The Pathogenic Yeast Metschnikowia bicuspidata var. bicuspidata in the Aquacultured Ecosystem and Its Biocontrol
Abstract
:1. Introduction
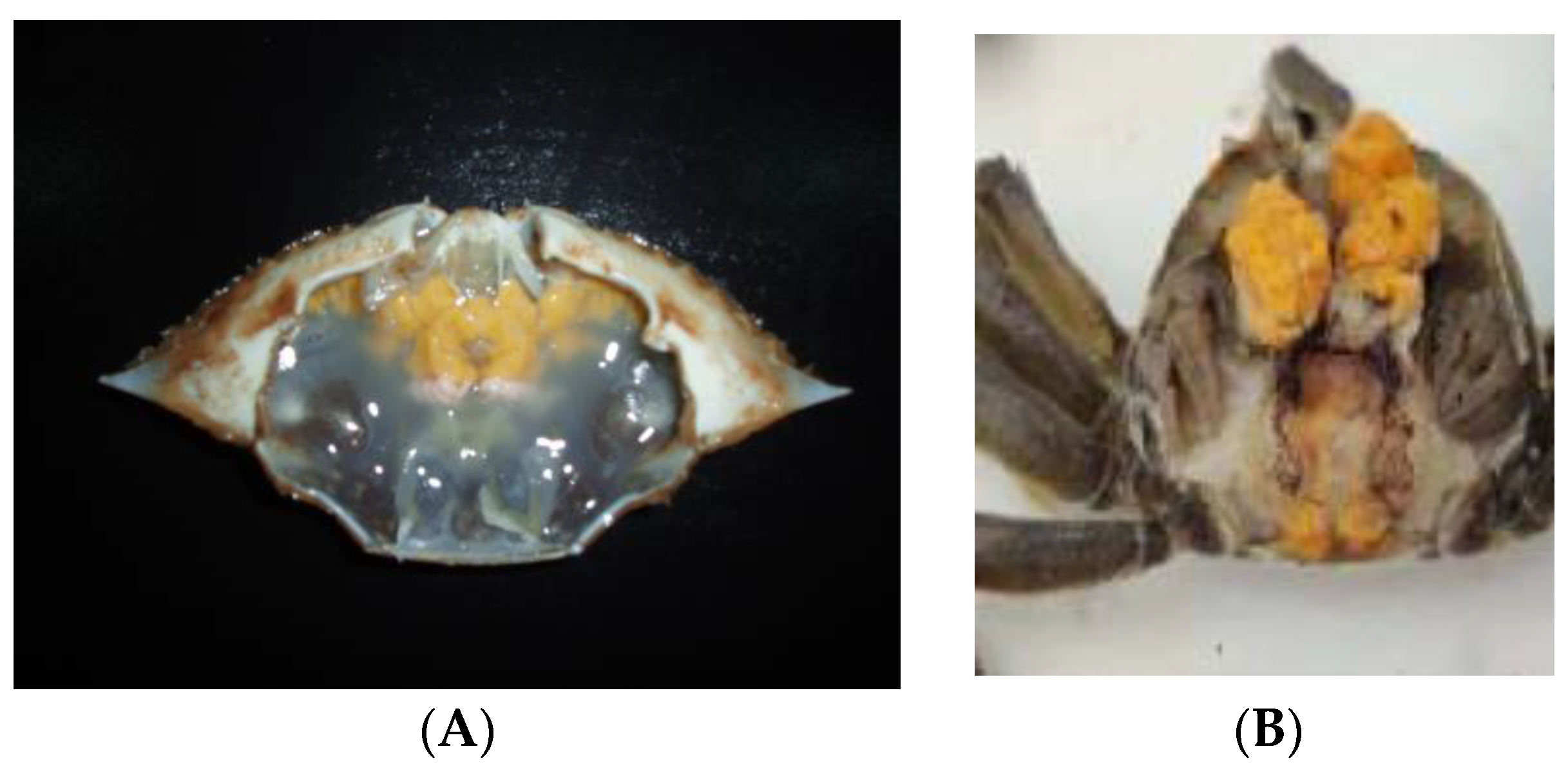
2. Milky Disease Caused by the Pathogenic M. bicuspidata var. bicuspidata
3. Possible Causes of the Milky Disease
4. Biocontrol of the Pathogenic M. bicuspidata, var. bicuspidata
4.1. Biocontrol of the Pathogenic Yeast M. bicuspidata var. bicuspidata Using Killer Toxins
4.2. Biocontrol of the Pathogenic Yeast M. bicuspidata var. bicuspidata Using Massoia Lactone Released from Liamocin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| YPD | Yeast Peptone Dextrose |
| ITS | Internal Transcribed Spacer |
| ABC | transporter ATP-binding-cassette transporter |
| MIC | Minimum Inhibitory Concentration |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical |
References
- Nguyen, N.H.; Suh, S.O.; Erbil, C.K.; Blackwell, M. Metschnikowia noctiluminum sp. nov., Metschnikowia corniflorae sp. nov., and Candida chrysomelidarum sp. nov., isolated from green lacewings and beetles. Mycol. Res. 2006, 110, 346–356. [Google Scholar] [CrossRef]
- Batista, T.M.; Hilário, H.O.; Moreira, R.G.; Furtado, C.; Godinho, V.M.; Rosa Franco, L.G.R.; Rosa, C.A. Draft genome sequence of Metschnikowia australis strain UFMG-CMY6158, an extremophile marine yeast endemic to Antarctica. Genom. Announ. 2017, 5, e00317–e00328. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T. The Yeasts—A Taxonomic Study, 5th ed.; Elsevier: London, UK; Burlington, NJ, USA; San Diego, CA, USA, 2011; Volume 2, pp. 584–585. [Google Scholar]
- Zhang, H.Q.; Chi, Z.; Liu, G.L.; Zhang, M.; Hu, Z.; Chi, Z.M. Metschnikowia bicuspidate associated with a milky disease in Eriocheir sinensis and its effectitve treatment by Massoia lactone. Microbiol. Res. 2021, 242, 126641. [Google Scholar] [CrossRef]
- Bao, J.; Jiang, H.; Shen, H.; Xing, Y.; Feng, C.C.; Li, X.; Chen, Q. First description of milky disease in the Chinese mitten crab Eriocheir sinensis caused by the yeast Metschnikowia bicuspidata. Aquaculture 2021, 532, 735984. [Google Scholar] [CrossRef]
- Jiang, H.; Bao, J.; Xing, Y.; Li, X.; Chen, Q. Comparative genomic analyses provide insight into the pathogenicity of Metschnikowia bicuspidata LNES0119. Front. Microbiol. 2022, 13, 939141. [Google Scholar] [CrossRef] [PubMed]
- Lachance, M.A.; Miranda, M.; Miller, M.W.; Phaff, H.J. Dehiscence and active spore release in pathogenic strains of the yeast Metschnikowia bicuspidata var. australis: Possible predatory implication. Can. J. Microbiol. 1976, 22, 1756–1761. [Google Scholar] [PubMed]
- Moore, M.M.; Strom, M.S. Infection and mortality by the yeast Metschnikowia bicuspidata var. bicuspidata in chinook salmon fed live adult brine shrimp (Artemia franciscana). Aquaculture 2003, 220, 43–57. [Google Scholar] [CrossRef]
- Xu, W.J.; Xu, H.X.; Jin, H.W. Studies on milky disease of Portunus trituberculatus. J. Oceanol. Zhejiang Prov. 2003, 3, 209–213. [Google Scholar]
- Wang, X.H.; Chi, Z.M.; Yue, L.X.; Li, J.; Li, M.J.; Wu, L.F. A marine killer yeast against the pathogenic yeast strain in crab (Portunus trituberculatus) and an optimization of the toxin production. Microbiol. Res. 2007, 162, 77–85. [Google Scholar] [CrossRef]
- Chen, S.C.; Chen, Y.C.; Kwang, J.; Manopo, I.; Wang, P.C.; Chaung, H.C.; Liaw, L.L.; Chiu, S.H. Metschnikowia bicuspidata dominates in Taiwanese cold-weather yeast infections of Macrobrachium rosenbergii. Dis. Aquat. Org. 2007, 75, 191–199. [Google Scholar] [CrossRef]
- Shi, W.; Zhao, R.; Hu, R.; Zhu, J.; Wan, X.; Qin, S. Proteomic analysis of the hepatopancreas of Exopalaemon carinicauda in response to Metschnikowia bicuspidata infection. Aquacul. Rep. 2023, 30, 101550. [Google Scholar] [CrossRef]
- Hube, B.; Sanglard, D.; Odds, F.C.; Hess, D.; Monod, M.; Schfer, W.; Brown, A.J.; Gow, N.A. Disruption of each of the secreted aspartyl proteinase genes SAP1, SAP2, and SAP3 of Candida albicans attenuates virulence. Infect. Immun. 1997, 65, 3529–3538. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Wright, L.C.; Santangelo, R.T.; Muller, M.; Moran, V.R.; Kuchel, P.W.; Sorrell, T.C. Identification of extracellular phospholipase B, lysophospholipase, and acyltransferase produced by Cryptococcus neoformans. Infect. Immun. 1997, 65, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.C.; Payne, J.; Santangelo, R.T.; Simpanya, M.F.; Chen, C.A.; Widmer, F.; Sorrell, T.C. Cryptococcal phospholipases: A novel lysophospholipase discovered in the pathogenic fungus Cryptococcus gattii. Biochem. J. 2004, 384, 377–384. [Google Scholar] [CrossRef]
- Schaller, M.; Borelli, C.; Korting, H.C.; Hube, B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses 2005, 48, 365–377. [Google Scholar] [CrossRef]
- Hruskova-Heidingsfeldova, O. Secreted proteins of Candida albicans. Front. Biosci. 2008, 13, 7227–7242. [Google Scholar] [CrossRef]
- Wei, X.; Chi, Z.; Liu, G.L.; Hu, Z.; Chi, Z.M. The genome-wide mutation shows the importance of cell wall integrity in growth of the psychrophilic yeast Metschnikowia australis W7-5 at different temperatures. Microb. Ecol. 2021, 81, 52–66. [Google Scholar] [CrossRef]
- Sun, N.; Bao, J.; Liang, F.; Liu, F.; Liang, H.B.; Li, X. Prevalence of ‘milky disease’ caused by Metschnikowia bicuspidata in Eriocheir sinensis in Panjin city, China. Aquacul. Res. 2022, 53, 1136–1140. [Google Scholar] [CrossRef]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microb. Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhang, M.; Chi, Z.; Liu, G.L.; Chi, Z.M. Genome-wide editing provides insights into role of unsaturated fatty acids in low temperature growth of the psychrotrophic yeast Metschnikowia bicuspidata var. australis W7-5. Mar. Biotechnol. 2023, 25, 70–82. [Google Scholar] [CrossRef]
- Ma, H.; Lu, X.; Liu, J.; Guo, S.; Zhao, X.; Ye, S. Metschnikowia bicuspidata isolated from milky diseased Eriocheir sinensis: Phenotypic and genetic characterization, antifungal susceptibility and challenge models. J. Fish Dis. 2022, 45, 41–49. [Google Scholar] [CrossRef]
- Reisa, A.C.; Kolvenbacha, B.A.; Nunesb, O.C.; Corvini, P.F.X. Biodegradation of antibiotics: The new resistance determinants—Part II. New Biotechnol. 2020, 54, 13–27. [Google Scholar] [CrossRef]
- Liu, G.L.; Chi, Z.; Wang, G.Y.; Wang, Z.P.; Li, Y.; Chi, Z.M. Yeast killer toxins, molecular mechanisms of their action and their applications. Crit. Rev. Biotechnol. 2015, 35, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Golubev, W. Differentiation between aquatic and terrestrial Metschnikowia species of based on their sensitivity to Pichia membranifaciens mycocins. Microbiology 2011, 80, 154–157. [Google Scholar] [CrossRef]
- Peng, Y.; Chi, Z.M.; Wang, X.H.; Li, J. β-1, 3-Glucanase inhibits activity of the killer toxin produced by the marine-derived yeast Williopsis saturnus WC91-2. Mar. Biotechnol. 2010, 12, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.X.; Jia, S.L.; Wei, X.; Zhang, M.; Liu, G.L.; Hu, Z.; Chi, Z.; Chi, Z.M. Liamocins biosynthesis, its regulation in Aureobasidium spp., and their bioactivities. Crit. Rev. Biotechnol. 2022, 42, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Price, N.P.J.; Manitchotpisit, P.; Vermillion, K.E.; Bowman, M.J.; Leathers, T.D. Structural characterization of novel extracellular liamocins (mannitol oils) produced by Aureobasidium pullulans strain NRRL 50380. Carboh. Res. 2013, 370, 24–32. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, M.; Chi, Z.; Liu, G.L.; Chi, Z.M. Liamocin overproduction by the mutants of Aureobasidium melanogenum 9–1 for effectively killing spores of the pathogenic fungi from diseased human skin by Massoia lactone. World J. Microbiol. Biotechnol. 2022, 38, 107. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Z.; Chi, Z.; Liu, G.L.; Chi, Z.M. Metabolic engineering of Aureobasidium melanogenum 9–1 for overproduction of liamocins by enhancing supply of acetyl-CoA and ATP. Microbiol. Res. 2022, 265, 127172. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, Z.C.; Chi, Z.; Wang, Z.; Liu, G.L.; Li, X.F.; Hu, Z.; Chi, Z.M. Massoia lactone displays strong antifungal property against many crop pathogens and its potential application. Microb. Ecol. 2022, 84, 376–390. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, H.Q.; Chi, Z.; Liu, G.L.; Chi, Z.M. Making of massoia lactone-loaded and food-grade nanoemulsions and their bioactivities against a pathogenic yeast. J. Mar. Sci. Eng. 2022, 10, 339. [Google Scholar] [CrossRef]
- Meng, M.; Feng, L.; Zhang, J. Synthesis of 5-hydroxy-2-decenoic acid lactone (Massoia Lactone). Chin. Acade. J. Electron Publish Hou. 2016, 1, 114–118. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansali, K.; Zhang, Z.-R.; Liu, G.-L.; Chi, Z.; Chi, Z.-M. The Pathogenic Yeast Metschnikowia bicuspidata var. bicuspidata in the Aquacultured Ecosystem and Its Biocontrol. J. Fungi 2023, 9, 1024. https://doi.org/10.3390/jof9101024
Hansali K, Zhang Z-R, Liu G-L, Chi Z, Chi Z-M. The Pathogenic Yeast Metschnikowia bicuspidata var. bicuspidata in the Aquacultured Ecosystem and Its Biocontrol. Journal of Fungi. 2023; 9(10):1024. https://doi.org/10.3390/jof9101024
Chicago/Turabian StyleHansali, Khalef, Zhao-Rui Zhang, Guang-Lei Liu, Zhe Chi, and Zhen-Ming Chi. 2023. "The Pathogenic Yeast Metschnikowia bicuspidata var. bicuspidata in the Aquacultured Ecosystem and Its Biocontrol" Journal of Fungi 9, no. 10: 1024. https://doi.org/10.3390/jof9101024
APA StyleHansali, K., Zhang, Z.-R., Liu, G.-L., Chi, Z., & Chi, Z.-M. (2023). The Pathogenic Yeast Metschnikowia bicuspidata var. bicuspidata in the Aquacultured Ecosystem and Its Biocontrol. Journal of Fungi, 9(10), 1024. https://doi.org/10.3390/jof9101024







