Screening of Antibacterial Activity of Some Resupinate Fungi, Reveal Gloeocystidiellum lojanense sp. nov. (Russulales) against E. coli from Ecuador
Abstract
1. Introduction
2. Materials and Methods
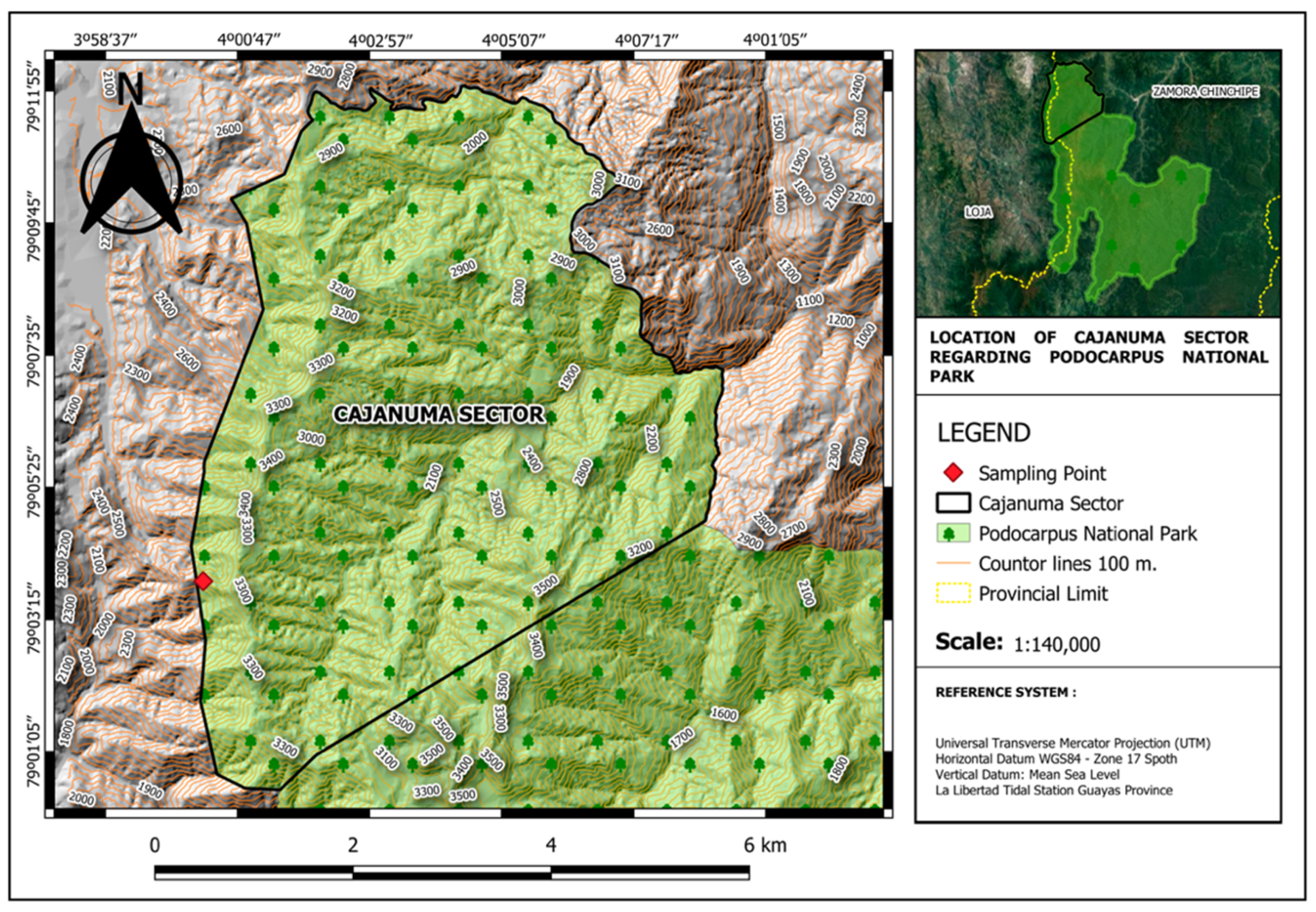
2.1. Study Area
2.2. Morphological Analysis
2.3. Molecular Analysis
2.4. Antibacterial Activity
2.4.1. Tested Bacterias
2.4.2. Antibacterial Activity Assay
2.5. Data Analysis
3. Results
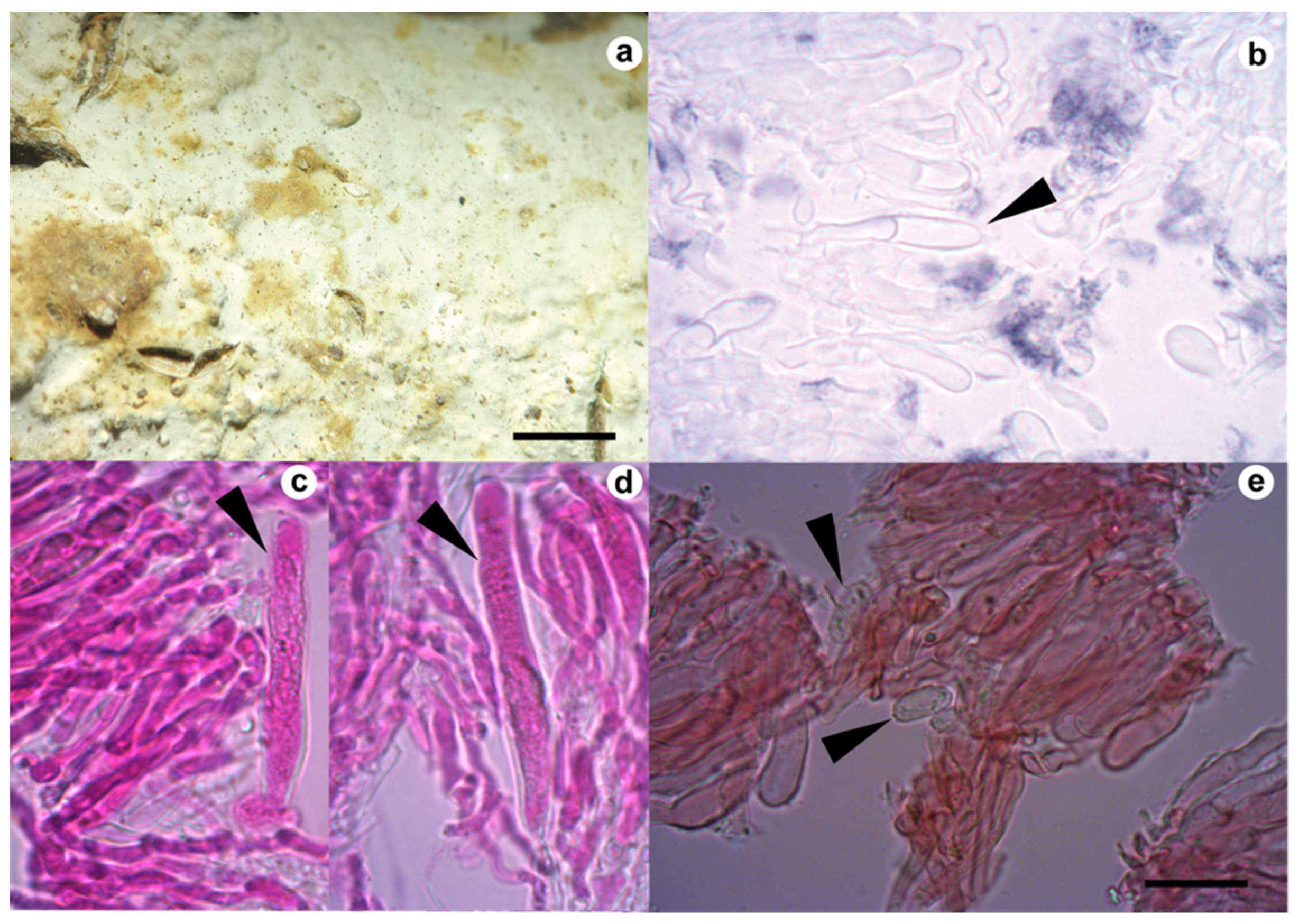
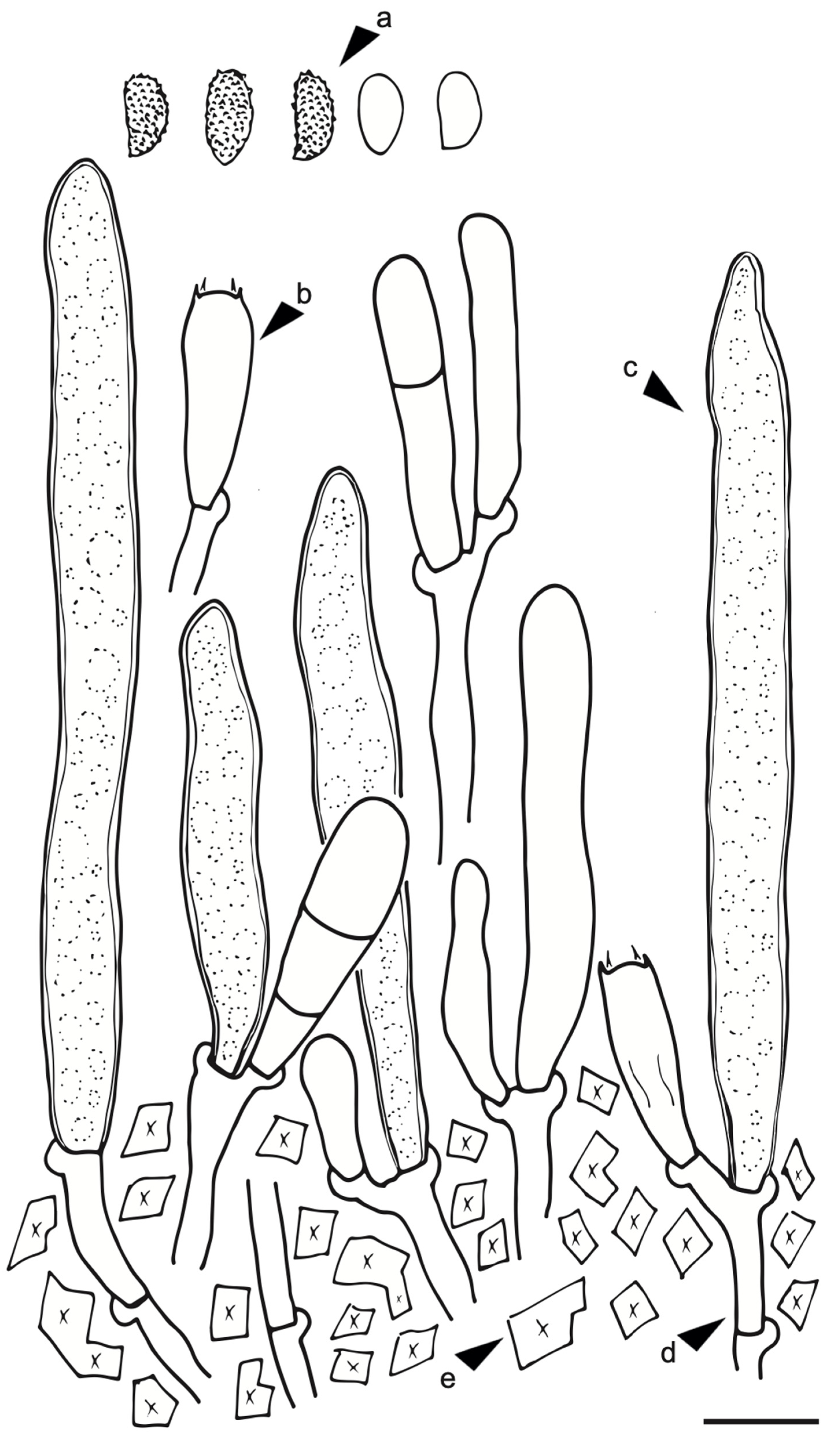
3.1. Taxonomy
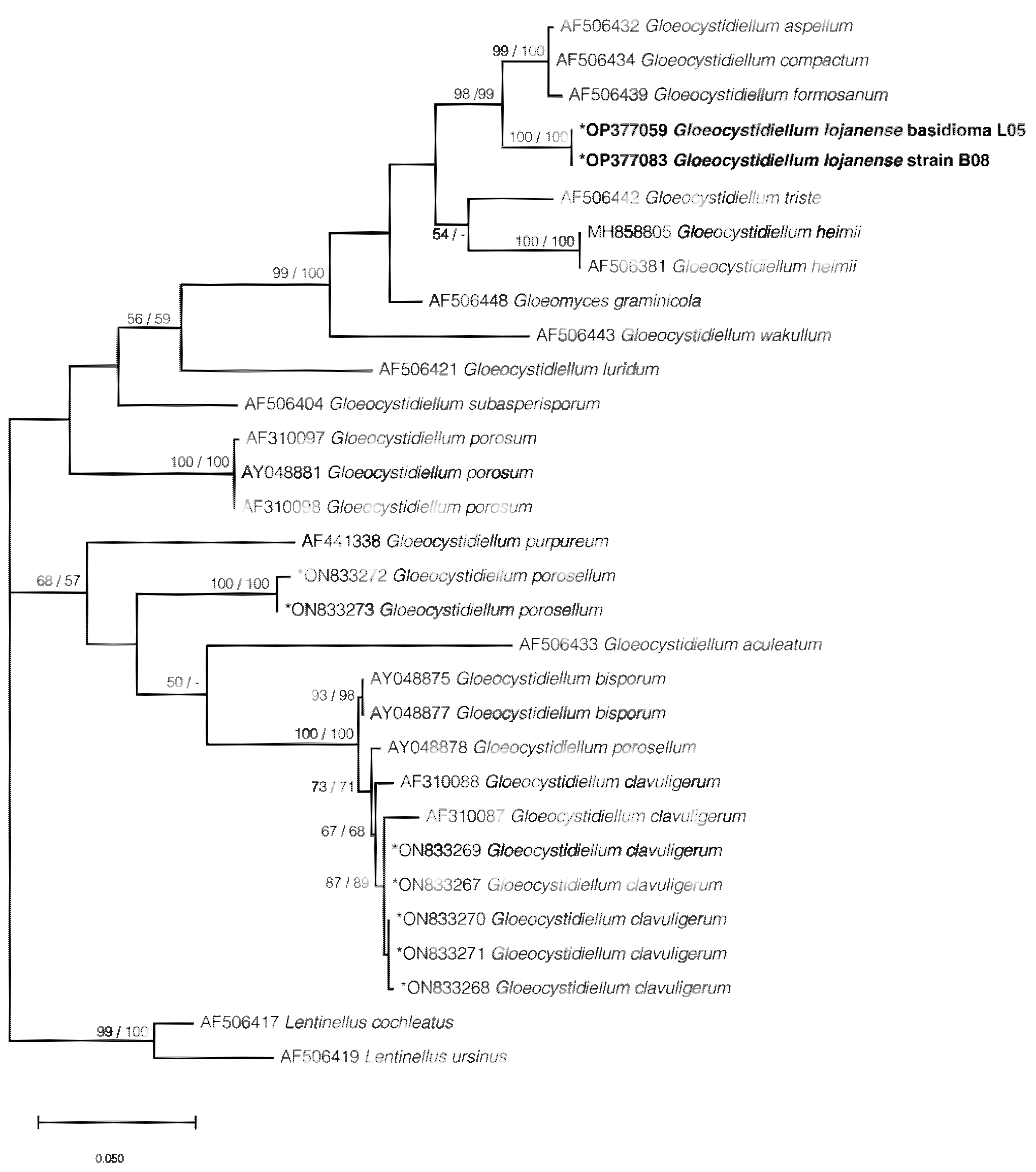
3.2. Phylogenetic Hypothesis for Gloeocystidiellum lojanense
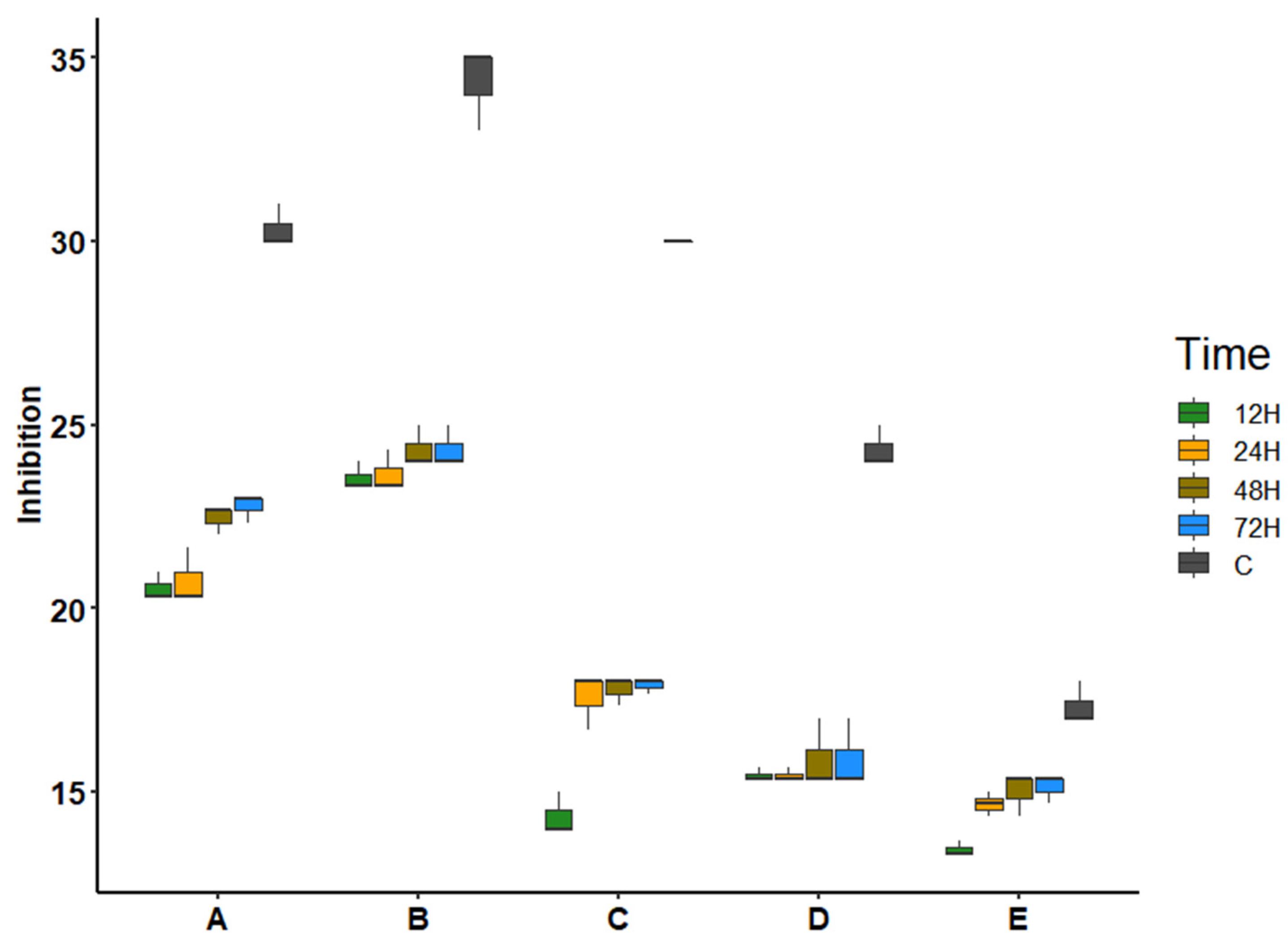
3.3. Antibacterial Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mir, M.; Rasool, U.; Aisha, S.; Alshehri, B.; Hamadani, S. Human pathogenic microbes (bacterial and fungal) and associated diseases. Hum. Pathog. Microbes 2022, 1–30. [Google Scholar] [CrossRef]
- Lagadinou, M.; Onisor, M.; Rigas, A.; Musetescu, D.; Gkentzi, D.; Assimakopoulos, S.; Panos, G.; Marangos, M. Antimicrobial properties on non-antibiotic drugs in the era of increased bacterial resistance. Antibiotics 2020, 9, 107. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, W. The mechanism of bacterial resistance and potential bacteriostatic strategies. Antibiotics 2022, 11, 1215. [Google Scholar] [CrossRef]
- Mena, Y.; Ramírez, L.; Arango, Á. Actividad antibacteriana y antioxidante del hongo Phanerochaete spp. Inf. Tecnol. 2021, 32, 69–78. [Google Scholar] [CrossRef]
- Pérez Guerrero, P.; Galán Sánchez, F.; Gutiérrez Saborido, D.; Guerrero Lozano, I. Infecciones por enterobacterias. Med.-Programa Form. Médica Contin. Acreditado 2014, 11, 3276–3282. [Google Scholar] [CrossRef]
- Janda, J.; Abbott, S. The changing face of the family Enterobacteriaceae (Order: “Enterobacterales”): New members, taxonomic issues, geographic expansion, and new diseases and disease syndromes. Clin. Microbiol. Rev. 2021, 34, e00174-20. [Google Scholar] [CrossRef] [PubMed]
- Staley, Z.; He, D.; Edge, T. Persistence of fecal contamination and pathogenic Escherichia coli O157:H7 in snow and snowmelt. J. Great Lakes Res. 2017, 43, 248–254. [Google Scholar] [CrossRef]
- Uprety, S.; Dangol, B.; Nakarmi, P.; Dhakal, I.; Sherchan, S.; Shisler, J.; Jutla, A.; Amarasiri, M.; Sano, D.; Nguyen, T. Assessment of microbial risks by characterization of Escherichia coli presence to analyze the public health risks from poor water quality in Nepal. Int. J. Hyg. Environ. Health 2020, 226, 113484. [Google Scholar] [CrossRef]
- Morosini, M.; del Campo, R. Urinary tract infections and antimicrobial resistance. Rev. Clín. Esp. 2019, 219, 149–150. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). “Vital signs: Carbapenem-resistant Enterobacteriaceae.” MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 165–170.
- Monteiro, A.; Cardoso, J.; Guerra, N.; Ribeiro, E.; Viegas, C.; Cabo Verde, S.; Sousa-Uva, A. Exposure and health effects of bacteria in healthcare units: An overview. Appl. Sci. 2022, 12, 1958. [Google Scholar] [CrossRef]
- Shen, S.; Qu, X.; Zhang, W.; Li, J.; Lv, Z. Infection against infection: Parasite antagonism against parasites, viruses and bacteria. Infect. Dis. Poverty 2019, 8, 1–12. [Google Scholar] [CrossRef]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.; Sulaiman, A.; Chee, J.; Lakshmanan, M.; Chin, C.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef]
- Morrill, H.; Pogue, J.; Kaye, K.; LaPlante, K. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect. Dis. 2015, 2, ofv050. [Google Scholar] [CrossRef]
- Ciabuschi, F.; Lindahl, O. The decline of innovation in the antibiotics industry and the global threat of antibiotic resistance: When entrepreneurial efforts are not enough. Entrep. Ind. Life Cycle 2018, 205–229. [Google Scholar] [CrossRef]
- Singh, D.; Thapa, S.; Mahawar, H.; Kumar, D.; Geat, N.; Singh, S. Prospecting potential of endophytes for modulation of biosynthesis of therapeutic bioactive secondary metabolites and plant growth promotion of medicinal and aromatic plants. Antonie Van Leeuwenhoek 2022, 115, 699–730. [Google Scholar] [CrossRef]
- Kamaruzaman, N.; Mohd Noor, N.; Radin Mohamed, R.; Al-Gheethi, A.; Ponnusamy, S.; Sharma, A.; Vo, D. Applicability of bio-synthesized nanoparticles in fungal secondary metabolites products and plant extracts for eliminating antibiotic-resistant bacteria risks in non-clinical environments. Environ. Res. 2022, 209, 112831. [Google Scholar] [CrossRef]
- Hyde, K.; Tennakoon, D.; Jeewon, R.; Bhat, D.; Maharachchikumbura, S.; Rossi, W.; Leonardi, M.; Lee, H.; Mun, H.; Houbraken, J.; et al. Fungal diversity notes 1036–1150: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2019, 96, 1–242. [Google Scholar] [CrossRef]
- Purvis, A.; Hector, A. Getting the measure of biodiversity. Nature 2000, 405, 212–219. [Google Scholar] [CrossRef]
- Baldrian, P.; Větrovský, T.; Lepinay, C.; Kohout, P. High-throughput sequencing view on the magnitude of global fungal diversity. Fungal Divers. 2021, 114, 539–547. [Google Scholar] [CrossRef]
- Sidorova, I.I.; Velikanov, L.L. Bioactive substances of agaricoid basidiomycetes and their possible role in regulation of myco- and microbiota structure in soils of forest ecosystems. I. Antibiotic activity of water extracts from basidioms of several dominant agaricoid basidiomycetes. Mikol. Fitopatol. 2000, 34, 11–17. [Google Scholar]
- Kumar, V.; Prasher, I. Antimicrobial potential of endophytic fungi isolated from Dillenia indica L. and identification of bioactive molecules produced by Fomitopsis meliae (Undrew.) Murril. Nat. Prod. Res. 2022, 36, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Alkhulaifi, M.; Awaad, A.; AL-Mudhayyif, H.; Alothman, M.; Alqasoumi, S.; Zain, S. Evaluation of antimicrobial activity of secondary metabolites of fungi isolated from Sultanate Oman soil. Saudi Pharm. J. 2019, 27, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Karwehl, S.; Stadler, M. Exploitation of fungal biodiversity for discovery of novel antibiotics. Curr. Top. Microbiol. Immunol. 2016, 303–338. [Google Scholar] [CrossRef]
- Venturini, M.; Rivera, C.; Gonzalez, C.; Blanco, D. Antimicrobial activity of extracts of edible wild and cultivated mushrooms against foodborne bacterial strains. J. Food Prot. 2008, 71, 1701–1706. [Google Scholar] [CrossRef]
- Winnie, C.S.; Stephen, A.I.; Josphat, C.M. Antimicrobial activity of basidiomycetes fungi isolated from a Kenyan tropical forest. Afr. J. Biotechnol. 2019, 18, 112–123. [Google Scholar] [CrossRef][Green Version]
- Batallas-Molina, R.; Moya-Marcalla, G.F.; Navas Muñoz, D. Listado de la colección de hongos (Ascomycota y Basidiomycota) del Herbario Nacional del Ecuador (QCNE) del Instituto Nacional de Biodiversidad (INABIO). Av. Cienc. Ing. 2021, 12, 38–71. [Google Scholar]
- Cruz, D.; Suárez, J.; Piepenbring, M. Morphological revision of Tulasnellaceae, with two new species of Tulasnella and new records of Tulasnella spp. for Ecuador. Nova Hedwig. 2016, 102, 279–338. [Google Scholar] [CrossRef]
- Gates, G.; Goyes, P.; Gundogdu, F.; Cruz, J.; Ratkowsky, D. Small plot surveying reveals high fungal diversity in the Ecuadorian Amazon–A case study. Curr. Res. Environ. Appl. Mycol. 2021, 11, 16–36. [Google Scholar] [CrossRef]
- Læssøe, T.; Petersen, J.H. Svampelivet på ækvator. Svampe 2008, 58, 1–53. [Google Scholar]
- Cruz, D.; Suárez, J.; Kottke, I.; Piepenbring, M.; Oberwinkler, F. Defining species in Tulasnella by correlating morphology and nrDNA ITS-5.8S sequence data of basidiomata from a tropical Andean forest. Mycol. Prog. 2011, 10, 229–238. [Google Scholar] [CrossRef]
- Cruz, D.; Suárez, J.; Kottke, I.; Piepenbring, M. Cryptic species revealed by molecular phylogenetic analysis of sequences obtained from basidiomata of Tulasnella. Mycol. Progress 2014, 106, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Toapanta-Alban, C.; Ordoñez, M.; Barnes, C.; Blanchette, R. Taxonomy of the major rhizomorphic species of the “Melanopus group” within Polyporaceae in Yasuní National Park, Ecuador. PLoS ONE 2021, 16, e0254567. [Google Scholar] [CrossRef] [PubMed]
- Toapanta-Alban, C.; Ordoñez, M.; Blanchette, R. New findings on the biology and ecology of the Ecuadorian Amazon fungus Polyporus leprieurii var. yasuniensis. J. Fungi 2022, 8, 203. [Google Scholar] [CrossRef]
- Sárközy, A.; Kúsz, N.; Zomborszki, Z.; Csorba, A.; Papp, V.; Hohmann, J.; Vanyolos, A. Isolation and characterization of chemical constituents from the Poroid Medicinal Mushroom Porodaedalea chrysoloma (Agaricomycetes) and their antioxidant activity. Int. J. Med. Mushrooms 2020, 22, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Trujillo, P.; Wartchow, F.; Cerón-Martinez, C.; Andi, D.; Uwinjin, P.; Grefa, G.; Entza, M.; Chimbo, E.; Chimbo, J.; Payaguaje, J.; et al. Edible mushrooms of Ecuador: Consumption, myths and implications for conservation. Ethnobot. Res. Appl. 2019, 18, 1–15. [Google Scholar] [CrossRef]
- Andrade, J.M.; Malagón, O.; Piepenbring, M.; Armijos, C. Etnomicología y valor nutricional de macrohongos silvestres de la comunidad indígena Saraguro en el Sur del Ecuador. Boletin Soc. Micológica Madr. 2012, 36, 193–201. [Google Scholar]
- Benítez, Á.; Cruz, D.; Vega, M.; González, L.; Jaramillo, N.; López, F.; Aguirre, Z. Briófitos y hongos (liquenizados y no liquenizados) del Parque Universitario Francisco Vivar Castro, Loja, Ecuador. Bosques Latid. Cero 2021, 11, 1–18. [Google Scholar] [CrossRef]
- Kuhar, F.; Furci, G.; Drechsler-Santos, E.; Pfister, D. Delimitation of Funga as a valid term for the diversity of fungal communities: The Fauna, Flora & Funga proposal (FF&F). IMA Fungus 2018, 9, A71–A74. [Google Scholar] [CrossRef]
- Binder, M.; Hibbett, D.; Larsson, K.; Larsson, E.; Langer, E.; Langer, G. The phylogenetic distribution of resupinate forms across the major clades of mushroom-forming fungi (Homobasidiomycetes). Syst. Biodivers. 2005, 3, 113–157. [Google Scholar] [CrossRef]
- Piepenbring, M.; López, F.; Cáceres, O. Colaboradores escondidos–La Importancia de los Hongos en los Ecosistemas Información para Educación Ambiental. Puente Biol. 2019, 8, 57–91. [Google Scholar]
- Hallenberg, N.; Ryberg, M.; Nilsson, R.; Wood, A.; Wu, S. Pseudolagarobasidium (Basidiomycota): On the reinstatement of a genus of parasitic, saprophytic, and endophytic resupinate fungi. Botany 2008, 86, 1319–1325. [Google Scholar] [CrossRef]
- Mosquera-Espinosa, A.; Bayman, P.; Prado, G.; Gómez-Carabalí, A.; Otero, J. The double life of Ceratobasidium: Orchid mycorrhizal fungi and their potential for biocontrol of Rhizoctonia solani sheath blight of rice. Mycologia 2013, 105, 141–150. [Google Scholar] [CrossRef]
- Sánchez-García, M.; Ryberg, M.; Khan, F.; Varga, T.; Nagy, L.; Hibbett, D. Fruiting body form, not nutritional mode, is the major driver of diversification in mushroom-forming fungi. Proc. Natl. Acad. Sci. USA 2020, 117, 32528–32534. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Melo, B.; Santos, M.; Rios, R.; Santos, C.; Júnior, K.; Maranhão, F.; Santos, T.; Fraga, A. Endophytic fungi: Benefits for plants and biotechnological potential. Res. Soc. Dev. 2022, 11, e9211427008. [Google Scholar] [CrossRef]
- Homeier, J.; Werner, F.A.; Gradstein, S.R. Potential vegetation and floristic composition of Andean forests in South Ecuador, with a focus on the RBSF. In Gradients in a Tropical Mountain Ecosystem of Ecuador: Ecological Studies; Beck, E., Bendix, J., Kottke, I., Makeschin, F., Mosandl, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 87–100. [Google Scholar] [CrossRef]
- Homeier, J.; Hertel, D.; Camenzind, T.; Cumbicus, N.L.; Maraun, M.; Martinson, G.O.; Poma, L.N.; Rillig, M.C.; Sandmann, D.; Scheu, S.; et al. Tropical Andean forests are highly susceptible to nutrient inputs-rapid effects of experimental N and P addition to an Ecuadorian montane forest. PLoS ONE 2012, 7, e47128. [Google Scholar] [CrossRef]
- Bendix, J.; Rollenbeck, R.; Göttlicher, D.; Cermak, J. Cloud occurrence and cloud properties in Ecuador. Clim. Res. 2006, 30, 133–147. [Google Scholar] [CrossRef]
- Xing, J.-H.; Song, J.; Decock, C.; Cui, B.-K. Morphological characters and phylogenetic analysis reveal a new species within the Ganoderma lucidum complex from South Africa. Phytotaxa 2016, 226, 115–224. [Google Scholar] [CrossRef]
- Wu, S.H. Studies on Gloeocystidiellum sensu lato (Basidiomycotina) in Taiwan. Mycotaxon 1996, 58, 1–68. [Google Scholar]
- Larsson, K.H.; Ryvarden, L. Corticioid fungi of Europe. Fungiflora 2021, 1, 253–257. [Google Scholar]
- Esquemas de Color Hexagonal, Pesquisa de Pintura, Paletas de Colores y Conversions. Available online: https://encycolorpedia.es/ (accessed on 11 July 2022).
- White, T.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. 1990, 18, 315–322. [Google Scholar]
- Katoh, K. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Quandt, D.; Müller, J.; Neinhuis, C. PhyDE-Phylogenetic Data Editor. Program Distributed by the Authors, Version 10.0. 2005. Available online: https://www.phyde.de (accessed on 2 May 2022).
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Gorjón, S.; Hallenberg, N. Some new species and a first checklist of corticioid fungi (Basidiomycota) from Chile. Mycol. Prog. 2012, 12, 185–192. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022; ISBN 978-1-68440-135-2. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests; CLSI Standard M02; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; ISBN 1-56238-835-5. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically; CLSI Standard M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; ISBN 1-56238-837-1. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Larsson, E.; Hallenberg, N. Species delimitation in the Gloeocystidiellum porosum-clavuligerum complex inferred from compatibility studies and nuclear rDNA sequence data. Mycologia 2001, 93, 907–914. [Google Scholar] [CrossRef]
- Larsson, E.; Larsson, K.H. Phylogenetic relationships of russuloid basidiomycetes with emphasis on aphyllophoralean taxa. Mycologia 2003, 95, 1037–1065. [Google Scholar] [CrossRef]
- Telleria, M.; Dueñas, M.; Melo, I.; Beltrán-Tejera, E.; Rodríguez-Armas, J.; Salcedo, I.; Martín, M. Gloeocystidiellum kenyense in Azores and Madeira. Mycotaxon 2012, 119, 337–343. [Google Scholar] [CrossRef]
- Miller, S.; Larsson, E.; Larsson, K.; Verbeken, A.; Nuytinck, J. Perspectives in the new Russulales. Mycologia 2006, 98, 960–970. [Google Scholar] [CrossRef]
- Nilsson, R.; Kristiansson, E.; Ryberg, M.; Hallenberg, N.; Larsson, K. Intraspecific ITS variability in the kingdom fungi as expressed in the international sequence databases and its implications for molecular species identification. Evol. Bioinform. 2008, 4, 193–201. [Google Scholar] [CrossRef]
- Shah, C.; Baral, R.; Bartaula, B.; Shrestha, L. Virulence factors of uropathogenic Escherichia coli (UPEC) and correlation with antimicrobial resistance. BMC Microbiol. 2019, 19, 204. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Estrada, L.; Ruíz-Rosas, M.; Molina-López, J.; Parra-Rojas, I.; González-Villalobos, E.; Castro-Alarcón, N. Relationship between virulence factors, resistance to antibiotics and phylogenetic groups of uropathogenic Escherichia coli in two locations in Mexico. Enferm. Infecc. Microbiol. Clin. 2017, 35, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, A.; Nagaya, Y.; Pradel, E.; Ooka, T.; Ogura, Y.; Katsura, K.; Kurokawa, K.; Oshima, K.; Hattori, M.; Parkhill, J.; et al. Genome evolution and plasticity of Serratia marcescens, an important multidrug-resistant nosocomial pathogen. Genome Biol. Evol. 2014, 6, 2096–2110. [Google Scholar] [CrossRef] [PubMed]
- Suay, I.; Arenal, F.; Asensio, F.J.; Basilio, A.; Angeles Cabello, M.; Teresa Díez, M.; García, J.B.; González del Val, A.; Gorrochategui, J.; Hernández, P.; et al. Screening of basidiomycetes for antimicrobial activities. Antonie Van Leeuwenhoek 2000, 78, 129–140. [Google Scholar] [CrossRef]
- Rosa, L.; Machado, K.; Jacob, C.; Capelari, M.; Rosa, C.; Zani, C. Screening of Brazilian basidiomycetes for antimicrobial activity. Memórias Instituto Oswaldo Cruz 2003, 98, 967–974. [Google Scholar] [CrossRef]
- Kohler, A.; Kuo, A.; Nagy, L.G.; Morin, E.; Barry, K.W.; Buscot, F.; Canbäck, B.; Choi, C.; Cichocki, N.; Clum, A.; et al. Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nat. Genet. 2015, 47, 410–415. [Google Scholar] [CrossRef]
- Barros, L.; Calhelha, R.; Vaz, J.; Ferreira, I.; Baptista, P.; Estevinho, L. Antimicrobial activity and bioactive compounds of Portuguese wild edible mushrooms methanolic extracts. Eur. Food Res. Technol. 2006, 225, 151–156. [Google Scholar] [CrossRef]
- Jørgensen, P.M.; León-Yánez, S. (Eds.) Catalogue of the vascular plants of Ecuador. Monogr. Syst. Bot. Mo. Bot. Gard. 1999, 75, 1–1182. [Google Scholar]
- Megadiverse Countries Definition. Biodiversity A-Z. Available online: https://www.biodiversitya-z.org/content/megadiverse-countries (accessed on 17 September 2022).
- Hawksworth, D.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. Fungal Kingd. 2017, 5, 79–95. [Google Scholar] [CrossRef]

| Taxon | Herbarium/Collection | Provenance | GenBank | Sequence Region |
|---|---|---|---|---|
| Gloeocystidiellum aculeatum | GB/2647 | Taiwan | AF265546 | ITS, nuLSU |
| Gloeocystidiellum aculeatum * | GB/2647 | Taiwan | AF506433 | ITS, nuLSU |
| Gloeocystidiellum aspellum * | GB/LIN625 | Taiwan | AF506432 | ITS, nuLSU |
| Gloeocystidiellum aspellum | HE4262 | China | KY860460 | nuLSU |
| Gloeocystidiellum aspellum | HE2041 | China | KY860461 | nuLSU |
| Gloeocystidiellum aspellum | HE1773 | China | KY860462 | nuLSU |
| Gloeocystidiellum bisporum | CBS/961.96 | Sweden | AY048875 | ITS, nuLSU |
| Gloeocystidiellum bisporum | GB/KHL4700 | Sweden | AY048876 | ITS |
| Gloeocystidiellum bisporum | GB/KHL11135 | Norway | AY048877 | ITS, nuLSU |
| Gloeocystidiellum clavuligerum | HHB1046 | USA, Mich. | AF310087 | ITS, nuLSU |
| Gloeocystidiellum clavuligerum | GB/NH11185 | Spain, Tenerife | AF310088 | ITS, nuLSU |
| Gloeocystidiellum clavuligerum | HE3313 | China | KY860441 | nuLSU |
| Gloeocystidiellum clavuligerum | MUCL/33964 | France | ON833267 | ITS, nuLSU |
| Gloeocystidiellum clavuligerum | MUCL/33965 | France | ON833268 | ITS, nuLSU |
| Gloeocystidiellum clavuligerum | MUCL/33966 | France | ON833269 | ITS, nuLSU |
| Gloeocystidiellum clavuligerum | MUCL/33967 | France | ON833270 | ITS, nuLSU |
| Gloeocystidiellum clavuligerum | MUCL/33968 | France | ON833271 | ITS, nuLSU |
| Gloeocystidiellum compactum | NMNS/WU880615-21 | Taiwan | AF506434 | ITS, nuLSU |
| Gloeocystidiellum formosanum | NMNS/WU9404-19 | Taiwan | AF506439 | ITS, nuLSU |
| Gloeocystidiellum friesii | CBS/323.66 | France | MH870446 | nuLSU |
| Gloeocystidiellum graminicola * | GB/WU9210-12 | Taiwan | AF506448 | ITS, nuLSU |
| Gloeocystidiellum heimii | LY/CBS321.66 | C. African Rep. | AF506381 | ITS, nuLSU |
| Gloeocystidiellum heimii | LY/CBS321.66 | C. African Rep. | MH858805 | ITS, nuLSU |
| Gloeocystidiellum kenyense | TFC/15278 | Portugal | FR878082 | ITS |
| Gloeocystidiellum kenyense | TFC/15309 | Portugal | FR878083 | ITS |
| Gloeocystidiellum kenyense | MA/Fungi80408 | Portugal | FR878084 | ITS |
| Gloeocystidiellum leucoxantha | CBS/454.86 | USA | AF287860 | nuLSU |
| Gloeocystidiellum lojanense * | HUTPL(F)/2181 | Ecuador | OP377059 | ITS, nuLSU |
| Gloeocystidiellum lojanense | HUTPL(F)/550 | Ecuador | OP377083 | ITS, nuLSU |
| Gloeocystidiellum luridum | HK9808 | Sweden | AF506421 | ITS, nuLSU |
| Gloeocystidiellum porosellum | HJM8851 | Sweden | AY048878 | ITS, nuLSU |
| Gloeocystidiellum porosellum | MUCL/35247 | France | ON833272 | ITS, nuLSU |
| Gloeocystidiellum porosellum | MUCL/35248 | France | ON833273 | ITS, nuLSU |
| Gloeocystidiellum porosum | CBS/51085 | Netherlands | AF310097 | ITS, nuLSU |
| Gloeocystidiellum porosum | CBS/27154 | France | AF310098 | ITS, nuLSU |
| Gloeocystidiellum porosum | GB/EB990923 | Sweden | AY048881 | ITS, nuLSU |
| Gloeocystidiellum porosum | WU1608-176 | Taiwan | LC430908 | nuLSU |
| Gloeocystidiellum purpurem * | GB/WU9310-45 | Sweden | AF441338 | ITS, nuLSU |
| Gloeocystidiellum rajchenbergii * | GB/NH16358 | Chile | JQ716940 | ITS1 |
| Gloeocystidiellum rajchenbergii | GB/NH16348 | Chile | JQ734554 | ITS1 |
| Gloeocystidiellum rajchenbergii | GB/NH16353 | Chile | JQ734555 | ITS1 |
| Gloeocystidiellum subasperisporum | GB/KHL8695 | Norway | AF506404 | ITS, nuLSU |
| Gloeocystidiellum triste | GB/KHL10334 | Puerto Rico | AF506442 | ITS, nuLSU |
| Gloeocystidiellum wakullum | O/OSLO-930107 | Tanzania | AF506443 | ITS, nuLSU |
| Lentinellus cochleatus | GB/KGN96-09-28 | Sweden | AF506417 | ITS, nuLSU |
| Lentinellus ursinus | GB/EL73-97 | USA, N.C | AF506419 | ITS, nuLSU |
| Code | Source | Closest Identified Relative | Query Cover (%) | Identity (%) | Culture From Fructification | Order | Closest Identified Relative | Query Cover (%) | Identity (%) |
|---|---|---|---|---|---|---|---|---|---|
| HUTPL(F)2181 | Basidiome | Gloeocystidiellum sp. | 93 | 96 | HUTPL(F)550 | Russulales | Gloeocystidiellum sp. | 94 | 95.93 |
| HUTPL(F)2162 | Basidiome | Tulasnella sp. | 98 | 95.53 | HUTPL(F)551 | Hypocreales | Hypocrea sp. | 98 | 97 |
| HUTPL(F)2166 | Basidiome | Oliveonia sp. | 99 | 94.2 | HUTPL(F)552 | Cantharellales | Oliveonia sp. | 97 | 93.79 |
| HUTPL(F)2183 | Basidiome | Phanerochaete sp. | 99 | 98.11 | HUTPL(F)553 | Polyporales | Phanerochaete sp. | 98 | 97.93 |
| HUTPL(F)2187 | Basidiome | Ceraceomyces sp. | 90 | 89.1 | HUTPL(F)554 | Amylocorticiales | Ceraceomyces sp. | 89 | 88.9 |
| HUTPL(F)2198 | Basidiome | Fuscoporia sp. | 97 | 90.12 | HUTPL(F)555 | Hymenochaetales | Fuscoporia sp. | 97 | 89.57 |
| HUTPL(F)2203 | Basidiome | Xenasmatella sp. | 96 | 97.21 | HUTPL(F)556 | Russulales | Xenasmatella sp. | 94 | 92.75 |
| Species | Basidiospores (L) | Basidiospores (W) | Gloeocystidia (L) | Gloecystidia (W) | Basidia (L) | Basidia (W) |
|---|---|---|---|---|---|---|
| Gloeocystidiellum rajchenbergii | 6–7 | 3–3.5 | 40–70 | 5.5–7.5 | 15–20 | 5–5.5 |
| Gloeocystidiellum formosanum | 6.5–7.5 | 2.8–3.2 | 100 | 6–12 | 16–22 | 5–6.5 |
| Gloeocystidiellum compactum | 5.8–6.5 | 3–3.4 | 100 | 5–10 | 17–27 | 5–6 |
| Gloeocystidiellum aspellum | 7–8 | 3.5–4 | 150 | 5–10 | 25–35 | 6–7 |
| Gloeocystidiellum lojanense | 6.5–8 | 3.4–4.5 | 80–90 | 7–8 | 25–35 | 5–6 |
| Species | Gloeocystidiellum lojanense | ||
|---|---|---|---|
| ITS1 | ITS1-5.8S-ITS2 | Partial LSU (D1/D2) | |
| Gloeocystidiellum aspellum | 3.69/3.56% | 6.90/7.02% | 0.81/0.78% |
| Gloeocystidiellum compactum | 2.94/2.81% | 6.45/6.59% | 0.81/0.78% |
| Gloeocystidiellum formosanum | 2.94/2.81% | 6.45/6.59% | 0.81/0.78% |
| Gloeocystidiellum rajchenbergii | 2.94/2.81% | - | - |
| Other species | 6.78–21.16/6.39–21.77% | 8.72–33.23/9.45–28.19% | 1.36–5.05/1.85–5.75% |
| Inhibition Zone Diameter (mm) | |||||
|---|---|---|---|---|---|
| Time (h) | |||||
| Pathogenic Bacteria | C | 12 | 24 | 48 | 72 |
| Enterobacter aerogenes | 25.67 ± 0.58 | - | - | - | - |
| Enterobacter cloacae | 26.00 ± 0.00 | - | - | - | - |
| Escherichia coliATCC 25922 FG: B2 | 30.33 ± 0.58 | 20.56 ± 0.38 | 20.78 ± 0.77 | 22.44 ± 0.38 | 22.78 ± 0.38 |
| Escherichia coliO157:H7 | 34.33 ± 1.15 | 23.56 ± 0.38 | 23.67 ± 0.58 | 24.33 ± 0.58 | 24.33 ± 0.58 |
| Escherichia coli UPEC FG: A | 30.00 ± 0.00 | 14.33 ± 0.58 | 17.56 ± 0.77 | 17.78 ± 0.38 | 17.89 ± 0.19 |
| Escherichia coli UPEC FG: B2 | 24.33 ± 0.58 | 15.44 ± 0.19 | 15.44 ± 0.19 | 15.89 ± 0.96 | 15.89 ± 0.96 |
| Klebsiella pneumoniae | 21.00 ± 0.00 | - | - | - | - |
| Klebsiella pneumoniae ATCC BAA-1706 | 20.00 ± 0.00 | - | - | - | - |
| Pseudomonas aeruginosa | 28.67 ± 0.58 | - | - | - | - |
| Serratia sp. | 17.33 ± 0.58 | 13.44 ± 0.19 | 14.67 ± 0.33 | 15.00 ± 0.58 | 15.11 ± 0.38 |
| Factors | df | SS | MS | F | p-Value |
|---|---|---|---|---|---|
| G. lojanense | 4 | 2.3629 | 0.5907 | 836 | <0.001 |
| Time | 4 | 1.5226 | 0.3807 | 538.7 | <0.001 |
| G. lojanense: Time | 16 | 0.2284 | 0.0143 | 20.2 | <0.001 |
| Residuals | 50 | 0.0353 | 0.0007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaramillo-Riofrío, A.; Decock, C.; Suárez, J.P.; Benítez, Á.; Castillo, G.; Cruz, D. Screening of Antibacterial Activity of Some Resupinate Fungi, Reveal Gloeocystidiellum lojanense sp. nov. (Russulales) against E. coli from Ecuador. J. Fungi 2023, 9, 54. https://doi.org/10.3390/jof9010054
Jaramillo-Riofrío A, Decock C, Suárez JP, Benítez Á, Castillo G, Cruz D. Screening of Antibacterial Activity of Some Resupinate Fungi, Reveal Gloeocystidiellum lojanense sp. nov. (Russulales) against E. coli from Ecuador. Journal of Fungi. 2023; 9(1):54. https://doi.org/10.3390/jof9010054
Chicago/Turabian StyleJaramillo-Riofrío, Andrea, Cony Decock, Juan Pablo Suárez, Ángel Benítez, Gabriel Castillo, and Darío Cruz. 2023. "Screening of Antibacterial Activity of Some Resupinate Fungi, Reveal Gloeocystidiellum lojanense sp. nov. (Russulales) against E. coli from Ecuador" Journal of Fungi 9, no. 1: 54. https://doi.org/10.3390/jof9010054
APA StyleJaramillo-Riofrío, A., Decock, C., Suárez, J. P., Benítez, Á., Castillo, G., & Cruz, D. (2023). Screening of Antibacterial Activity of Some Resupinate Fungi, Reveal Gloeocystidiellum lojanense sp. nov. (Russulales) against E. coli from Ecuador. Journal of Fungi, 9(1), 54. https://doi.org/10.3390/jof9010054






