OsCERK1 Contributes to Cupric Oxide Nanoparticles Induced Phytotoxicity and Basal Resistance against Blast by Regulating the Anti-Oxidant System in Rice
Abstract
:1. Introduction
2. Materials and Methods
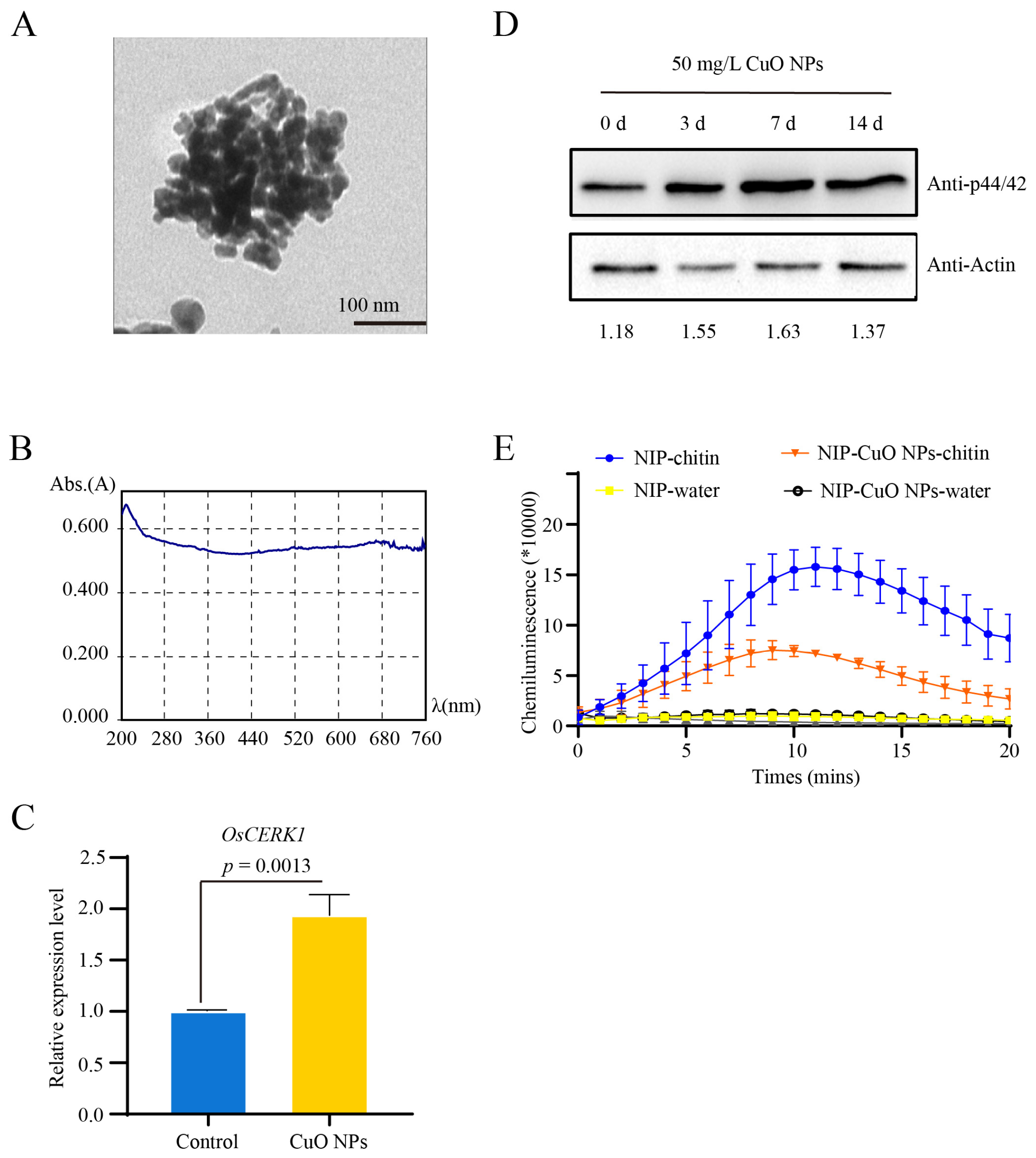
2.1. Characterization of CuO NPs
2.2. Culture and CuO NPs Treatment of Rice Seedlings
2.3. Rice Seedling Infection Assay
2.4. Quantitative RT-PCR Analysis
2.5. MAPK Assay
2.6. Measurement of ROS
2.7. Measurement of Hydrogen Peroxide (H2O2) Content
2.8. Measurement of Activities of Anti-Oxidant Enzymes
2.9. Investigation of Physiological Features
2.10. Data Analysis
3. Results
3.1. Basal Resistance Gene OsCERK1 Is Involved in Response to CuO NPs Stress in Rice
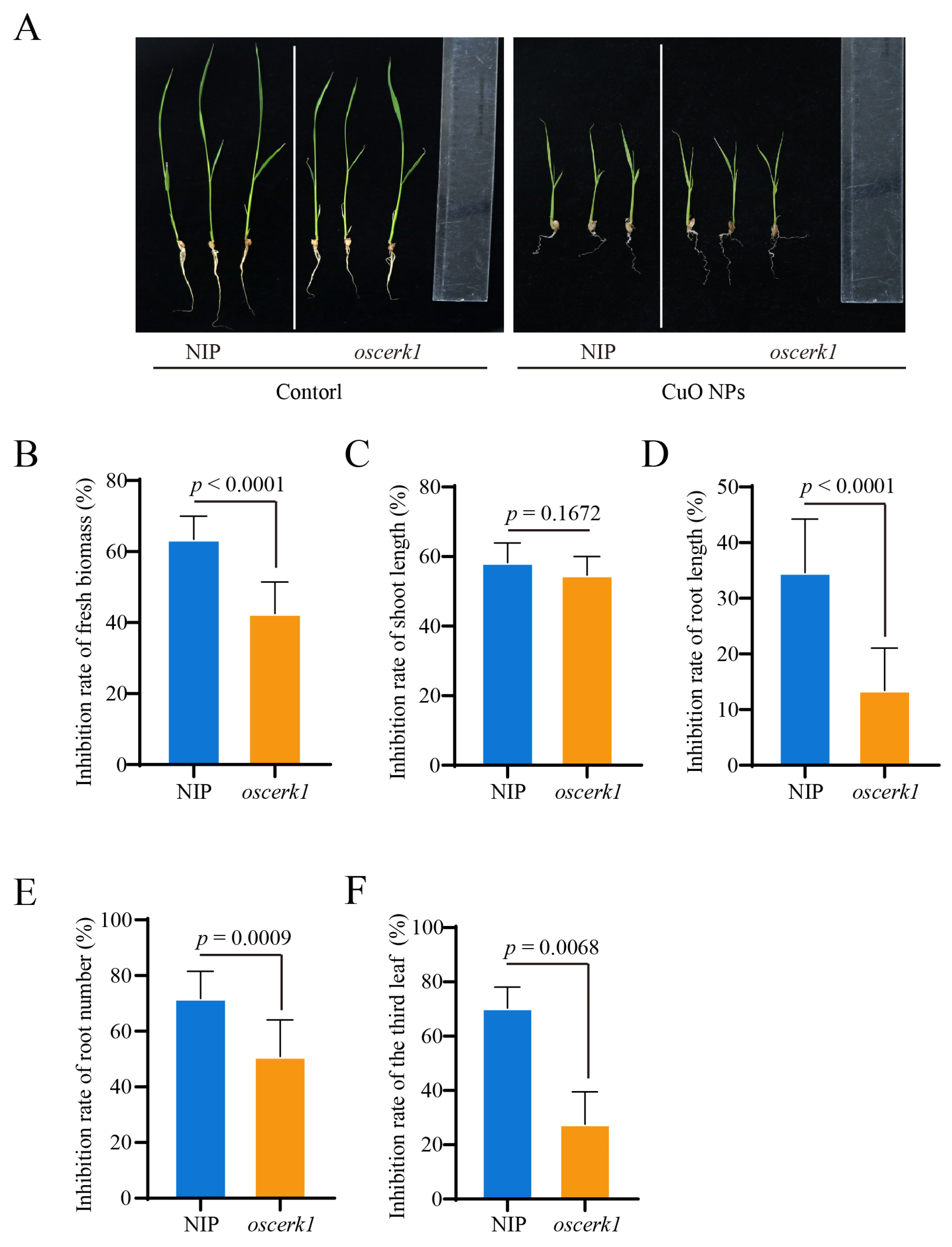
3.2. OsCERK1 Regulates Phytotoxicity of CuO NPs Stress to Rice
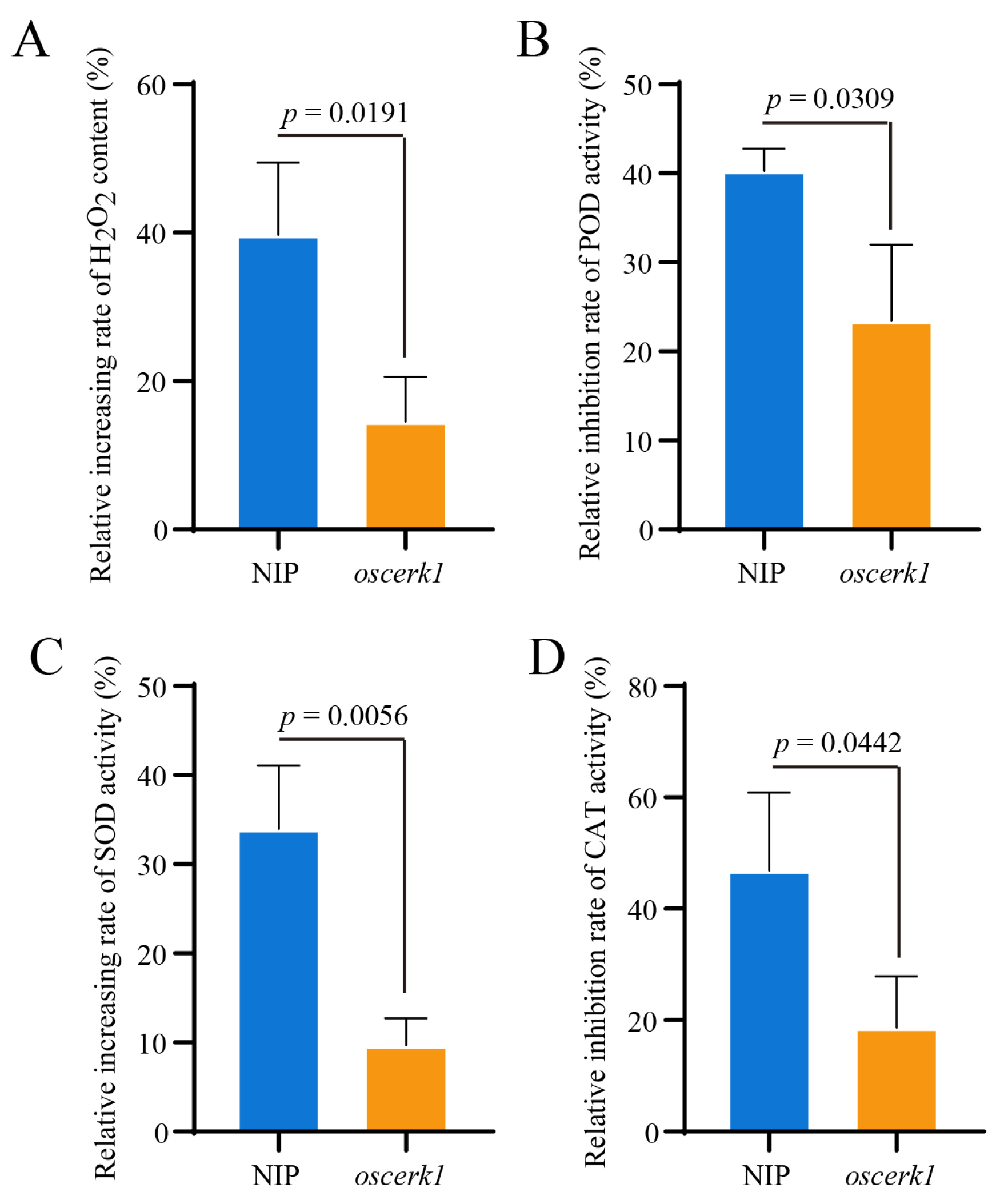
3.3. OsCERK1 Regulates Anti-Oxidative System in Response to CuO NPs Stress
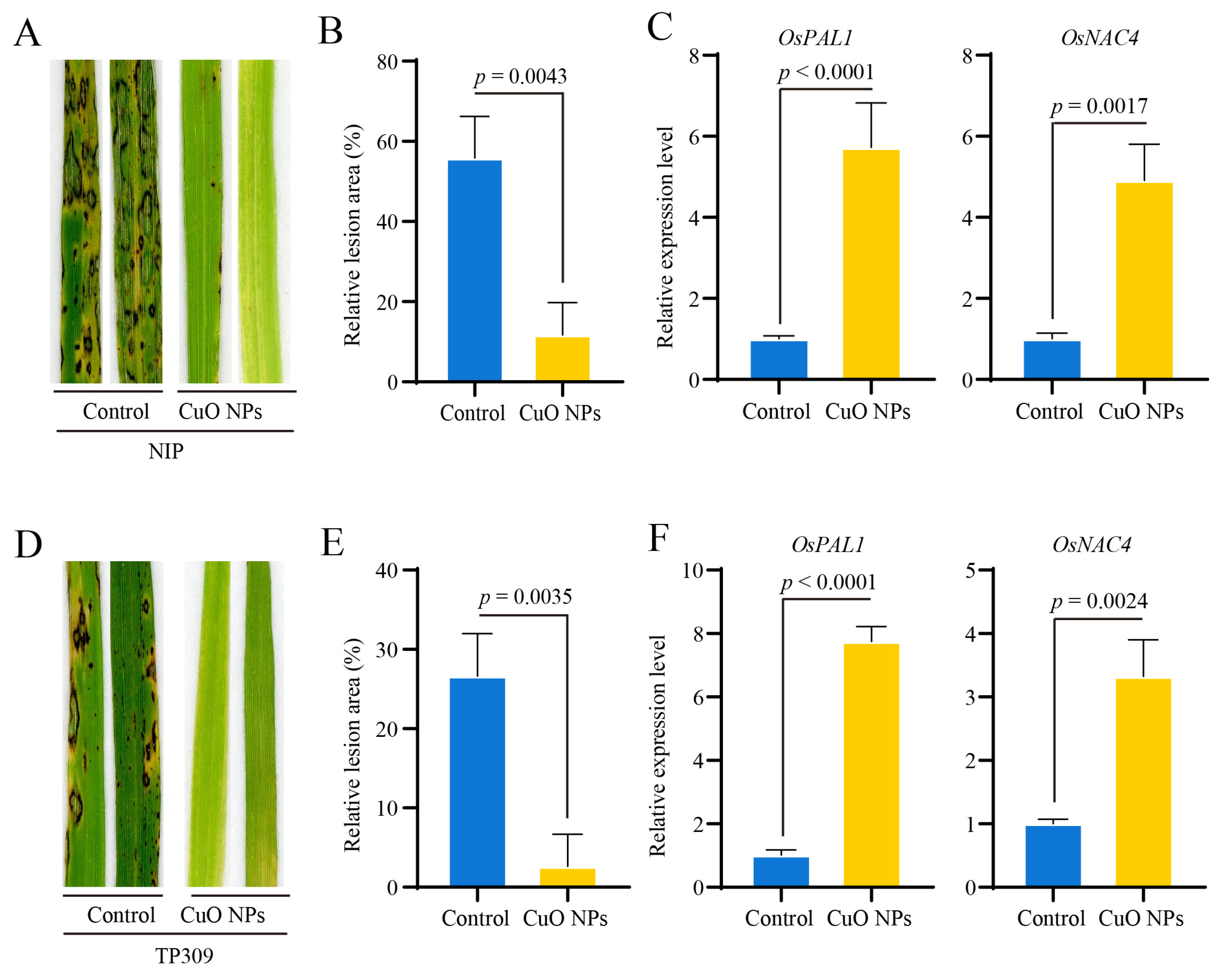
3.4. CuO NPs Enhance the Basal Resistance against M. oryzae in Rice
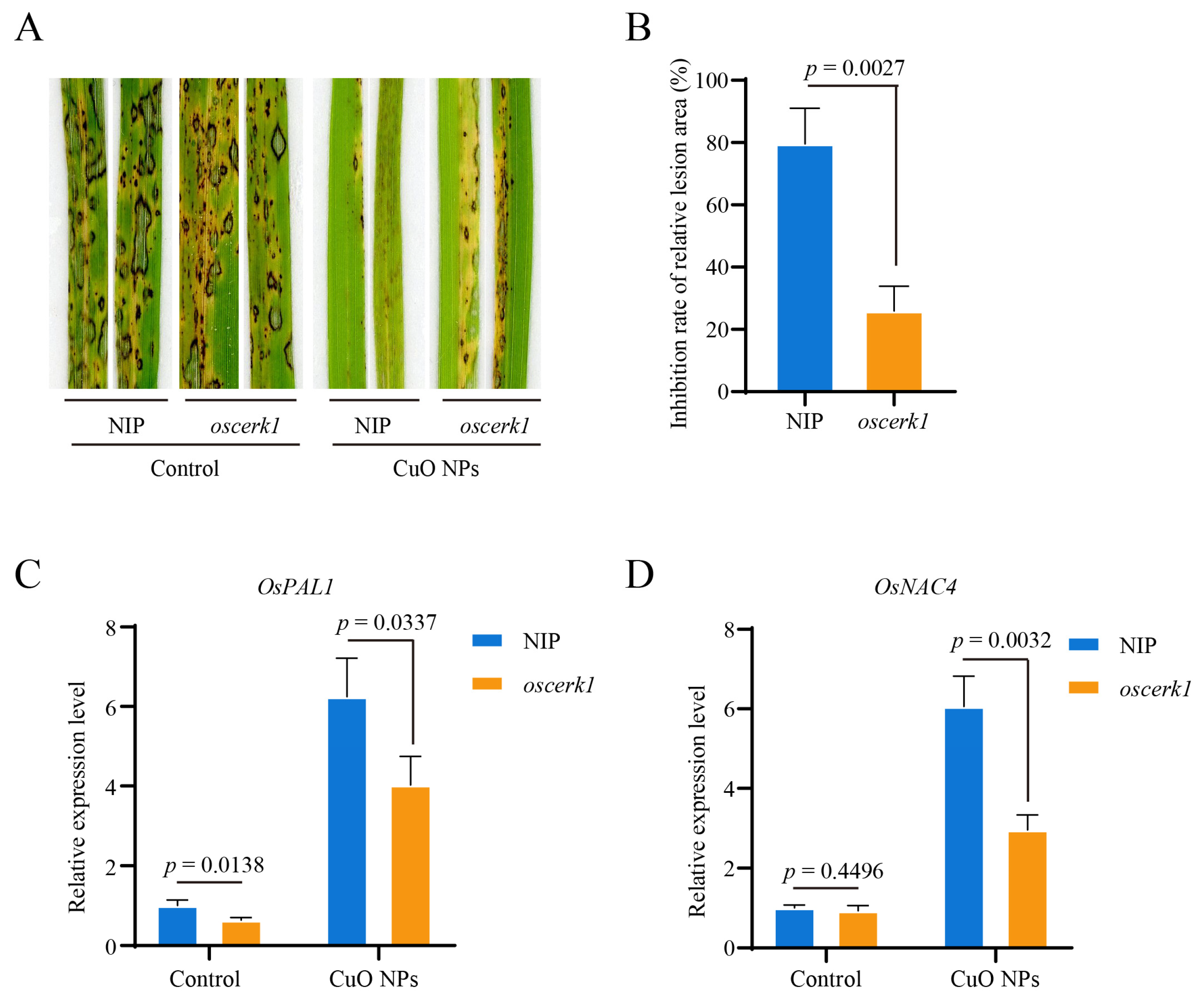
3.5. OsCERK1 Contributes to the CuO NPs-Modulated Basal Resistance against M. oryzae in Rice
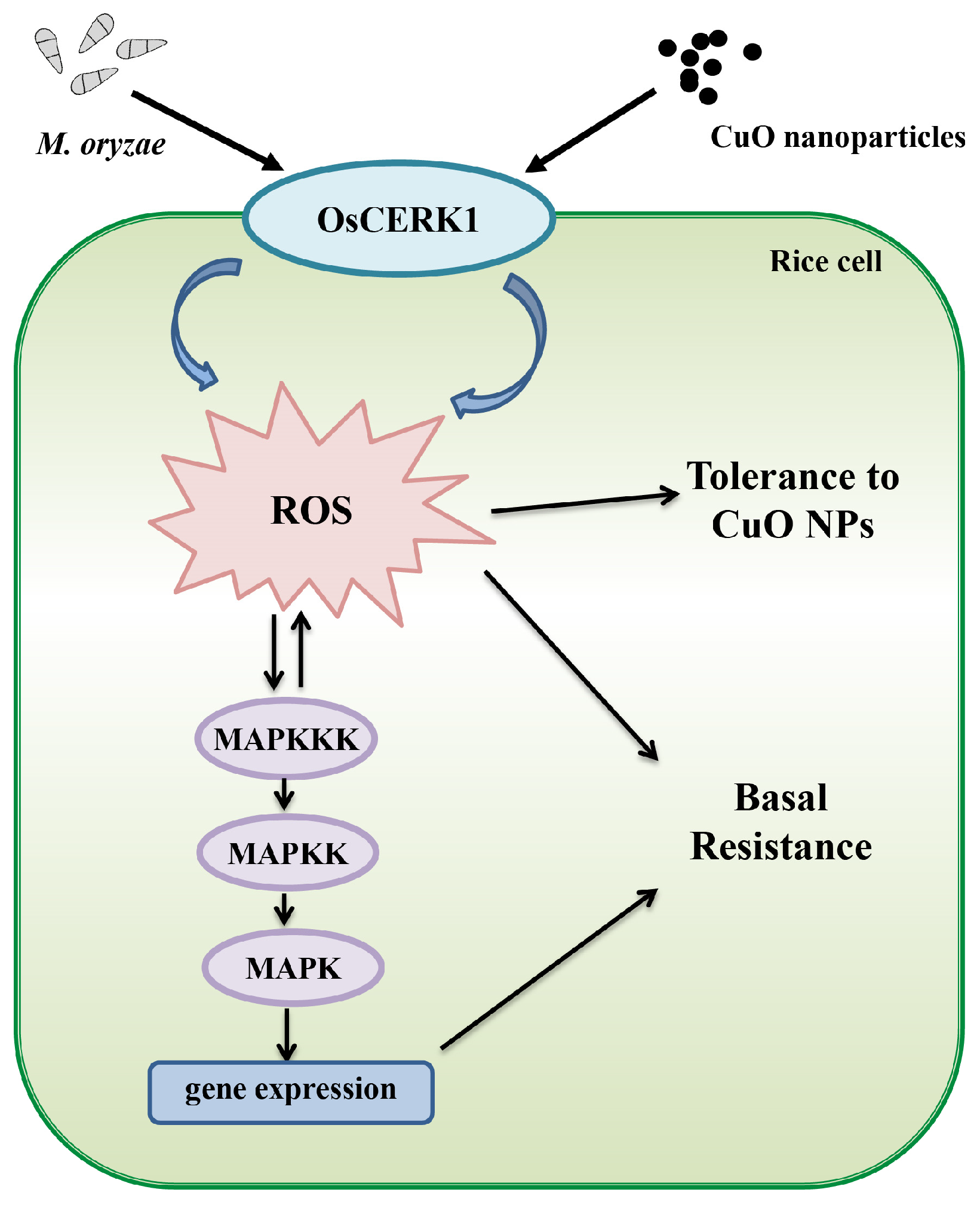
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ye, J.; Fang, H.; Zhang, S.; Xu, C. Effects of copper oxide nanoparticles on paddy soil properties and components. Nanomaterials 2018, 8, 839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abudayyak, M.; Guzel, E.; Özhan, G. Cupric oxide nanoparticles induce cellular toxicity in liver and intestine cell lines. Adv. Pharm. Bull. 2020, 10, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Hanna, S.K.; Miller, R.J.; Zhou, D.; Keller, A.A.; Lenihan, H.S. Accumulation and toxicity of metal oxide nanoparticles in a soft-sediment estuarine amphipod. Aquat. Toxicol. 2013, 142, 441–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moos, N.; Maillard, L.; Slaveykova, V.I. Dynamics of sub-lethal effects of nano-CuO on the microalga Chlamydomonas reinhardtii during short-term exposure. Aquat. Toxicol. 2015, 161, 267–275. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Z.; Zhao, J.; Xu, L.; Xu, L.; Yu, X.; Wei, Y.; Xing, B. Interaction of CuO nanoparticles with plant cells: Internalization, oxidative stress, electron transport chain disruption, and toxicogenomic responses. Environ. Sci. Nano 2018, 5, 2269–2281. [Google Scholar] [CrossRef]
- Nair PM, G.; Chung, I.M. Study on the correlation between copper oxide nanoparticles induced growth suppression and enhanced lignification in Indian mustard (Brassica juncea L.). Ecotoxicol. Environ. Saf. 2015, 113, 302–313. [Google Scholar] [CrossRef]
- Sui, H.J.; Zhang, J.Z.; Wang, Z.Y. Toxicity of copper oxide engineered nanoparticles to maize (Zea Mays L.) at different aging times. Adv. Mat. Res. 2014, 881–883, 972–975. [Google Scholar] [CrossRef]
- Le Van, N.; Ma, C.; Shang, J.; Rui, Y.; Liu, S.; Xing, B. Effects of CuO nanoparticles on insecticidal activity and phytotoxicity in conventional and transgenic cotton. Chemosphere 2016, 144, 661–670. [Google Scholar] [CrossRef]
- Yue, L.; Zhao, J.; Yu, X.; Kunmiao, L.; Wang, Z.; Xing, B. Interaction of CuO nanoparticles with duckweed (Lemna minor. L): Uptake, distribution and ROS production sites. Environ. Pollut. 2018, 243, 543–552. [Google Scholar] [CrossRef]
- Yang, Z.; Xiao, Y.; Jiao, T.; Zhang, Y.; Chen, J.; Gao, Y. Effects of copper oxide nanoparticles on the growth of rice (Oryza sativa L.) seedlings and the relevant physiological responses. Int. J. Public Health 2020, 17, 1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, E.P. Bioactive food components and health properties of rice bran. J. Am. Vet. Med. Assoc. 2011, 238, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Duan, D.; Xu, C.; Chen, Y.; Sun, L.; Zhang, H.; Yuan, X.; Zheng, L.; Yang, Y.; Yang, J.; et al. Translocation and biotransformation of CuO nanoparticles in rice (Oryza sativa L.) plants. Environ. Pollut. 2015, 197, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Elmer, W.; De La Torre-Roche, R.; Pagano, L.; Majumdar, S.; Zuverza-Mena, N.; Dimkpa, C.; Gardea-Torresdey, J.; White, J.C. Effect of metalloid and metal oxide nanoparticles on fusarium wilt of watermelon. Plant Dis. 2018, 102, 1394–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Han, J.; Xu, W.; Chen, X. Resistance and control effect of CuO NPs and Fe2O3 NPs suspension on cucumber downy mildew. Guizhou Agric. Sci. 2022, 50, 8. [Google Scholar]
- Hsd, A.; Mab, A.; Sr, A.; Sp, B.; Ahw, B.; Mas, A. Biosynthesis and antifungal activities of CuO and Al2O3 nanoparticles. Compr. Anal. Chem. 2021, 94, 533–546. [Google Scholar]
- Simkhada, K.; Thapa, R. Rice Blast, A major threat to the rice production and its various management techniques. TUR. J. Agric. Food. Sci. Tech. 2022, 10, 147–157. [Google Scholar] [CrossRef]
- Tsuda, K.; Katagiri, F. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr. Opin. Plant. Biol. 2010, 13, 459–465. [Google Scholar] [CrossRef]
- Qiu, J.; Xie, J.; Chen, Y.; Shen, Z.; Shi, H.; Naqvi, N.I.; Qian, Q.; Liang, Y.; Kou, Y. Warm temperature compromises JA-regulated basal resistance to enhance Magnaporthe oryzae infection in rice. Mol. Plant. 2022, 15, 723–739. [Google Scholar] [CrossRef]
- Miya, A.; Albert, P.; Shinya, T.; Desaki, Y.; Ichimura, K.; Shirasu, K.; Narusaka, Y.; Kawakami, N.; Kaku, H.; Shibuya, N. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 19613–19618. [Google Scholar] [CrossRef] [Green Version]
- Gong, B.Q.; Wang, F.Z.; Li, J.F. Hide-and-seek: Chitin-triggered plant immunity and fungal counterstrategies. Trends Plant Sci. 2020, 25, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Erwig, J.; Ghareeb, H.; Kopischke, M.; Hacke, R.; Matei, A.; Petutschnig, E.; Lipka, V. Chitin-induced and CHITIN ELICITOR RECEPTOR KINASE1 (CERK1) phosphorylation-dependent endocytosis of Arabidopsis thaliana LYSIN MOTIF-CONTAINING RECEPTOR-LIKE KINASE5 (LYK5). New Phytol. 2017, 215, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, A.; Wong, H.L.; Fujiwara, M.; Okuda, J.; Nishide, K.; Uno, K.; Lmai, K.; Umemura, K.; Kawasaki, T.; Kawano, Y.; et al. An OsCEBiP/OsCERK1-OsRacGEF1-OsRac1 module is an essential early component of chitin-induced rice immunity. Cell Host Microbe 2013, 13, 465–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Liu, B.; Chen, S.; Gong, B.; Chen, L.; Zhou, Q.; Xiong, F.; Wang, M.; Feng, D.; Li, J.; et al. A tyrosine phosphorylation cycle regulates fungal activation of a plant receptor Ser/Thr kinase. Cell Host Microbe 2018, 23, 241–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carotenuto, G.; Chabaud, M.; Miyata, K.; Capozzi, M.; Takeda, N.; Kaku, H.; Shibuya, N.; Nakagawa, T.; Barker, D.; Genre, A. The rice LysM receptor-like kinase OsCERK1 is required for the perception of short-chain chitin oligomers in arbuscular mycorrhizal signaling. New Phytol. 2017, 214, 1440–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinoza, C.; Liang, Y.; Stacey, G. Chitin receptor CERK1 links salt stress and chitin-triggered innate immunity in Arabidopsis. Plant J. 2017, 89, 984–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, T.; Araújo, O.; Pereira, R.; Almeida, A.C.; Cravo, A.; Bebianno, M.J. Genotoxicity of copper oxide and silver nanoparticles in the mussel Mytilus galloprovincialis. Mar. Environ. Res. 2013, 84, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Azhar, W.; Khan, A.R.; Muhammad, N.; Liu, B.; Song, G.; Hussain, A.; Yasin, M.U.; Khan, S.; Munir, R.; Gan, Y. Ethylene mediates CuO NP-induced ultrastructural changes and oxidative stress in Arabidopsis thaliana leaves. Environ. Sci. Nano 2020, 7, 938–953. [Google Scholar] [CrossRef]
- Yang, C.; Liu, R.; Pang, J.; Ren, B.; Liu, J. Poaceae-specific cell wall-derived oligosaccharides activate plant immunity via OsCERK1 during Magnaporthe oryzae infection in rice. Nat. Commun. 2021, 12, 2178. [Google Scholar] [CrossRef]
- Da Costa, M.V.J.; Sharma, P.K. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 2016, 54, 110–119. [Google Scholar] [CrossRef]
- Kou, Y.; Tan, Y.H.; Ramanujam, R.; Naqvi, N.I. Structure-function analyses of the Pth11 receptor reveal an important role for CFEM motif and redox regulation in rice blast. New Phytol. 2017, 214, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Meng, S.; Qiu, J.; Wang, C.; Shu, Y.; Luo, C.; Kou, Y. MoWhi2 regulates appressorium formation and pathogenicity via the MoTor signalling pathway in Magnaporthe oryzae. Mol. Plant Pathol. 2021, 22, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, J.; Rui, M.; Yang, L.; Shen, J.; Chu, H.; Song, S.; Chen, Y. OsFTIP7 determines metallic oxide nanoparticles response and tolerance by regulating auxin biosynthesis in rice. J. Hazard. Mater. 2021, 403, 123946. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013, 8, 811–816. [Google Scholar] [CrossRef] [Green Version]
- Shaw, A.K.; Hossain, Z. Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere. 2013, 93, 906–915. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere 2014, 112, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, L.; Song, K.; Zhu, Y.; Ding, W. Nonphytotoxic copper oxide nanoparticles are powerful “nanoweapons” that trigger resistance in tobacco against the soil-borne fungal pathogen Phytophthora nicotianae. J. Integr. Agric. 2022, 21, 3245–3262. [Google Scholar] [CrossRef]
- Hou, J.; Wang, X.; Hayat, T.; Wang, X. Ecotoxicological effects and mechanism of CuO nanoparticles to individual organisms. Environ. Pollut. 2017, 221, 209–217. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignificaion, and molecular level changes. Environ. Sci. Pollut. Res. 2014, 21, 12709–12722. [Google Scholar] [CrossRef]
- Wang, C.; Wang, G.; Zhang, C.; Zhu, P.; Dai, H.; Yu, N.; He, Z.; Xu, L.; Wang, E. OsCERK1-mediated chitin perception and immune signaling requires receptor-like cytoplasmic kinase 185 to activate an MAPK cascade in rice. Mol. Plant. 2017, 10, 619–633. [Google Scholar] [CrossRef] [Green Version]
- Kilasi, N.L.; Singh, J.; Vallejos, C.E.; Ye, C.; Jagadish, S.V.K.; Kusolwa, P.; Rathinasabapathi, B. Heat stress tolerance in rice (oryza sativa L.): Identification of quantitative trait loci and candidate genes for seedling growth under heat stress. Front. Plant Sci. 2018, 871, 1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haller, E.; Iven, T.; Feussner, I.; Stahl, M.; Fröhlich, K.; Löffelhardt, B.; Gust, A.A.; Nürnberger, T. ABA-Dependent salt stress tolerance attenuates botrytis immunity in Arabidopsis. Front. Plant Sci. 2020, 11, 594827. [Google Scholar] [CrossRef]
- Kim, Y.; Chung, Y.S.; Lee, E.; Tripathi, P.; Heo, S.; Kim, K.H. Root response to drought stress in rice (Oryza sativa L.). Int. J. Mol. Sci. 2020, 21, 1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Shetehy, M.; Moradi, A.; Maceroni, M.; Reinhardt, D.; Petri-Fink, A.; Rothen-Rutishauser, B.; Mauch, F.; Schwab, F. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat. Nanotechnol. 2021, 16, 344–353. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Liu, Z.; Meng, S.; Shen, Z.; Shi, H.; Qiu, J.; Lin, F.; Zhang, S.; Kou, Y. OsCERK1 Contributes to Cupric Oxide Nanoparticles Induced Phytotoxicity and Basal Resistance against Blast by Regulating the Anti-Oxidant System in Rice. J. Fungi 2023, 9, 36. https://doi.org/10.3390/jof9010036
Chen Y, Liu Z, Meng S, Shen Z, Shi H, Qiu J, Lin F, Zhang S, Kou Y. OsCERK1 Contributes to Cupric Oxide Nanoparticles Induced Phytotoxicity and Basal Resistance against Blast by Regulating the Anti-Oxidant System in Rice. Journal of Fungi. 2023; 9(1):36. https://doi.org/10.3390/jof9010036
Chicago/Turabian StyleChen, Ya, Zhiquan Liu, Shuai Meng, Zhenan Shen, Huanbin Shi, Jiehua Qiu, Fucheng Lin, Shu Zhang, and Yanjun Kou. 2023. "OsCERK1 Contributes to Cupric Oxide Nanoparticles Induced Phytotoxicity and Basal Resistance against Blast by Regulating the Anti-Oxidant System in Rice" Journal of Fungi 9, no. 1: 36. https://doi.org/10.3390/jof9010036
APA StyleChen, Y., Liu, Z., Meng, S., Shen, Z., Shi, H., Qiu, J., Lin, F., Zhang, S., & Kou, Y. (2023). OsCERK1 Contributes to Cupric Oxide Nanoparticles Induced Phytotoxicity and Basal Resistance against Blast by Regulating the Anti-Oxidant System in Rice. Journal of Fungi, 9(1), 36. https://doi.org/10.3390/jof9010036






