A New Genotype of Trichophyton quinckeanum with Point Mutations in Erg11A Encoding Sterol 14-α Demethylase Exhibits Increased Itraconazole Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Plate Assay for Resistance Estimation of T. schoenleinii and T. quinckeanum
2.3. Microplate Laser Nephelometry Assays
2.4. DNA Isolation and DNA Amplification
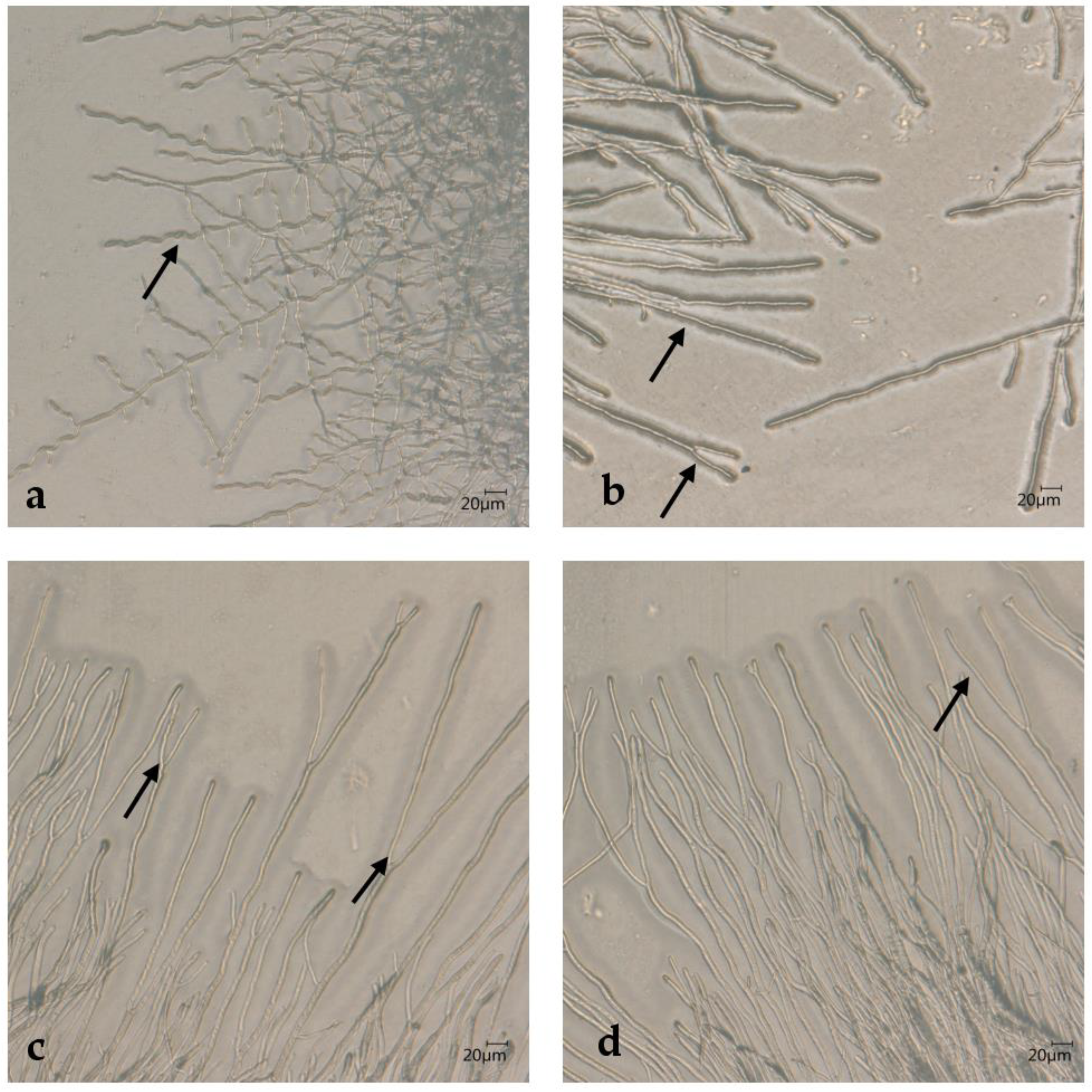
2.5. Microscopy
3. Results
3.1. Resistant Phenotypes of T. quinckeanum Strains
3.2. Gene Analyses for Putative Association with Resistance Phenotypes
3.3. T. quinckeanum and T. schoenleinii Represent Opposite Mating Types
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quincke, H.I. Ueber Favuspilze. Arch. Exp. Pathol. Pharmakol. 1886, 22, 62–67. [Google Scholar] [CrossRef]
- Ilkit, M. Favus of the scalp: An overview and update. Mycopathologia 2010, 170, 143–154. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, M.S.; Pereiro, M.; Pereiro, M.M.; Toribio, J. Favus due to Trichophyton mentagrophytes var. quinckeanum. Dermatology 1997, 194, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Beguin, H.; Pyck, N.; Hendrickx, M.; Planard, C.; Stubbe, D.; Detandt, M. The taxonomic status of Trichophyton quinckeanum and T. interdigitale revisited: A multigene phylogenetic approach. Med. Mycol. 2012, 50, 871–882. [Google Scholar] [CrossRef]
- Iwasa, K.; Ogawa, K.; Azukizawa, H.; Tanabe, H.; Iwanaga, T.; Anzawa, K.; Mochizuki, T.; Asada, H. Revival. of favus in Japan caused by Trichophyton schoenleinii. J. Dermatol. 2019, 46, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, D.M.; Burmester, A.; Ludriksone, L.; Darr-Foit, S.; Hipler, U.-C.; Wiegand, C. Renaissance of mouse favus. Retrospective analysis of Trichophyton quinckeanum infections at Jena University Hospital in the period 2015–2020. Hautarzt 2021, 72, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Erntebericht 2020. Available online: https://infrastruktur-landwirtschaft.thueringen.de/fileadmin/Landwirtschaft/07_Pflanzliche_Erzeugung_Gartenbau_und_Sonderkulturen/Erntebericht_2020.pdf (accessed on 18 August 2022).
- De Hoog, G.S.; Dukik, K.; Monod, M.; Packeu, A.; Stubbe, D.; Hendrickx, M.; Kupsch, C.; Stielow, J.B.; Freeke, J.; Göker, M.; et al. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia 2017, 182, 5–31. [Google Scholar] [CrossRef]
- Uhrlaß, S.; Schroedl, W.; Mehlhorn, C.; Krüger, C.; Hubka, V.; Maier, T.; Gräser, Y.; Paasch, U.; Nenoff, P. Molecular epidemiology of Trichophyton quinckeanum—A zoophilic dermatophyte on the rise. J. Dtsch. Dermatol. Ges. 2018, 16, 21–32. [Google Scholar] [CrossRef]
- Nenoff, P.; Uhrlaß, S.; Bethge, A.; Pöge, A.; Krüger, C.; Kohl, M. Tinea capitis profunda durch Trichophyton quinckeanum. Derm. Prakt. Dermatol. 2018, 1, 12–23. [Google Scholar]
- Lysková, P.; Dobiáš, R.; Čmoková, A.; Kolařík, M.; Hamal, P.; Šmatláková, K.; Hušek, J.; Mencl, K.; Mallátová, N.A.; Poláčková, Z.; et al. An outbreak of Trichophyton quinckeanum zoonotic infections in the Czech Republic transmitted from Cats and Dogs. J. Fungi 2021, 7, 684. [Google Scholar] [CrossRef] [PubMed]
- Drouot, S.; Mignon, B.; Fratti, M.; Roosje, P.; Monod, M. Pets as the main source of two zoonotic species of the Trichophyton mentagrophytes complex in Switzerland, Arthroderma vanbreuseghemii and Arthroderma benhamiae. ESVD ACVD 2008, 20, 13–18. [Google Scholar] [CrossRef]
- Ajello, L.; Bostick, L.; Cheng, S.-H. The relationship of Trichophyton quinckeanum to Trichophyton mentagrophytes. Mycologia 1968, 60, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Weitzman, I.; Padhye, A.A. Is Arthroderma simii the perfect state of Trichophyton quinckeanum? Sabouraudia 1976, 14, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Weitzman, I.; Summerbell, R.C. The dermatophytes. Clin. Microbiol. Rev. 1995, 8, 240–259. [Google Scholar] [CrossRef] [PubMed]
- Gräser, Y.; Kuijpers, A.F.A.; Presber, W.; De Hoog, S.B. Molecular taxonomy of Trichophyton mentagrophytes and T. tonsurans. Med. Mycol. 1999, 37, 315–330. [Google Scholar] [CrossRef]
- Čmoková, A.; Kolařík, M.; Dobiáš, R.; Hoyer, L.L.; Janouškovcová, H.; Kano, R.; Kuklová, I.; Lysková, P.; Machová, L.; Maier, T.; et al. Resolving the taxonomy of emerging zoonotic pathogens in the Trichophyton benhamiae complex. Fungal Divers. 2020, 104, 333–387. [Google Scholar] [CrossRef]
- Symoens, F.; Jousson, O.; Packeu, A.; Fratti, M.; Staib, P.; Mignon, B.; Monod, M. The dermatophyte species Arthroderma benhamiae: Intraspecies variability and mating behavior. J. Med. Microbiol. 2013, 62, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Nenoff, P.; Verma, S.B.; Uhrlaß, S.; Burmester, A.; Gräser, Y. A clarion call for preventing taxonomical errors of dermatophytes using the example of the novel Trichophyton mentagrophytes genotype VIII uniformly isolated in the Indian epidemic of superficial dermatophytosis. Mycoses 2019, 62, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Kong, X.; Ahmed, S.A.; Thakur, R.; Chowdhary, A.; Nenoff, P.; Uhrlass, S.; Verma, S.B.; Meis, J.F.; Kandemir, H.; et al. Taxonomy of the Trichophyton mentagrophytes/T. interdigitale species complex harboring the highly virulent, multiresistant genotype T. indotineae. Mycopathologia 2021, 186, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Masih, A.; Khurana, A.; Singh, P.K.; Gupta, M.; Hagen, F.; Meis, J.F.; Chowdhary, A. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses 2018, 61, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.; Monod, M.; Salamin, K.; Burmester, A.; Uhrlaß, S.; Wiegand, C.; Hipler, U.C.; Krüger, C.; Koch, D.; Wittig, F.; et al. Alarming India-wide phenomenon of antifungal resistance in dermatophytes: A multicentre study. Mycoses 2020, 63, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Burmester, A.; Hipler, U.-C.; Elsner, P.; Wiegand, C. Point mutations in the squalene epoxidase erg1 and sterol 14-α demethylase erg11 gene of T. indotineae isolates indicate that the resistant mutant strains evolved independently. Mycoses 2021, 65, 97–102. [Google Scholar] [CrossRef]
- Metin, B.; Heitman, J. She loves me, she loves me not: On the dualistic asexual/sexual nature of dermatophyte fungi. Mycopathologia 2020, 185, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Takashio, M. Is Arthroderma behamiae the perfect state of Trichophyton mentagrophytes? Sabouraudia 1972, 10, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Burmester, A.; Hipler, U.-C.; Elsner, P.; Wiegand, C. Mating analyses of Trichophyton benhamiae offspring reveals linkage of genetic markers used in taxonomy. Med. Mycol. 2019, 57, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Guirges, S.Y. Viability of Trichophyton schoenleinii in epilated hairs. Sabouraudia 1981, 19, 155–156. [Google Scholar] [CrossRef] [PubMed]
- IHEM Species Details. Available online: https://bccm.belspo.be/catalogues/ihem-species-details?NUM=IHEM (accessed on 6 September 2023).
- CBS 318.56 Species Details. Available online: https://wi.knaw.nl/fungal_table (accessed on 6 September 2023).
- Guinea, J.; Verweij, P.E.; Meletiadis, J.; Mouton, J.W.; Barchiesi, F.; Arendrup, M.C. How to: EUCAST recommendations on the screeningprocedure E.Def 10.1 for the detection of azole resistance in Aspergillus fumigatus isolates using four-well azole-containing agar plates. Clin. Microbiol. Infect. 2019, 25, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Burmester, A.; Hipler, U.-C.; Uhrlaß, S.; Nenoff, P.; Singal, A.; Verma, S.B.; Elsner, P.; Wiegand, C. Indian Trichophyton mentagrophytes squalene epoxidase erg1 double mutants show high proportion of combined fluconazole and terbinafine resistance. Mycoses 2020, 63, 1175–1180. [Google Scholar] [CrossRef]
- Fink, S.; Burmester, A.; Hipler, U.-C.; Neumeister, C.; Götz, M.R.; Wiegand, C. Efficacy of antifungal agents against fungal spores: An in vitro study using microplate laser nephelometry and an artificially infected 3D skin model. MicrobiologyOpen 2022, 11, e1257. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, G.S.; van den Ende, A.H.G.G. Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses 1998, 41, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, S.; Monod, M.; Gräser, Y. Signature polymorphisms in the internal transcribed spacer region relevant for the differentiation of the zoophilic and anthropophilic strains of Trichophyton interdigitale and other species of T. mentagrophytes sensu lato. Br. J. Dermatol. 2010, 162, 282–295. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Ge, L.-Y.; Liu, J.; Zheng, H.-L.; Mei, H.; Liang, G.-Z.; Liu, W.-D. Comprehensive genome and transcriptome analysis of the dermatophyte Trichophyton schoenleinii reveals the candidate pathogenic genes. Mycoses 2021, 64, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Rosam, K.; Monk, B.C.; Lackner, M. Sterol 14α-demethylase ligand-binding pocket-mediated acquired and intrinsic azole resistance in fungal pathogens. J. Fungi 2021, 7, 1. [Google Scholar] [CrossRef]
- Burmester, A.; Shelest, E.; Glöckner, G.; Heddergott, C.; Schindler, S.; Staib, P.; Heidel, A.; Felder, M.; Petzold, A.; Szafranski, K.; et al. Comparative and functional genomics provide insights into the pathogenicity of dermatophytic fungi. Genome Biol. 2011, 12, R7. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.-M.; Faria-Ramos, I.; Cruz, L.C.; Pina-Vaz, C.; Rodrigues, A.G. Genesis of Azole Antifungal Resistance from Agriculture to Clinical Settings. J. Agric. Food Chem. 2015, 63, 7463–7468. [Google Scholar] [CrossRef]
- Meis, J.; Chowdhary, A.; Rhodes, J.L.; Fisher, M.C.; Verweij, P.E. Clinical implications of globally emerging azole resistance in Aspergillus fumigatus. Phil. Trans. R. Soc. B 2016, 371, 20150460. [Google Scholar] [CrossRef] [PubMed]
- Langfeldt, A.; Gold, J.A.W.; Chiller, T. Emerging fungal infections: From the fields to the clinic, resistant Aspergillus fumigatus and dermatophyte species: A one health perspective on an urgent public health problem. Curr. Clin. Microbiol. Rep. 2022, 9, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, H. Meiosis: An overview of key differences from mitosis. Cold Spring Harb. Perspect. Biol. 2015, 7, a015859. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, B.P.S.; James, T.Y. The frequency of sex in fungi. Phil. Trans. R. Soc. B 2016, 371, 20150540. [Google Scholar] [CrossRef] [PubMed]
- Billiard, S.; Pez-Villavicencio, M.L.; Hood, M.E.; Giraud, T. Sex, outcrossing and mating types: Unsolved questions in fungi and beyond. J. Evol. Biol. 2012, 25, 1020–1038. [Google Scholar] [CrossRef] [PubMed]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; Ugene Team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Phylogenetic Tree Online Tool. Available online: https://www.ncbi.nlm.nih.gov/blast/treeview (accessed on 5 June 2023).
- Baert, F.; Stubbe, D.; D’hooge, E.; Packeu, A.; Hendrickx, M. Updating the taxonomy of dermatophytes of the BCCM/IHEM collection according to the new standard: A phylogenetic approach. Mycopathologia 2020, 185, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.-O.; Grosso, K.M.; Carrion, M.E. Multilocus phylogeny of the Trichophyton mentagrophytes species complex and the application of matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) mass spectrometry for the rapid identification of dermatophytes. Mycology 2018, 110, 118–130. [Google Scholar] [CrossRef] [PubMed]
| Species | Strain 1 | Synonyms 1 | Collected at Year | Country | Host | Age, Sex | Diagnosis |
|---|---|---|---|---|---|---|---|
| T. quinckeanum | UKJ 1505/12 | 2012 | Germany | Human | 6 y, female | Tinea corporis | |
| UKJ 621/13 | 2013 | Germany | Human | male | Tinea corporis | ||
| UKJ 1547/14 | 2014 | Germany | Human | 7 y, female | Tinea capitis | ||
| UKJ 1905/19 | 2019 | Germany | Human | 25 y, female | Tinea manum | ||
| UKJ 1953/19 | 2019 | Germany | Cat | Infected hairs | |||
| UKJ 1254/20 | 2020 | Germany | Cat | Infected hairs | |||
| UKJ 1506/20 | 2020 | Germany | Human | 69 y, female | Tinea corporis | ||
| UKJ 1891/20 | 2020 | Germany | Human | 21 y, female | Tinea corporis | ||
| IHEM 13570 | CDC X395 | Before 1968 2 | USA | Dog | Favus 4 | ||
| IHEM 13572 | 1964 | Australia | Rodent | Favus 4 | |||
| IHEM 13697 | CDC X393 | Before 1968 2 | USA | Mouse | Favus 4 | ||
| IHEM 26522 | CBS 318.56 | 1955 | Netherlands | Human | Deep trichophytosis 4 | ||
| T. schoenleinii | IHEM 13515 | 1966 | Morocco | Human | Favus 4 | ||
| UKJ 1317/12 | ap. 1972 | ||||||
| T. mentagrophytes | ATCC 46950 | Before 1980 3 | Iraq | Human | Infected epilated hairs |
| Strain | Itr | Vor | Ser | Clt | Ter | Amo | Nys | Cic |
|---|---|---|---|---|---|---|---|---|
| UKJ 1505/12 | 0.45 ± 0.13 | 0.71 ± 0.04 | 2.78 ± 0.66 | 0.42 ± 0.02 | 0.0038 ± 0.0006 | 0.021 ± 0.004 | 16.4 ± 1.9 | 7.5 ± 1.0 |
| UKJ 621/13 | 0.12 ± 0.001 | 0.70 ± 0.10 | 3.81 ± 0.11 | 0.39 ± 0.01 | 0.0030 ± 0.0005 | 0.053 ± 0.0004 | 15.6 ± 0.5 | 7.4 ± 0.8 |
| UKJ 1547/14 | 0.29 ± 0.11 | 0.59 ± 0.06 | 1.88 ± 0.22 | 0.25 ± 0.02 | 0.0030 ± 0.0002 | 0.026 ± 0.001 | 11.0 ± 1.5 | 6.4 ± 0.9 |
| UKJ 1905/19 | 0.11 ± 0.004 | 0.55 ± 0.04 | 2.03 ± 0.01 | 0.20 ± 0.004 | 0.0030 ± 0.0002 | 0.027 ± 0.001 | 10.0 ± 0.2 | 8.7 ± 2.4 |
| UKJ 1953/19 | 0.25 ± 0.13 | 0.99 ± 0.28 | 1.60 ± 0.23 | 0.22 ± 0.06 | 0.0030 ± 0.0005 | 0.019 ± 0.001 | 18.9 ± 2.6 | 6.4 ± 0.3 |
| UKJ 1254/20 | 0.11 ± 0.02 | 0.88 ± 0.01 | 1.48 ± 0.34 | 0.18 ± 0.06 | 0.0026 ± 0.0002 | 0.018 ± 0.0004 | 19.4 ± 0.9 | 7.5 ± 1.5 |
| UKJ 1506/20 | 0.34 ± 0.061 | 1.03 ± 0.15 | 1.49 ± 0.13 | 0.25 ± 0.03 | 0.0029 ± 0.0004 | 0.020 ± 0.0001 | 10.5 ± 0.3 | 7.0 ± 0.9 |
| UKJ 1891/20 | 0.091 ± 0.02 | 0.47 ± 0.06 | 1.96 ± 0.44 | 0.22 ± 0.01 | 0.0028 ± 0.0002 | 0.034 ± 0.001 | 9.9 ± 0.6 | 6.7 ± 0.9 |
| IHEM 13570 | 0.0033 ± 0.0004 | 0.11 ± 0.009 | 0.81 ± 0.16 | 0.06 ± 0.02 | 0.0026 ± 0.0003 | 0.014 ± 0.0001 | 5.4 ± 0.4 | 4.9 ± 1.8 |
| IHEM 13572 | 0.070 ± 0.007 | 0.53 ± 0.10 | 1.88 ± 0.17 | 0.26 ± 0.02 | 0.0036 ± 0.0007 | 0.024 ± 0.004 | 10.1 ± 3.1 | 7.9 ± 0.9 |
| IHEM 13697 | 0.0030 ± 0.00007 | 0.17 ± 0.003 | 0.67 ± 0.19 | 0.08 ± 0.01 | 0.0016 ± 0.0001 | 0.015 ± 0.001 | 4.1 ± 0.6 | 12.5 ± 0.4 |
| IHEM 26522 | 0.019 ± 0.001 | 0.62 ± 0.07 | 1.66 ± 0.13 | 0.22 ± 0.04 | 0.0020 ± 0.0002 | 0.025 ± 0.002 | 10.8 ± 0.7 | 7.1 ± 0.3 |
| Species | Strain * | Flu 0.4 µg/mL | Flu 4 µg/mL | Flu 40 µg/mL | Itr 0.005 µg/mL | Itr 0.05 µg/mL | Itr 0.5 µg/mL |
|---|---|---|---|---|---|---|---|
| T. quinckeanum | UKJ 1505/12 | n.t. | + | + | n.t. | + | + |
| UKJ 621/13 | n.t. | + | + | n.t. | + | + | |
| UKJ 1547/14 | n.t. | + | + | n.t. | + | + | |
| UKJ 1905/19 | n.t. | + | + | n.t. | + | + | |
| UKJ 1953/19 | n.t. | + | + | n.t. | + | + | |
| UKJ 1254/20 | n.t. | + | + | n.t. | + | + | |
| UKJ 1506/20 | n.t. | + | + | n.t. | + | + | |
| UKJ 1891/20 | n.t. | + | + | n.t. | + | + | |
| IHEM 13570 | n.t. | + | + | n.t. | +/− | − | |
| IHEM 13572 | n.t. | + | + | n.t. | +/− | − | |
| IHEM 13697 | n.t. | + | + | n.t. | +/− | − | |
| IHEM 26522 | n.t. | + | + | n.t. | +/− | − | |
| T. schoenleinii | UKJ 1317/12 | + | + | − | + | − | − |
| IHEM 13515 | + | + | − | + | − | − | |
| T. mentagrophytes | ATCC 46950 | + | − | − | − | − | − |
| Species | Strain Alignment in bp * | Erg11A 1509 bp | Erg11B 1722 bp | Erg1 1420 bp | Mat1-1-1 571 bp | Mat1-2-1 1121 bp |
|---|---|---|---|---|---|---|
| T. quinckeanum | UKJ 1505/12 | 15 | 0 | 0 | 1 | absent |
| UKJ 621/13 | 15 | 0 | 0 | 1 | absent | |
| UKJ 1547/14 | 15 | 0 | 0 | 1 | absent | |
| UKJ 1905/19 | 15 | 0 | 0 | 1 | absent | |
| UKJ 1953/19 | 15 | 0 | 0 | 1 | absent | |
| UKJ 1254/20 | 15 | 0 | 0 | 1 | absent | |
| UKJ 1506/20 | 15 | 0 | 0 | 1 | absent | |
| UKJ 1891/20 | 15 | 0 | 0 | 1 | absent | |
| IHEM 13570 | 0 | 0 | 0 | 1 | absent | |
| IHEM 13572 | 1 | 0 | 0 | 1 | absent | |
| IHEM 13697 | 0 | 0 | 0 | 0 | absent | |
| IHEM 26522 | 0 | 0 | 0 | 1 | absent | |
| T. schoenleinii | UKJ 1317/12 | 8 | 0 | 0 | absent | 0 |
| HEM 13515 | 9 | 0 | 0 | absent | 0 | |
| CMCC (F)T2s | 8 | 0 | 0 | absent | 0 | |
| T. simii | HEM 4420 | 20 | 30 | 22 | 7 | absent |
| T. mentagrophytes | ATCC 46950 | 75 | 66 | 65 | 39 | absent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winter, P.; Burmester, A.; Tittelbach, J.; Wiegand, C. A New Genotype of Trichophyton quinckeanum with Point Mutations in Erg11A Encoding Sterol 14-α Demethylase Exhibits Increased Itraconazole Resistance. J. Fungi 2023, 9, 1006. https://doi.org/10.3390/jof9101006
Winter P, Burmester A, Tittelbach J, Wiegand C. A New Genotype of Trichophyton quinckeanum with Point Mutations in Erg11A Encoding Sterol 14-α Demethylase Exhibits Increased Itraconazole Resistance. Journal of Fungi. 2023; 9(10):1006. https://doi.org/10.3390/jof9101006
Chicago/Turabian StyleWinter, Paula, Anke Burmester, Jörg Tittelbach, and Cornelia Wiegand. 2023. "A New Genotype of Trichophyton quinckeanum with Point Mutations in Erg11A Encoding Sterol 14-α Demethylase Exhibits Increased Itraconazole Resistance" Journal of Fungi 9, no. 10: 1006. https://doi.org/10.3390/jof9101006
APA StyleWinter, P., Burmester, A., Tittelbach, J., & Wiegand, C. (2023). A New Genotype of Trichophyton quinckeanum with Point Mutations in Erg11A Encoding Sterol 14-α Demethylase Exhibits Increased Itraconazole Resistance. Journal of Fungi, 9(10), 1006. https://doi.org/10.3390/jof9101006





