermatol. 2015, 151, 1263–1264. [Google Scholar] [CrossRef]
- Malik, R.; Capoor, M.R.; Vanidassane, I.; Gogna, A.; Singh, A.; Sen, B.; Rudramurthy, S.M.; Honnavar, P.; Gupta, S.; Chakrabarti, A. Disseminated Emmonsia pasteuriana infection in India: A case report and a review. Mycoses 2016, 59, 127–132. [Google Scholar] [CrossRef]
- Wang, P.; Kenyon, C.; de Hoog, S.; Guo, L.; Fan, H.; Liu, H.; Li, Z.; Sheng, R.; Yang, Y.; Jiang, Y.; et al. A novel dimorphic pathogen, Emergomyces orientalis (Onygenales), agent of disseminated infection. Mycoses 2017, 60, 310–319. [Google Scholar] [CrossRef]
- Koneru, H.; Penupolu, S. Pulmonary Adiaspiromycosis: An Emerging Fungal Infection. Chest 2017, 152, A162. [Google Scholar] [CrossRef]
- Crombie, K.; Spengane, Z.; Locketz, M.; Dlamini, S.; Lehloenya, R.; Wasserman, S.; Maphanga, T.G.; Govender, N.P.; Kenyon, C.; Schwartz, I.S. Paradoxical worsening of Emergomyces africanus infection in an HIV-infected male on itraconazole and antiretroviral therapy. PLoS Negl. Trop. Dis. 2018, 12, e0006173. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Sanche, S.; Wiederhold, N.P.; Patterson, T.F.; Sigler, L. Emergomyces canadensis, a Dimorphic Fungus Causing Fatal Systemic Human Disease in North America. Emerg. Infect. Dis. 2018, 24, 758–761. [Google Scholar] [CrossRef]
- Gast, K.B.; van der Hoeven, A.; de Boer, M.G.J.; van Esser, J.W.J.; Kuijper, E.J.; Verweij, J.J.; van Keulen, P.H.J.; van der Beek, M.T. Two cases of Emergomyces pasteurianus infection in immunocompromised patients in the Netherlands. Med. Mycol. Case Rep. 2019, 24, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Moodley, A.; Mosam, A.; Govender, N.P.; Mahabeer, Y.; Chateau, A.V. Emergomyces africanus: The Mimicking Fungus. Dermatopathology 2019, 6, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Rooms, I.; Mugisha, P.; Gambichler, T.; Hadaschik, E.; Esser, S.; Rath, P.-M.; Haase, G.; Wilmes, D.; McCormick-Smith, I.; Rickerts, V. Disseminated Emergomycosis in a Person with HIV Infection, Uganda. Emerg. Infect. Dis. 2019, 25, 1750–1751. [Google Scholar] [CrossRef] [PubMed]
- Capoor, M.R.; Mishra, N.; Kolte, S.; Singla, G.; Gogna, A.; Rudramurthy, S.; Prakash, H.; Chakrabarti, A. Disseminated Emergomyces pasteurianus Infection in India: A Case Report and a Review. Mycopathologia 2020, 185, 193–200. [Google Scholar] [CrossRef]
- Chik, K.K.; To, W.K. Autochthonous Emergomyces pasteurianus pneumonia in an immunocompromised patient in Hong Kong: A case report. Hong Kong Med. J./Xianggang Yi Xue Za Zhi 2020, 26, 446–448. [Google Scholar] [CrossRef]
- He, D.; Quan, M.; Zhong, H.; Chen, Z.; Wang, X.; He, F.; Qu, J.; Zhou, T.; Lv, X.; Zong, Z. Emergomyces orientalis Emergomycosis Diagnosed by Metagenomic Next-Generation Sequencing. Emerg. Infect. Dis. 2021, 27, 2740–2742. [Google Scholar] [CrossRef]
- Kuzyk, A.C.; Burbidge, T.; Mydlarski, P.R. Cutaneous Emmonsia infection in a renal transplant recipient. JAAD Case Reports 2021, 11, 44–46. [Google Scholar] [CrossRef]
- Mah, J.; Bakker, A.; Tseng, C.; Lafay-Cousin, L.; Kuhn, S.; Brundler, M.-A.; Lisboa, L.F. Isolated Pulmonary Emergomycosis in an Immunocompetent Patient in Alberta, Canada. Open Forum Infect. Dis. 2022, 9, ofac021. [Google Scholar] [CrossRef]
- Schwartz, I.S.; McLoud, J.D.; Berman, D.; Botha, A.; Lerm, B.; Colebunders, R.; Levetin, E.; Kenyon, C. Molecular detection of airborne Emergomyces africanus, a thermally dimorphic fungal pathogen, in Cape Town, South Africa. PLoS Negl. Trop. Dis. 2018, 12, e0006174. [Google Scholar] [CrossRef]
- Wheat, L.J.; Freifeld, A.G.; Kleiman, M.B.; Baddley, J.W.; McKinsey, D.S.; Loyd, J.E.; Kauffman, C.A. Infectious Diseases Society of America Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2007, 45, 807–825. [Google Scholar] [CrossRef]
- Chapman, S.W.; Dismukes, W.E.; Proia, L.A.; Bradsher, R.W.; Pappas, P.G.; Threlkeld, M.G.; Kauffman, C.A. Infectious Diseases Society of America Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 1801–1812. [Google Scholar] [CrossRef]
- Kauffman, C.A.; Bustamante, B.; Chapman, S.W.; Pappas, P.G. Infectious Diseases Society of America Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2007, 45, 1255–1265. [Google Scholar] [CrossRef]
- Dukik, K.; Al-Hatmi, A.M.S.; Curfs-Breuker, I.; Faro, D.; de Hoog, S.; Meis, J.F. Antifungal Susceptibility of Emerging Dimorphic Pathogens in the Family Ajellomycetaceae. Antimicrob. Agents Chemother. 2018, 62, 1–6. [Google Scholar] [CrossRef]
- Maphanga, T.G.; Britz, E.; Zulu, T.G.; Mpembe, R.S.; Naicker, S.D.; Schwartz, I.S.; Govender, N.P. In Vitro Antifungal Susceptibility of Yeast and Mold Phases of Isolates of Dimorphic Fungal Pathogen Emergomyces africanus (Formerly Emmonsia sp.) from HIV-Infected South African Patients. J. Clin. Microbiol. 2017, 55, 1812–1820. [Google Scholar] [CrossRef]
| Parameters | Total N (%) | HIV-Infected Patients N (%) | HIV-Uninfected Patients N (%) | p Value |
|---|---|---|---|---|
| Total number of patients | 77 (100) | 61 (79.2) | 16 (20.8) | - |
| Age (in range) a | 3–80 | 3–68 | 17–80 | - |
| Mean age in years (±SD) a | 39.25 (13.28) | 36.38 (10.48) | 50 (17.12) | - |
| Paediatric (0–16 years) a | 1 (1.3) | 1 (1.3) | 0 | >0.99 |
| Adult (>16 years) a | 75 (98.7) | 59 (78.7) | 16 (21.3) | >0.99 |
| Male and female ratio | 1.57:1 | 1.26:1 | 4.3:1 | - |
| Male | 47 (61) | 34 (44.2) | 13 (16.9) | 0.085 |
| Female | 30 (39) | 27 (35.1) | 03 (3.9) | 0.085 |
| Cases reported across continents b | ||||
| Africa | 57 (74) | 56 (72.7) | 1 (1.3) | 0.0001 * |
| Asia | 7 (9.1) | 2 (2.6) | 5 (6.5) | 0.004 * |
| North America | 7 (9.1) | 2 (2.6) | 5 (6.5) | 0.004 * |
| Europe | 5 (6.5) | 1 (1.3) | 4 (5.2) | 0.006 * |
| South America | 1 (1.3) | 0 | 1 (1.3) | 0.208 |
| Underlying diseases and risk factors | ||||
| CD4+ T-cell counts (<100 cells/mm3) | 55 (71.4) | 55 (71.4) | 0 | 0.0001 * |
| Anaemia | 32 (41.6) | 30 (39) | 2 (2.6) | 0.01 * |
| Thrombocytopenia | 17 (22.1) | 17 (22.1) | 0 | 0.016 * |
| Immunosuppressive drugs | 11 (14.3) | 1 (1.3) | 10 (13) | 0.0001 * |
| Renal diseases | 9 (11.7) | 1 (1.3) | 8 (10.4) | 0.0001 * |
| Transplant c | 7 (9.1) | 1 (1.3) | 6 (7.8) | 0.0001 * |
| Malignancy d | 5 (6.5) | 2 (2.6) | 3 (3.9) | 0.058 |
| Diabetes mellitus | 5 (6.5) | 1 (1.3) | 4 (5.2) | 0.006 * |
| Immunocompetent host | 4 (5.2) | 0 | 4 (5.2) | 0.001 * |
| History of past pulmonary tuberculosis | 17 (22.1) | 17 (22.1) | 0 | 0.016 * |
| Cholestatic disease | 20 (26) | 20 (26) | 0 | 0.008 * |
| Acute kidney injury | 9 (11.7) | 9 (11.7) | 0 | 0.191 |
| Antifungal Treatment e | ||||
| Patients with antifungal treatment | 64 (83.1) | 50 (64.9) | 14 (18.2) | 0.740 |
| No antifungal treatment | 9 (11.7) | 8 (10.4) | 1 (1.3) | 0.740 |
| Amphotericin B alone | 8 (10.4) | 7 (9.1) | 1 (1.3) | >0.99 |
| Triazole alone | 19 (24.7) | 12 (15.6) | 7 (9.1) | 0.058 |
| Amphotericin B and azole combination therapy | 34 (44.2) | 30 (39) | 4 (5.2) | 0.098 |
| Amphotericin B, azoles and echinocandins | 3 (3.9) | 1 (1.3) | 2 (2.6) | 0.108 |
| The outcome of the disease f | ||||
| Survived | 44 (57.1) | 33 (42.9) | 11 (14.3) | 0.397 |
| Death | 33 (42.9) | 28 (36.4) | 5 (6.5) | |
| Diagnostic Methods | Total N (%) | HIV-Infected Patients N (%) | HIV-Uninfected Patients N (%) | p Value |
|---|---|---|---|---|
| Histology and culture | 50 (64.9) | 38 (49.4) | 12 (15.6) | - |
| Culture only | 22 (28.6) | 20 (26) | 2 (2.6) | - |
| Histology and molecular detection from direct clinical specimens | 5 (6.5) | 3 (3.9) | 2 (2.6) | - |
| Specimens positive via histology a | ||||
| Skin biopsy | 47 (61) | 41 (53.2) | 6 (7.8) | 0.044 * |
| Respiratory specimens | 12 (15.6) | 2 (2.6) | 10 (13) | 0.0001 * |
| Bone marrow | 3 (3.9) | 3 (3.9) | 0 | >0.99 |
| Liver | 2 (2.6) | 2 (2.6) | 0 | >0.99 |
| Blood | 1 (1.3) | 1 (1.3) | 0 | >0.99 |
| Clinical specimens positive via culture or molecular methods b | ||||
| Skin biopsy | 35 (45.5) | 30 (39) | 5 (6.5) | 0.263 |
| Blood | 25 (32.5) | 23 (29.9) | 2 (2.6) | 0.074 |
| Respiratory specimens | 15 (19.5) | 3 (3.9) | 12 (15.6) | 0.0001 * |
| Bone marrow | 14 (18.2) | 14 (18.2) | 0 | 0.034 * |
| Causative agents c | ||||
| Emergomyces africanus | 55 (71.4) | 54 (70.1) | 1 (1.3) | 0.0001 * |
| Emergomyces pasteurianus | 9 (11.7) | 4 (5.2) | 5 (6.5) | 0.016 * |
| Emergomyces canadensis | 5 (6.5) | 2 (2.6) | 3 (3.9) | 0.058 |
| Emergomyces crescens d | 2 (2.6) | 0 | 2 (2.6) | 0.041 * |
| Emergomyces orientalis | 2 (2.6) | 0 | 2 (2.6) | 0.041 * |
| Emergomyces europaeus | 1 (1.3) | 0 | 1 (1.3) | 0.208 |
| Emergomyces species d | 3 (3.9) | 1 (1.3) | 2 (2.6) | 0.108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinayagamoorthy, K.; Gangavaram, D.R.; Skiada, A.; Prakash, H. Emergomycosis, an Emerging Thermally Dimorphic Fungal Infection: A Systematic Review. J. Fungi 2023, 9, 1039. https://doi.org/10.3390/jof9101039
Vinayagamoorthy K, Gangavaram DR, Skiada A, Prakash H. Emergomycosis, an Emerging Thermally Dimorphic Fungal Infection: A Systematic Review. Journal of Fungi. 2023; 9(10):1039. https://doi.org/10.3390/jof9101039
Chicago/Turabian StyleVinayagamoorthy, Kalaiselvi, Dinesh Reddy Gangavaram, Anna Skiada, and Hariprasath Prakash. 2023. "Emergomycosis, an Emerging Thermally Dimorphic Fungal Infection: A Systematic Review" Journal of Fungi 9, no. 10: 1039. https://doi.org/10.3390/jof9101039
APA StyleVinayagamoorthy, K., Gangavaram, D. R., Skiada, A., & Prakash, H. (2023). Emergomycosis, an Emerging Thermally Dimorphic Fungal Infection: A Systematic Review. Journal of Fungi, 9(10), 1039. https://doi.org/10.3390/jof9101039






