Unraveling the Polysaccharide Biosynthesis Potential of Ganoderma lucidum: A Chromosome-Level Assembly Using Hi-C Sequencing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Information
2.2. Sample Preparation and Sequencing
2.3. Analysis of the WGS Data: Pre-Processing, Assembly and Quality Assessment
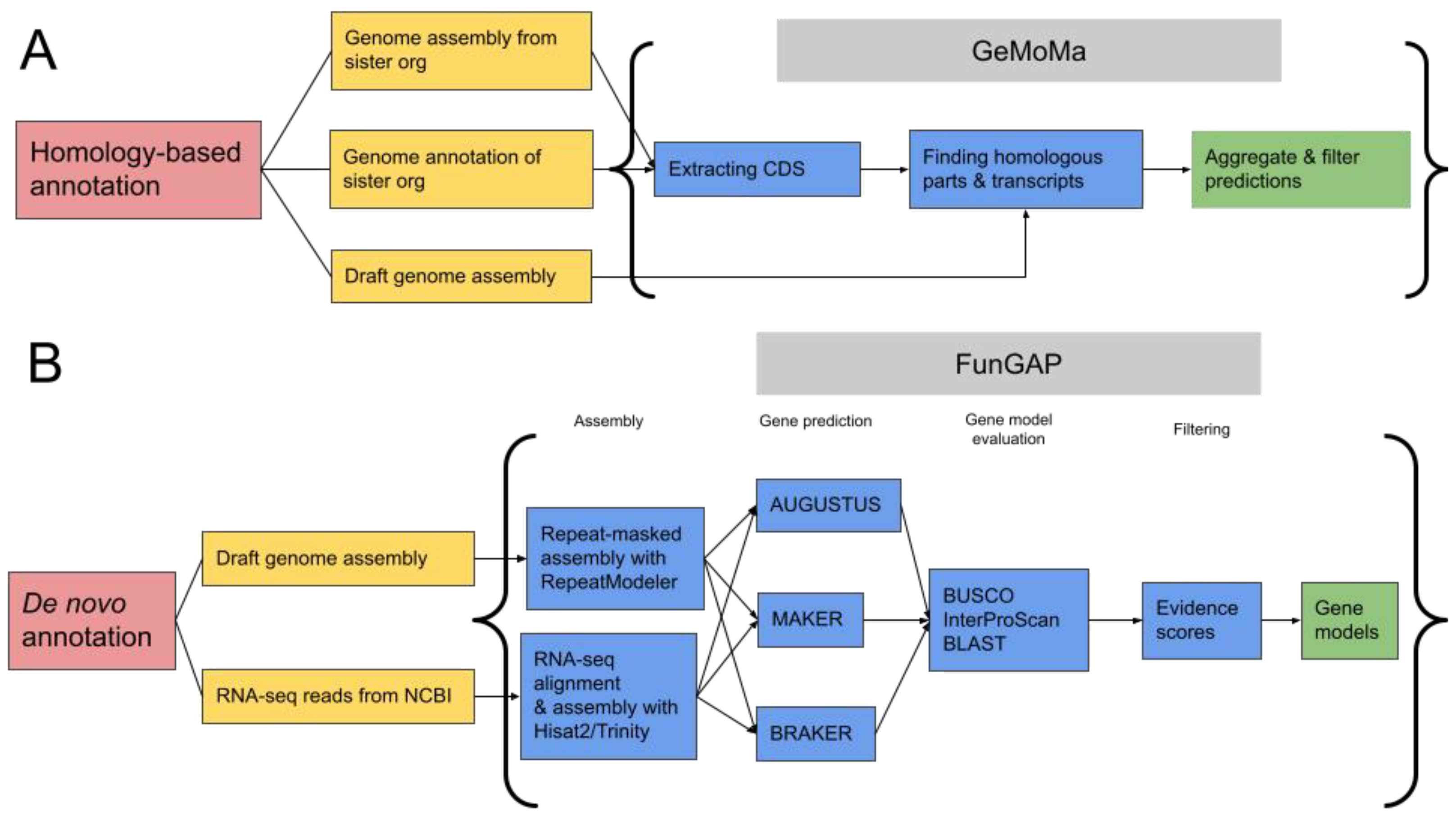
2.4. Total De Novo and Homology-Based Gene Annotation
2.5. Identification of the Genes Potentially Involved in the Synthesis of Xylomannan and Fucogalactan
2.6. Analysis of the Hi-C Data
3. Results
3.1. Genome Assembly of Ganoderma Lucidum Strain 5.1
3.2. Homology-Based Search of the Genes Involved in Xylomannan and Fucogalactan Biosynthesis
3.3. Search of the Genes Involved in Xylomannan and Fucogalactan Biosynthesis Based on the De Novo Annotation Results in WGS Assembly
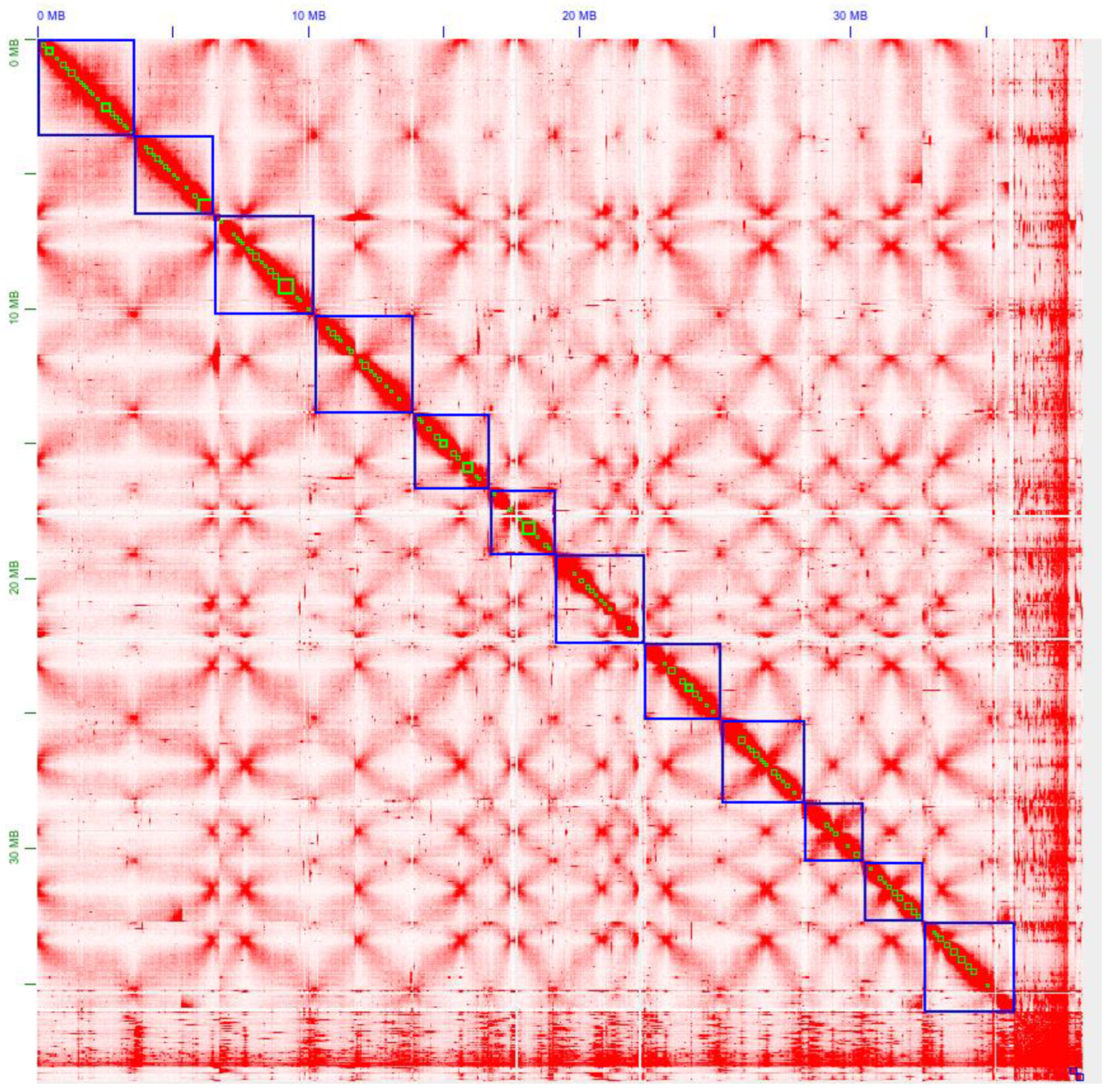
3.4. Improving G. lucidum Genome Assembly Using the Chromosome Conformation Data
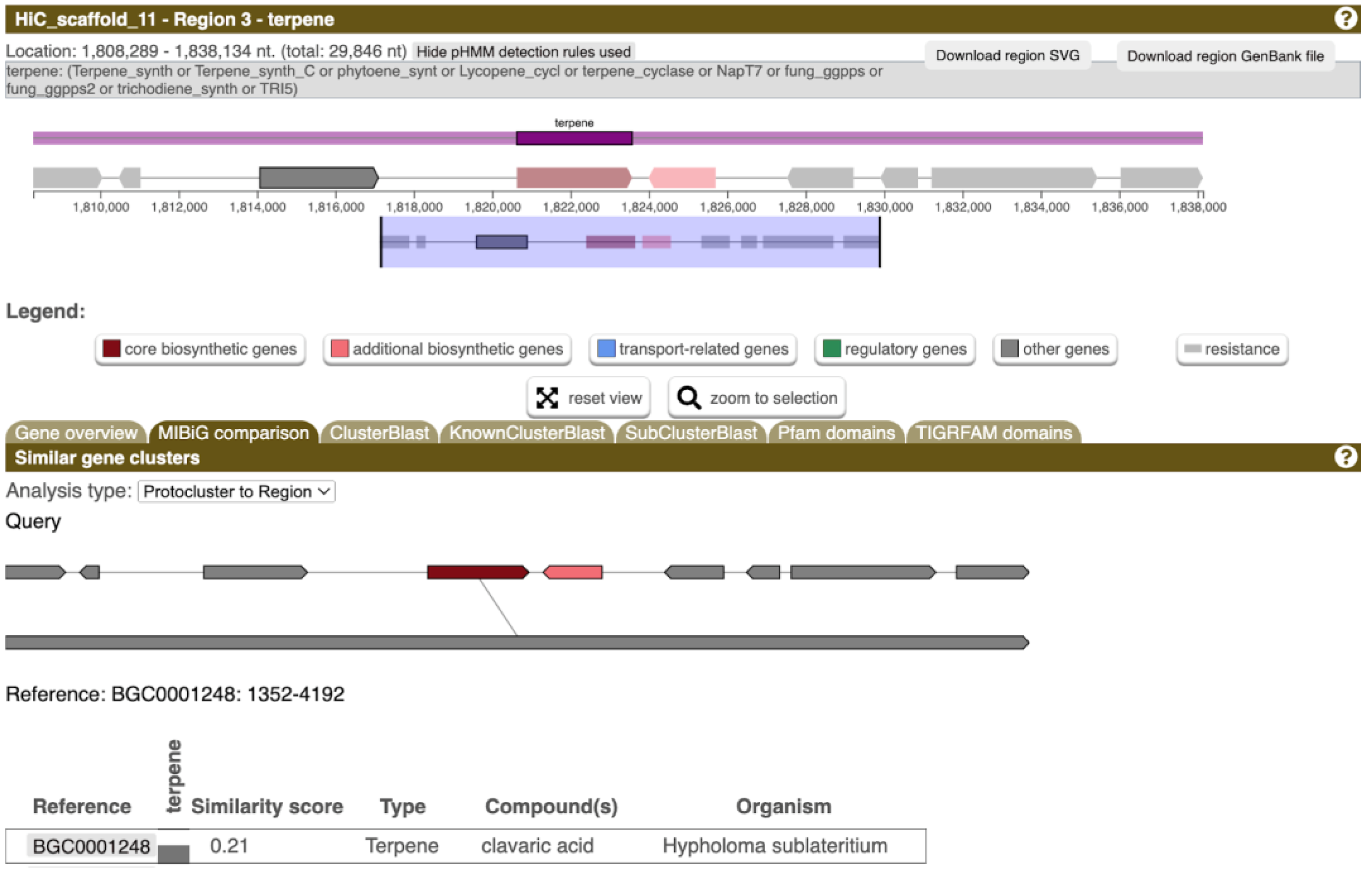
3.5. Prediction of Biosynthesis Genes Extends the Potential of Bioactive Compounds Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, M.-Q.; Zhao, R.-L.; Liu, D.-M.; Denchev, T.T.; Begerow, D.; Yurkov, A.; Kemler, M.; Millanes, A.M.; Wedin, M.; McTaggart, A.R.; et al. Species diversity of Basidiomycota. Fungal Divers. 2022, 114, 281–325. [Google Scholar] [CrossRef]
- Gressler, M.; Löhr, N.A.; Schäfer, T.; Lawrinowitz, S.; Seibold, P.S.; Hoffmeister, D. Mind the mushroom: Natural product biosynthetic genes and enzymes of Basidiomycota. Nat. Prod. Rep. 2021, 38, 702–722. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.; Sharma, A.; Tuli, H.S.; Batra, P.; Beniwal, V.; Gupta, G.K.; Sharma, A.K. Bioactive metabolites of Ganoderma lucidum: Factors, mechanism and broad spectrum therapeutic potential. J. Herb. Med. 2019, 17–18, 100268. [Google Scholar] [CrossRef]
- Lu, J.; He, R.; Sun, P.; Zhang, F.; Linhardt, R.J.; Zhang, A. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int. J. Biol. Macromol. 2020, 150, 765–774. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, F.A.; Khan, M.I.; Alsayegh, A.A.; Wahab, S.; Alam, M.I.; Ahmed, F. Ganoderma lucidum: A potential source to surmount viral infections through β-glucans immunomodulatory and triterpenoids antiviral properties. Int. J. Biol. Macromol. 2021, 187, 769–779. [Google Scholar] [CrossRef]
- Ahmad, R.; Riaz, M.; Khan, A.; Aljamea, A.; Algheryafi, M.; Sewaket, D.; Alqathama, A. Ganoderma lucidum (Reishi) an edible mushroom; a comprehensive and critical review of its nutritional, cosmeceutical, mycochemical, pharmacological, clinical, and toxicological properties. Phytother. Res. 2021, 35, 6030–6062. [Google Scholar] [CrossRef]
- Seweryn, E.; Ziała, A.; Gamian, A. Health-promoting of polysaccharides extracted from Ganoderma lucidum. Nutrients 2021, 13, 2725. [Google Scholar] [CrossRef]
- Xu, J.; Shen, R.; Jiao, Z.; Chen, W.; Peng, D.; Wang, L.; Yu, N.; Peng, C.; Cai, B.; Song, H.; et al. Current advancements in antitumor properties and mechanisms of medicinal components in edible mushrooms. Nutrients 2022, 14, 2622. [Google Scholar] [CrossRef]
- de Camargo, M.R.; Frazon, T.F.; Inacio, K.K.; Smiderle, F.R.; Amôr, N.G.; Dionísio, T.J.; Santos, C.F.; Rodini, C.O.; Lara, V.S. Ganoderma lucidum polysaccharides inhibit in vitro tumorigenesis, cancer stem cell properties and epithelial-mesenchymal transition in oral squamous cell carcinoma. J. Ethnopharmacol. 2022, 286, 114891. [Google Scholar] [CrossRef]
- Li, J.; Gu, F.; Cai, C.; Hu, M.; Fan, L.; Hao, J.; Yu, G. Purification, structural characterization, and immunomodulatory activity of the polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 2020, 143, 806–813. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Ling, J. Medicinal fungi with antiviral effect. Molecules 2022, 27, 4457. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, S.-X.; Wu, F.-Y.; Wu, K.-J.; Shi, R.-P.; Qin, L.-H.; Lu, C.-F.; Wang, F.-F.; Zhou, S. Effects and mechanism of polysaccharides in the treatment of diabetic nephropathy in streptozotocin-induced diabetic rats. BioMed Res. Int. 2022, 2022, 4314415. [Google Scholar] [CrossRef]
- Wen, L.; Sheng, Z.; Wang, J.; Jiang, Y.; Yang, B. Structure of water-soluble polysaccharides in spore of Ganoderma lucidum and their anti-inflammatory activity. Food Chem. 2022, 373, 131374. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Wang, Z.; Zhang, J.; Jia, L. Ganoderma lucidum polysaccharides improve lipid metabolism against high-fat diet-induced dyslipidemia. J. Ethnopharmacol. 2023, 309, 116321. [Google Scholar] [CrossRef]
- Tian, B.; Zhao, Q.; Xing, H.; Xu, J.; Li, Z.; Zhu, H.; Yang, K.; Sun, P.; Cai, M. Gastroprotective effects of polysaccharides with different molecular weights on ethanol-induced acute gastric injury in rats. Nutrients 2022, 14, 1476. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Wang, W.; Jia, L.; Zhang, J. Characterization and hepatoprotections of polysaccharides against multiple organ dysfunction syndrome in mice. Oxidative Med. Cell. Longev. 2021, 2021, 9703682. [Google Scholar] [CrossRef]
- Li, H.; Xiao, Y.; Han, L.; Jia, Y.; Luo, S.; Zhang, D.; Zhang, L.; Wu, P.; Xiao, C.; Kan, W.; et al. Ganoderma lucidum polysaccharides ameliorated depression-like behaviors in the chronic social defeat stress depression model via modulation of Dectin-1 and the innate immune system. Brain Res. Bull. 2021, 171, 16–24. [Google Scholar] [CrossRef]
- Liu, X.; Yang, L.; Li, G.; Jiang, Y.; Zhang, G.; Ling, J. A novel promising neuroprotective agent: Ganoderma lucidum polysaccharide. Int. J. Biol. Macromol. 2023, 229, 168–180. [Google Scholar] [CrossRef]
- Hu, F.; Yan, Y.; Wang, C.-W.; Liu, Y.; Wang, J.-J.; Zhou, F.; Zeng, Q.-H.; Zhou, X.; Chen, J.; Wang, A.-J.; et al. Article effect and mechanism of Ganoderma lucidum polysaccharides on human fibroblasts and skin wound healing in mice. Chin. J. Integr. Med. 2019, 25, 203–209. [Google Scholar] [CrossRef]
- Kang, Q.; Chen, S.; Li, S.; Wang, B.; Liu, X.; Hao, L.; Lu, J. Comparison on characterization and antioxidant activity of polysaccharides from Ganoderma lucidum by ultrasound and conventional extraction. Int. J. Biol. Macromol. 2019, 124, 1137–1144. [Google Scholar] [CrossRef]
- Evsenko, M.S.; Shashkov, A.S.; Avtonomova, A.V.; Krasnopolskaya, L.M.; Usov, A.I. Polysaccharides of basidiomycetes. alkali-soluble polysaccharides from the mycelium of white rot fungus Ganoderma lucidum (Curt.: Fr.) P. Karst. Biochemistry 2009, 74, 533–542. [Google Scholar] [CrossRef]
- Bleha, R.; Třešnáková, L.; Sushytskyi, L.; Capek, P.; Čopíková, J.; Klouček, P.; Jablonský, I.; Synytsya, A. Polysaccharides from Basidiocarps of the Polypore Fungus: Isolation and Structure. Polymers 2022, 14, 255. [Google Scholar] [CrossRef]
- Abdullah, N.R.; Sharif, F.; Azizan, N.H.; Hafidz, I.F.M.; Supramani, S.; Usuldin, S.R.A.; Ahmad, R.; Al Qadr Imad Wan-Mohtar, W.A. Pellet diameter of Ganoderma lucidum in a repeated-batch fermentation for the trio total production of biomass-exopolysaccharide-endopolysaccharide and its anti-oral cancer beta-glucan response. AIMS Microbiol. 2020, 6, 379–400. [Google Scholar] [CrossRef]
- Fryssouli, V.; Zervakis, G.I.; Polemis, E.; Typas, M.A. A global meta-analysis of ITS rDNA sequences from material belonging to the genus Ganoderma (Basidiomycota, Polyporales) including new data from selected taxa. MycoKeys 2020, 75, 71–143. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, S.-H.; Dai, Y.-C. Species clarification of the prize medicinal Ganoderma mushroom “Lingzhi”. Fungal Divers. 2012, 56, 49–62. [Google Scholar] [CrossRef]
- Loyd, A.L.; Barnes, C.W.; Held, B.W.; Schink, M.J.; Smith, M.E.; Smith, J.A.; Blanchette, R.A. Elucidating “lucidum”: Distinguishing the diverse laccate Ganoderma species of the United States. PLoS ONE 2018, 13, e0199738. [Google Scholar] [CrossRef]
- Loyd, A.L.; Richter, B.S.; Jusino, M.A.; Truong, C.; Smith, M.E.; Blanchette, R.A.; Smith, J.A. Identifying the “mushroom of immortality”: Assessing the species composition in commercial reishi products. Front Microbiol. 2018, 9, 1557. [Google Scholar] [CrossRef]
- Yarina, M.; Krasnopolskaya, L.; Usov, A.; Marakhonov, A. Biologically active polysaccharide was isolated from submergedly cultured mycelium of the genus Ganoderma P. Karst. In Biotechnology: State of the Art and Perspectives; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017; Volume 2, pp. 593–594. [Google Scholar]
- Usov, A.I.; Evsenko, M.S.; Shashkov, A.S.; Avtonomova, A.V.; Krasnopolskaya, L.M. The Structure of Fucogalactan from Ganoderma lucidum Mycelium. Immunopathol. Allergol. Infectology 2009, 2, 45. Available online: http://www.immunopathology.com/ru/article.php?carticle=29 (accessed on 10 June 2023).
- Krasnopolskaya, L.M.; Yarina, M.S.; Avtonomova, A.V.; Usov, A.I.; Isakova, E.B.; Bukchman, V.M. Antitumor Activity of Polysaccharides from Ganoderma lucidum Mycelium: In Vivo Comparative Study. Antibiot. Chemother. 2015, 60, 29–34. [Google Scholar]
- Free, S.J. Fungal cell wall organization and biosynthesis. Adv. Genet. 2013, 81, 33–82. [Google Scholar]
- Blyakher, M.S.; Aleshkin, A.V.; Fedorova, I.M.; Ramazanova, Z.K.; Kapustin, I.V.; Koteleva, S.I.; Krasnopolskaya, L.M.; Yarina, M.S. The effect of xylomannan isolated from Ganoderma lucidum on cytokine production by human peripheral blood mononuclear cells. Cytokines Inflamm. 2017, 16, 27–30. [Google Scholar]
- Krasnopolskaya, L.M.; Avtonomova, A.V.; Scheglovitova, O.N.; Sklyznkina, N.N.; Guschin, P.A. Production of Ganoderma lucidum strains submerged mycelium and interferon-inducing activity of endopolysaccharides. Bashkirski Him. Zhurnal 2012, 19, 83–88. [Google Scholar]
- Almyasheva, N.R.; Yarina, M.S.; Golyshkin, A.V.; Dzhavakhyan, B.R.; Krasnopolskaya, L.M. Antioxidant properties of water-soluble polysaccharides and ethanolic extracts of xylotrophic Basidiomycetes mycelium. Antibiot. I Khimioterapiya 2017, 62, 8–12. [Google Scholar]
- Qian, J.; Xu, H.; Song, J.; Xu, J.; Zhu, Y.; Chen, S. Genome-wide analysis of simple sequence repeats in the model medicinal mushroom Ganoderma lucidum. Gene 2013, 512, 331–336. [Google Scholar] [CrossRef]
- Binder, M.; Justo, A.; Riley, R.; Salamov, A.; Lopez-Giraldez, F.; Sjökvist, E.; Copeland, A.; Foster, B.; Sun, H.; Larsson, E.; et al. Phylogenetic and phylogenomic overview of the Polyporales. Mycologia 2013, 105, 1350–1373. [Google Scholar] [CrossRef]
- Kong, W.; Wang, Y.; Zhang, S.; Yu, J.; Zhang, X. Recent advances in assembly of plant complex genomes. In Genomics Proteomics Bioinformatics; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Ivanova, V.; Chernevskaya, E.; Vasiluev, P.; Ivanov, A.; Tolstoganov, I.; Shafranskaya, D.; Ulyantsev, V.; Korobeynikov, A.; Razin, S.V.; Beloborodova, N.; et al. Hi-C metagenomics in the ICU: Exploring clinically relevant features of gut microbiome in chronically critically ill patients. Front Microbiol. 2021, 12, 770323. [Google Scholar] [CrossRef]
- Smukowski Heil, C.; Burton, J.N.; Liachko, I.; Friedrich, A.; Hanson, N.A.; Morris, C.L.; Schacherer, J.; Shendure, J.; Thomas, J.H.; Dunham, M.J. Identification of a novel interspecific hybrid yeast from a metagenomic spontaneously inoculated beer sample using Hi-C. Yeast 2018, 35, 71–84. [Google Scholar] [CrossRef]
- Utomo, C.; Tanjung, Z.A.; Aditama, R.; Buana, R.F.N.; Pratomo, A.D.M.; Tryono, R.; Liwang, T. High quality genome sequence reveals the 12 pseudo-chromosomes of Ganoderma boninense. bioRxiv 2019. [Google Scholar] [CrossRef]
- Ulianov, S.V.; Khrameeva, E.E.; Gavrilov, A.A.; Flyamer, I.M.; Kos, P.; Mikhaleva, E.A.; Penin, A.A.; Logacheva, M.D.; Imakaev, M.V.; Chertovich, A.; et al. Active chromatin and transcription play a key role in chromosome partitioning into topologically associating domains. Genome Res. 2016, 26, 70–84. [Google Scholar] [CrossRef]
- Kajitani, R.; Toshimoto, K.; Noguchi, H.; Toyoda, A.; Ogura, Y.; Okuno, M.; Yabana, M.; Harada, M.; Nagayasu, E.; Maruyama, H.; et al. Efficient de novo assembly of highly heterozygous genomes from whole-genome shotgun short reads. Genome Res. 2014, 24, 1384–1395. [Google Scholar] [CrossRef]
- Kajitani, R.; Yoshimura, D.; Okuno, M.; Minakuchi, Y.; Kagoshima, H.; Fujiyama, A.; Kubokawa, K.; Kohara, Y.; Toyoda, A. Platanus-allee is a de novo haplotype assembler enabling a comprehensive access to divergent heterozygous regions. Nat. Commun. 2019, 10, 1702. [Google Scholar] [CrossRef]
- Nurk, S.; Bankevich, A.; Antipov, D.; Gurevich, A.; Korobeynikov, A.; Lapidus, A.; Prjibelsky, A.; Pyshkin, A.; Sirotkin, A.; Sirotkin, Y.; et al. Assembling genomes and mini-metagenomes from highly chimeric reads. In Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2013; pp. 158–170. [Google Scholar] [CrossRef]
- Seppey, M.; Manni, M.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness. Methods Mol. Biol. 2019, 1962, 227–245. [Google Scholar]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Menzel, P.; Ng, K.L.; Krogh, A. Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat. Commun. 2016, 7, 11257. [Google Scholar] [CrossRef]
- Pryszcz, L.P.; Gabaldón, T. Redundans: An assembly pipeline for highly heterozygous genomes. Nucleic Acids Res. 2016, 44, e113. [Google Scholar] [CrossRef]
- Keilwagen, J.; Hartung, F.; Paulini, M.; Twardziok, S.O.; Grau, J. Combining RNA-seq data and homology-based gene prediction for plants, animals and fungi. BMC Bioinform. 2018, 19, 189. [Google Scholar] [CrossRef]
- Min, B.; Grigoriev, I.V.; Choi, I.-G. FunGAP: Fungal Genome Annotation Pipeline using evidence-based gene model evaluation. Bioinformatics 2017, 33, 2936–2937. [Google Scholar] [CrossRef]
- Shao, J.; Chen, H.; Yang, D.; Jiang, M.; Zhang, H.; Wu, B.; Li, J.; Yuan, L.; Liu, C. Genome-wide identification and characterization of natural antisense transcripts by strand-specific RNA sequencing in Ganoderma lucidum. Sci. Rep. 2017, 7, 5711. [Google Scholar] [CrossRef]
- Törönen, P.; Medlar, A.; Holm, L. PANNZER2: A rapid functional annotation web server. Nucleic Acids Res. 2018, 46, W84–W88. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: New and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef]
- Durand, N.C.; Shamim, M.S.; Machol, I.; Rao, S.S.P.; Huntley, M.H.; Lander, E.S.; Aiden, E.L. Juicer provides a one-click system for analyzing loop-resolution Hi-C experiments. Cell Syst. 2016, 3, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Dudchenko, O.; Batra, S.S.; Omer, A.D.; Nyquist, S.K.; Hoeger, M.; Durand, N.C.; Shamim, M.S.; Machol, I.; Lander, E.S.; Aiden, A.P.; et al. De novo assembly of the Aedes aegypti genome using Hi-C yields chromosome-length scaffolds. Science 2017, 356, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Dudchenko, O.; Shamim, M.S.; Batra, S.S.; Durand, N.C.; Musial, N.T.; Mostofa, R.; Pham, M.; St Hilaire, B.G.; Yao, W.; Stamenova, E. The Juicebox Assembly Tools module facilitates de novo assembly of mammalian genomes with chromosome-length scaffolds for under $1000. bioRxiv 2018. [Google Scholar] [CrossRef]
- Liu, D.; Gong, J.; Dai, W.; Kang, X.; Huang, Z.; Zhang, H.M.; Liu, W.; Liu, L.; Ma, J.; Xia, Z.; et al. The genome of Ganderma lucidum provides insights into triterpense biosynthesis and wood degradation. PLoS ONE 2012, 7, e36146. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, J.; Sun, C.; Zhou, S.; Xu, H.; Nelson, D.R.; Qian, J.; Song, J.; Luo, H.; Xiang, L.; et al. Chromosome-level genome map provides insights into diverse defense mechanisms in the medicinal fungus Ganoderma sinense. Sci. Rep. 2015, 5, 11087. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, J.; Liu, C.; Zhu, Y.; Nelson, D.R.; Zhou, S.; Li, C.; Wang, L.; Guo, X.; Sun, Y.; et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat. Commun. 2012, 3, 913. [Google Scholar] [CrossRef]
- Jayasuriya, H.; Silverman, K.C.; Zink, D.L.; Jenkins, R.G.; Sanchez, M.; Pelaez, F.; Vilella, D.; Lingham, R.B.; Singh, S.B. Clavaric acid: A triterpenoid inhibitor of farnesyl-protein transferase from Clavariadelphus truncatus. J. Nat. Prod. 1998, 61, 1568–1570. [Google Scholar] [CrossRef]
- Ho, C.-L. Comparative genomics analysis of Ganoderma orthologs involved in plant-pathogenesis. Trees Livelihoods 2023, 14, 653. [Google Scholar] [CrossRef]
- Smirnov, A.; Anisimkin, V.; Krasnopolskaya, L.; Guliy, O.; Sinev, I.; Simakov, V.; Golyshkin, A.; Almyasheva, N.; Ageykin, N.; Kuznetsova, I. Features of the formation of sensitive films based on mycelium of higher fungi for surface and plate acoustic waves gas sensors. Sensors 2023, 23, 2216. [Google Scholar] [CrossRef]
- Danninger, D.; Pruckner, R.; Holzinger, L.; Koeppe, R.; Kaltenbrunner, M. MycelioTronics: Fungal mycelium skin for sustainable electronics. Sci. Adv. 2022, 8, eadd7118. [Google Scholar] [CrossRef]
- Ma, L.; Yang, C.; Xiao, D.; Liu, X.; Jiang, X.; Lin, H.; Ying, Z.; Lin, Y. Chromosome-level assembly of Dictyophora rubrovolvata genome using third-generation DNA sequencing and Hi-C analysis. G3 2023, 13, jkad102. [Google Scholar] [CrossRef] [PubMed]
- Lewin, H.A.; Richards, S.; Aiden, E.L.; Lieberman Allende, M.; Archibald, J.M.; Bálint, M.; Barker, K.B.; Baumgartner, B.; Belov, K.; Bertorelle, G.; et al. The Earth BioGenome Project 2020, Starting the clock. Proc. Natl. Acad. Sci. USA 2022, 119, e2115635118. [Google Scholar] [CrossRef] [PubMed]
- Onoue, T.; Tanaka, Y.; Hagiwara, D.; Ekino, K.; Watanabe, A.; Ohta, K.; Kamei, K.; Shibata, N.; Goto, M.; Oka, T. Identification of two mannosyltransferases contributing to biosynthesis of the fungal-type galactomannan α-core-mannan structure in Aspergillus fumigatus. Sci. Rep. 2018, 8, 16918. [Google Scholar] [CrossRef] [PubMed]
| WGS + Hi-C Assembly | WGS Assembly | |
|---|---|---|
| 38,961,136 | 39,106,447 | Total assembly length, bp |
| 2731 | 2270 | Number of contigs |
| 3,048,500 | 72,187 | Scaffold N50, bp |
| 4,258,832 | 594,110 | Maximum scaffold length |
| 55.61 | 55.57 | GC% |
| AA Sequence Identity, % | Gene ID in G. lucidum Strain 5.1 Assembly | Reference Gene ID in Ganoderma sp. 10597 |
|---|---|---|
| 100 | MRNA_403_R0 | jgi|Gansp1|38124|e_gw1.1.2377.1 |
| 99.42 | MRNA_2449_R0 | jgi|Gansp1|41514|e_gw1.2.2688.1 |
| 99.74 | MRNA_1789_R0 | jgi|Gansp1|42603|e_gw1.2.2497.1 |
| 98.33 | MRNA_7121_R0 | jgi|Gansp1|51293|e_gw1.7.902.1 |
| 99.13 | MRNA_7035_R0 | jgi|Gansp1|51570|e_gw1.7.313.1 |
| 98.97 | MRNA_10444_R0 | jgi|Gansp1|55798|e_gw1.11.587.1 |
| 93.11 | MRNA_10444_R1 | jgi|Gansp1|56496|e_gw1.11.376.1 |
| 99.74 | MRNA_6022_R0 | jgi|Gansp1|69736|estExt_Genewise1.C_6_t10369 |
| 99.48 | MRNA_8855_R0 | jgi|Gansp1|73716|estExt_Genewise1.C_9_t20352 |
| 99.01 | MRNA_4151_R0 | jgi|Gansp1|84501|estExt_Genewise1Plus.C_4_t10328 |
| 96.12 | MRNA_6859_R0 | jgi|Gansp1|88381|estExt_Genewise1Plus.C_7_t10263 |
| 99.27 | MRNA_7000_R0 | jgi|Gansp1|88625|estExt_Genewise1Plus.C_7_t20055 |
| 98.83 | MRNA_4436_R0 | jgi|Gansp1|127772|fgenesh1_pm.4_#_322 |
| 100 | MRNA_7163_R0 | jgi|Gansp1|129696|fgenesh1_pm.7_#_370 |
| 97.8 | MRNA_11372_R0 | jgi|Gansp1|132299|fgenesh1_pm.13_#_149 |
| 99.86 | MRNA_1100_R0 | jgi|Gansp1|113974|fgenesh1_kg.1_#_1092_#_isotig08803 |
| 99.59 | MRNA_2242_R0 | jgi|Gansp1|115010|fgenesh1_kg.2_#_583_#_isotig10382 |
| 99.74 | MRNA_6364_R0 | jgi|Gansp1|118777|fgenesh1_kg.6_#_527_#_isotig08567 |
| 99.85 | MRNA_7916_R0 | jgi|Gansp1|120249|fgenesh1_kg.8_#_247_#_isotig07580 |
| 97.78 | MRNA_11130_R0 | jgi|Gansp1|123003|fgenesh1_kg.12_#_593_#_isotig07483 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonets, I.V.; Dovidchenko, N.V.; Ulianov, S.V.; Yarina, M.S.; Koshechkin, S.I.; Razin, S.V.; Krasnopolskaya, L.M.; Tyakht, A.V. Unraveling the Polysaccharide Biosynthesis Potential of Ganoderma lucidum: A Chromosome-Level Assembly Using Hi-C Sequencing. J. Fungi 2023, 9, 1020. https://doi.org/10.3390/jof9101020
Sonets IV, Dovidchenko NV, Ulianov SV, Yarina MS, Koshechkin SI, Razin SV, Krasnopolskaya LM, Tyakht AV. Unraveling the Polysaccharide Biosynthesis Potential of Ganoderma lucidum: A Chromosome-Level Assembly Using Hi-C Sequencing. Journal of Fungi. 2023; 9(10):1020. https://doi.org/10.3390/jof9101020
Chicago/Turabian StyleSonets, Ignat V., Nikita V. Dovidchenko, Sergey V. Ulianov, Maria S. Yarina, Stanislav I. Koshechkin, Sergey V. Razin, Larissa M. Krasnopolskaya, and Alexander V. Tyakht. 2023. "Unraveling the Polysaccharide Biosynthesis Potential of Ganoderma lucidum: A Chromosome-Level Assembly Using Hi-C Sequencing" Journal of Fungi 9, no. 10: 1020. https://doi.org/10.3390/jof9101020
APA StyleSonets, I. V., Dovidchenko, N. V., Ulianov, S. V., Yarina, M. S., Koshechkin, S. I., Razin, S. V., Krasnopolskaya, L. M., & Tyakht, A. V. (2023). Unraveling the Polysaccharide Biosynthesis Potential of Ganoderma lucidum: A Chromosome-Level Assembly Using Hi-C Sequencing. Journal of Fungi, 9(10), 1020. https://doi.org/10.3390/jof9101020








