Abstract
Chronic pulmonary aspergillosis (CPA) often occurs in patients that have been previously treated for pulmonary tuberculosis (PTB). A limited number of studies have looked at the development of CPA at different times following the completion of a PTB treatment course. This prospective longitudinal study aimed to determine the incidence of CPA at two timepoints, at the end of the PTB treatment (T1) and six months post-treatment (T2). Patients with confirmed PTB from a previous study who were placed on anti-TB medication were followed up and screened for CPA at T1 and T2 by assessing their symptoms, evaluating their quality of life, and screening them for Aspergillus infection by performing antibody testing and cultures. CPA was defined by the Global Action for Fungal Infections (GAFFI) diagnostic algorithm. Forty-one patients were enrolled, of whom thirty-three patients (80%) and twenty-eight patients (68%) were resurveyed at T1 and T2, respectively. The rate of new CPA was 3.3% (1/33) and 7.4% (2/27) at T1 and T2, respectively, with an overall incidence of 10.7% (3/28) among the patients at both T1 and T2. A positive Aspergillus-specific antibody test was an indicator for CPA in all three patients. Aspergillus-specific antibody screening during and after the end of an anti-TB treatment regimen may be important for early detection of CPA in high-PTB-burden settings.
1. Introduction
Pulmonary tuberculosis (PTB) remains a major global health problem, with a high burden in low- and middle-income countries (LMICs). In 2020, there were 4.8 million people diagnosed with PTB globally, and 59% of these cases had been bacteriologically confirmed [1]. About 85% of the people diagnosed with PTB are generally successfully treated with a 6 month drug regimen [1]. Unfortunately, patients with PTB after a successful treatment become more exposed to secondary respiratory infections that are uncommon in patients without prior PTB [2]. These infections can be chronic, and they are associated with high morbidity and mortality rates. Chronic pulmonary aspergillosis (CPA) is one of the common infections, and the high-risk group are patients with PTB with overt lung cavities [3]. The prevalence of CPA as a sequel of PTB worldwide was estimated at 1.2 million in 2011 [4]. Several cross-sectional studies have been conducted on CPA as a secondary infection of PTB in different countries, and they have reported varying rates [5,6,7,8,9]. However, none of these studies have solely focused on bacteriologically confirmed PTB, and so, they may have included individuals without prior PTB, and this may not be a true definition of the post-PTB complications. In recent times, there has been an interest in evaluating the timing of the incidence of CPA from diagnosis, during treatment and to post-treatment of PTB [3,10,11]. Such data could help guide screening strategies for early CPA diagnosis.
In addition, CPA may rarely co-exist with PTB as a primary CPA-PTB co-infection [12,13,14]. Genetic and environmental factors may also influence the development of CPA at different times among individuals [10,15]. Presently, CPA has been identified in PTB patients from the beginning of the PTB treatment and close to 40 years after completing the treatment [3,10,16]. The current prospective longitudinal study was conducted to evaluate the incidence of CPA at two timepoints following the completion of a PTB treatment regimen in a cohort of new bacteriologically confirmed PTB patients using Aspergillus-specific antibody testing as a screening tool.
2. Materials and Methods
Adult (≥18 years) patients with MTB detected in their sputum in a previous study [17] and were subsequently placed on an anti-TB treatment were enrolled for this study. The patients were receiving care for suspected PTB at the Chest Clinic of the Korle-Bu Teaching Hospital, Accra, Ghana, or they were referred from various health facilities across the country to the Chest Clinic TB laboratory for GeneXpert MTB and rifampicin (RIF) resistance testing (Xpert® MTB/RIF, Cepheid, California, CA, USA). Patients with a previous history of PTB were excluded.
The patients’ demographics and baseline CPA screening findings including laboratory and chest radiograph reports were extracted from the primary research data. The patients were then followed-up and further screened for CPA at two timepoints; one was within one month after completing treatment (T1, 6–7 months from diagnosis) and the other was 6–7 months post-treatment (T2, 12–13 months from diagnosis). The CPA screening involved an assessment of the symptoms, Aspergillus-specific antibody testing, sputum Aspergillus culture, chest radiograph and/or a computed tomography (CT) scan.
Serum samples were obtained from all the patients for Aspergillus-specific antibody testing with the LDBio Aspergillus IgG & IgM LFA (LDBio Diagnostics, Lyon, France) following the manufacturer’s instructions. A sputum Aspergillus culture was performed for all of the patients using a modified version of the high-volume culture method by inoculating an aliquot (1–2 mL) of undiluted sputum on Sabouraud dextrose agar, and then, it was incubated at 37 °C for up to 8 days [17]. A chest CT scan was performed for the patients with positive Aspergillus serology or cavitation on baseline chest radiograph with new or persistent respiratory symptoms. A consultant radiologist (HG) who was blinded to the clinical and laboratory data evaluated all imaging investigations. Xpert MTB/RIF and/or acid-fast bacilli (AFB) smear results were retrieved from the laboratory records. Additionally, the quality of life (QoL) of the patients were evaluated at both timepoints using the St. George’s Respiratory Questionnaire (SGRQ), which scores patients from 1 (excellent health) to 100 (very ill) [18,19]. CPA was defined using the Global Action for Fungal Infections (GAFFI) diagnostic criteria (2018) [20].
The data were analyzed with SPSS version 25 (IBM, New York, NY, USA) at a 5% significance level using Chi Square test. The summary statistics are presented using frequencies and percentages for the categorical variables. Fisher’s exact tests were employed to compare the proportions of the various characteristics of the patients recruited at both, timepoints.
3. Results
There were 47 Xpert MTB positive cases, of whom, 41 were diagnosed as new cases. Of the 41 new PTB patients, 33 (80%) and 28 (68%) were resurveyed at T1 and T2, respectively. In all, three of the thirteen patients who were not resurveyed at T1 and T2 died, and the remainder were lost during follow-up for varying reasons. Details of enrolled patients at both timepoints including those lost during follow-up are shown in Figure 1.
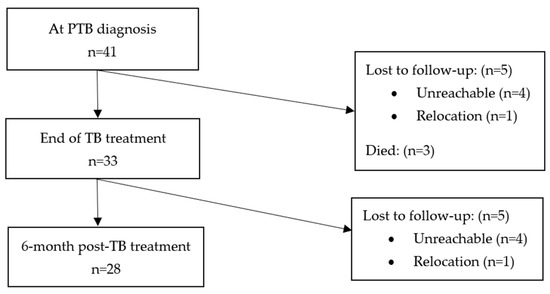
Figure 1.
Overview of 41 patients enrolled.
There were more male patients (78%, n = 32), and the mean age was 40.2 years (range, 18–75 years) (Table 1). Of the 41 positive Xpert MTB cases, majority of them (78%, n = 32) had a high or medium MTB load or concentration. No RIF resistance was recorded, but there were three cases of RIF indeterminate results (and so resistance could not be established). At baseline, Aspergillus-specific antibody was negative in all the patients. Cavitation was visible on chest radiograph in 18 patients at baseline. There were 10 patients whose sputum grew Aspergillus spp. All the 41 patients were placed on a standard first-line anti-TB regimen, comprising rifampicin, isoniazid, pyrazinamide, and ethambutol. The baseline SGRQ score was higher than 50 in 32% (13/41) of patients, with an average of 43.1 (Table 1).

Table 1.
Patients’ characteristics at the three time points.
Of the 33 patients resurveyed at T1, 90.9% of them (30/33) had completed TB treatment and achieved microbiological cure (AFB smear negative), and three patients were declared to have failed the treatment due to persisting symptoms and a positive AFB smear. The Aspergillus-specific antibody was positive in one patient who was presumed to have failed the treatment due to persisting symptoms, but his AFB smear was negative, and he was placed on a second-line treatment. This patient met the criteria for CPA with suggestive image findings on a CT scan (Figure 2) and sputum growing A. fumigatus and A. niger (Table 2). The SGRQ score improved significantly at T1 for patients without CPA or treatment failure, with an average score of 51.4 falling to 3.8, signifying PTB treatment success. The SGRQ for the CPA patient decreased from 45 at baseline to 29 at T1. The three patients who failed TB treatment had a SGRQ reduction from an average of 51 to 26.3, respectively.
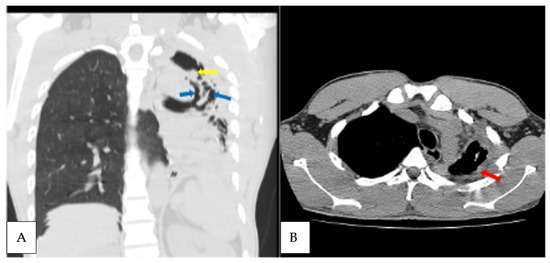
Figure 2.
Axial non contrast CT scan, coronal reformatted lung, and axial mediastinal windows of the chest of the patient diagnosed with CPA at T1 (A). Extensive left lung traction bronchiectasis (blue arrows) with ipsilateral lung volume loss, left apical lung cavity with intracavitary material (yellow arrow); (B) left apical lung pericavitary pleural thickening (red arrow).

Table 2.
Demographics, symptoms, laboratory, imaging and QoL details of the three CPA patients.
The 28 patients rescreened at T2, included the previous CPA patient. Twenty-seven patients had achieved microbiological cure, while one was confirmed as having had a PTB relapse. Of the 27 patients with a previous negative Aspergillus antibody test, 7.4% of them (2/27) seroconverted. These two new patients also met the criteria for CPA, with one of them having a fungal ball (Figure 3 and Figure 4), and both had A. fumigatus growing from sputum. One of the CPA patients was HIV positive. Again, the two new patients who developed CPA at T2 were symptomatically worse with increasing SGRQ scores from 14.5 at T1 to 36 at T2. Generally, among the patients resurveyed at both time points, the average SGRQ scores for those without CPA had improved significantly compared to those with CPA. The other reasons for a higher SGRQ score were anti-TB treatment failure and PTB relapse.
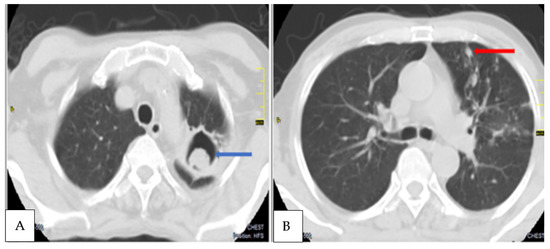
Figure 3.
Axial contrast CT scans, axial lung window of chest of another CPA patient diagnosed at T2 showing (A) a soft tissue mass within a left apical lung cavity (blue arrow) with a characteristic crescent of air around it, the ‘Monod sign’, indicative of an aspergilloma. (B). Nodular opacities (red arrow) and non pericavitary fibrotic changes in the left upper lobe.
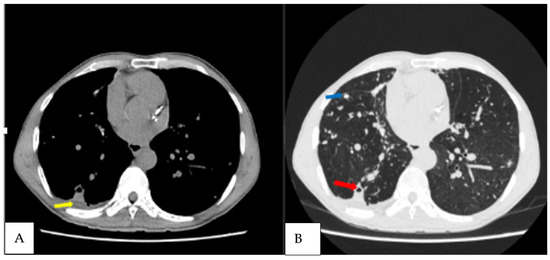
Figure 4.
Axial non contrast CT scan, axial mediastinal and lung windows of the chest of the third CPA patient diagnosed at T3 patient, demonstrating (A) right lung nodules (blue arrow); (B) small right lower lobe cavity with pericavitary infiltration (red arrow) as well as a small pleural effusion (yellow arrow).
The overall incidence of CPA among the resurveyed patients over 12 months from the time of PTB diagnosis was 10.7% (3/28). The incidence of CPA at end of the PTB treatment (T1) was 3% (1/33), which later increased to 7.4% (2/27) at 6 months post-treatment (T2). This represents 42.9% (3/7) of the patients with new or persisting symptoms suggestive of PTB after either 6 months or 12 months post-initial PTB diagnosis. Haemoptysis was only noted in patients who had CPA when they were resurveyed at T1 and T2. The common CT scan findings among the three CPA patients were cavitation, pleural thickening, pericavitary fibrosis and bronchiectasis (Table 2). Two of the three CPA patients had cavitation based on a baseline chest radiograph, representing 11.1% (2/18) of all of those with cavitation.
4. Discussion
We prospectively followed up new bacteriologically confirmed PTB patients from a previous study [17] to evaluate the incidence of CPA from the time of PTB diagnosis to the time after completing the TB treatment, and finally, six months after the treatment regimen. A CT scan was performed in all the CPA patients in our study. This makes the diagnosis more robust because a CT scan is very important for confirming a CPA diagnosis with a maximum score point in the recently published CPA EQUAL scores [21]. This study adds to previous studies that have indicated the need to regularly screen for CPA post-PTB treatment [3,5,10]. It also contributes to the emerging evidence that CPA may develop during or early after completion of anti-TB medications, as previously reported [10]. The current study also shows the increasing incidence rate of CPA among post-PTB patients when they are resurveyed over time, which is similar to previous findings [10].
Interestingly, at the baseline, no patient met the criteria for CPA, that is, we found no PTB-CPA coinfection at the time of the confirmed PTB diagnosis. However, it possible for CPA to develop two months after initiating anti-TB treatment, as reported by two recently published studies in Indonesia [22] and in Uganda [6]. First, Setianingrum et al. [10] in Indonesia, reported a CPA incidence of 7.9% among PTB patients who were within 2 months into an anti-TB treatment. It is important to note, however, that their cohort included at least 42% new non-bacteriologically confirmed PTB cases, and some of them probably had CPA alongside other respiratory disorders, including asthma and chronic pulmonary obstructive disease (COPD) as an underlying condition. Similarly, another study in Uganda reported ~20% CPA incidence among PTB patients with persisting symptoms after 2 months of TB treatment [6]. In a recently published study, a possible PTB-CPA coinfection was reported, but there are still doubts with regard to the existence of a true PTB infection [17]. As more studies are being conducted on CPA in patients presenting with PTB, the phenomenon of co-infection may be further explored.
In the current study, the T1 CPA patient resurveyed after six months, continued to have features of CPA including a positive Aspergillus-specific antibody test and Aspergillus culture which were accompanied by a worsening SGRQ score. Similar observations have been made in other studies [3,10]. However, it has been reported that some of these patients may no longer have the features of CPA when they are resurveyed without any antifungal treatment, surgical procedure or radiotherapy [10]. The data available on this phenomenon are scanty, and notwithstanding, it is widely accepted that some cases of CPA may be self-resolving or remain static for long periods [13].
Aspergillus specific-antibody testing was critical in identifying potential cases of CPA and distinguishing them from treatment failures or PTB recurrent cases with CT scan investigations. It is worthy to note that, all the PTB patients who were eventually diagnosed with CPA had a positive Aspergillus-specific antibody test, and was helpful in indicating, the need for advanced imaging seeking radiological features suggestive of CPA. Aspergillus-specific antibody can be detected by several methods including, precipitins, counter immuno-electrophoresis, immunodiffusion, complement fixation, enzyme immunoassay or immunochromatography. All of these tests have limitations including, false positives and false negatives [22,23]. In fact, evaluation studies and the clinical use evaluations of the LDBio Aspergillus-specific IgG and IgM assay employed in our study have reported varying sensitivities and specificities pooled at 90% and 91%, respectively, among different populations [6,8,24,25,26,27,28]. Although, an elevated Aspergillus-specific IgG level is superior compared to other immunoglobulins in the diagnosis of CPA, it is possible for some CPA patients to have normal levels of Aspergillus-specific IgG but raised levels of Aspergillus-specific IgM, which may be positive in about half of the CPA patients [22]. Thus, an Aspergillus-specific IgG and IgM assay may offer additional sensitivity. Like Aspergillus-specific IgM, Aspergillus-specific IgA and IgE can also be positive when the symptoms and imaging features suggestive of CPA are observed and the Aspergillus-specific IgG levels are normal. Aspergillus-specific IgE may be linked to allergic aspergillosis as the underlying condition for CPA, but it also may be independently elevated.
The performances of some Aspergillus-specific antibody assays are negatively affected by the relatively common minor or subtle immunodeficiencies found in CPA patients. However, the LDBio Aspergillus-specific IgG and IgM assay has been previously reported to be minimally affected by immunodeficiency, and thus, it may perform acceptably well in both HIV seronegative and seropositive patients [29]. Notwithstanding, CPA is rarely associated with HIV, and it is more common in patients without apparent or with subtle immunodeficiency. Our study suggests the LDBio Aspergillus-specific IgG and IgM can be used with the GAFFI diagnostic algorithm, in accord with a previous report from Uganda [27]. However, the algorithm relies mainly on chest radiographs, which would have missed one CPA patient in this series who had CPA-suggestive image findings demonstrated only on CT scan images. Clinicians thus need to consider Aspergillus-specific antibody testing in successfully treated PTB patients who return with new or persistent respiratory symptoms without radiological progression on chest radiographs. Most of these cases are usually presumed to have anti-TB treatment failure, PTB relapse or reinfection, but studies have shown that over 50% actually have CPA [9,17,30]. The current finding corroborates a recent study in Ghana, where CPA was present in 50% of the patients with presumed recurrent PTB [17]. Although the Aspergillus culture was positive in eight more patients at T1, none of them had symptoms or a previous chest radiograph suggestive of CPA, and so culture alone should not be used to diagnose CPA in the absence of characteristic radiological findings.
The major limitation of our study was that our sample size was small, and thus, it may not be sufficiently representative. A validation in a larger population will carry more statistical weight. Additionally, some of the patients lost during follow-up may have developed CPA, and thus, we may have underreported the frequency of this problem as in other studies with a significant proportion of study subjects who were lost during follow-up.
5. Conclusions
The present study indicates that CPA may develop during and after completing an anti-TB treatment regimen among new bacteriologically confirmed PTB patients. Aspergillus-specific antibody testing is instrumental in screening patients prior to performing CT scans to confirm the cases of CPA in resource-constrained settings, where advanced imaging is mostly unavailable or expensive to access. We recommend the validation of these findings in a larger cohort study. Additionally, subsequent studies may also consider investigations at 3 months or half of the duration into anti-TB treatment regimen. This will be important to contribute to efforts in identifying strategies for early detection of CPA cases, particularly in high-PTB-burden settings to minimize the number of inappropriate retreatments for PTB and late presentations with aspergilloma.
Author Contributions
Conceptualization, B.K.O., C.K. and D.W.D.; Methodology, B.K.O., B.O., H.G., C.K. and D.W.D.; Validation, C.K., J.S.A.-M., D.W.D. and J.A.O.; Formal analysis, B.K.O., B.O., H.G., C.K. and D.W.D.; Investigation, B.K.O., B.O. and H.G.; Resources, B.K.O., J.S.A.-M., J.A.O., C.K. and D.W.D.; Data curation, B.K.O., B.O. and H.G.; Writing—Original Draft Preparation, B.K.O.; Writing—Review and Editing, B.O., H.G., C.K., J.S.A.-M., D.W.D. and J.A.O.; Supervision, C.K., D.W.D., J.S.A.-M. and J.A.O.; Funding Acquisition, D.W.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CARIGEST SA as part of the ‘Epidemiology of Invasive Fungal Infections in Ghana/West Africa’ research award to DWD which includes a studentship award to BKO. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Korle-Bu Teaching Hospital (STC/IRB/00058/2020) and University Research and Ethics Committee of the University of Manchester (Ref: 2020-9368-16168).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The research data associated with this paper are available from the corresponding author upon reasonable request.
Acknowledgments
The authors are grateful for the support of the staff of Chest Clinic and the research team from the Noguchi Memorial Institute of Medical Research based at the Chest Clinic for their invaluable contribution to the study, with special thanks being given to Emmanuel Tetteh. The authors also acknowledge the organizational support of Eric Sampane-Donkor, and the laboratory assistance offered by Mary-Magdalene Osei, Joseph Anamsi and Amos Akumwena from the Department of Medical Microbiology, University of Ghana Medical School. Finally, Leonard Okine is appreciated for his support with analysing the research data.
Conflicts of Interest
B.K.O., B.O., H.G., J.S.A.M. and J.A.O. declare no conflict of interest. C.K. has received speaker’s fees from Pfizer Inc. D.W.D. and family hold Founder shares in F2G Ltd., a University of Manchester spin-out antifungal discovery company and share options in TFF Pharma. He acts or has recently acted as a consultant to Pulmatrix, Pulmocide, Biosergen, TFF Pharmaceuticals, Pfizer, Omega, Novacyt and Cipla. He sat on the DSMB for a SARS CoV2 vaccine trial. In the last 3 years, he has been paid for talks on behalf of Hikma, Gilead, BioRad, Basilea, Mylan, and Pfizer. He is a longstanding member of the Infectious Disease Society of America Aspergillosis Guidelines group, the European Society for Clinical Microbiology and Infectious Diseases Aspergillosis Guidelines group and recently joined the One World Guideline for Aspergillosis.
References
- World Health Organization. Factsheet Global Tuberculosis Report 2021; WHO: Geneva, Switzerland, 2021.
- Hsu, D.; Irfan, M.; Jabeen, K.; Iqbal, N.; Hasan, R.; Migliori, G.B.; Tiberi, S. Post tuberculosis treatment infectious complications. Int. J. Infect. Dis. 2020, 92, S41–S45. [Google Scholar] [CrossRef] [PubMed]
- Page, I.D.; Byanyima, R.; Hosmane, S.; Onyachi, N.; Opira, C.; Richardson, M.; Sawyer, R.; Sharman, A.; Denning, D.W. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur. Respir. J. 2019, 53, 1801184. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Pleuvry, A.; Cole, D. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull. World Health Organ. 2011, 89, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Oladele, R.O.; Irurhe, N.K.; Foden, P.; Akanmu, A.S.; Gbaja-Biamila, T.; Nwosu, A.; Ekundayo, H.A.; Ogunsola, F.T.; Richardson, M.D.; Denning, D.W. Chronic pulmonary aspergillosis as a cause of smear-negative TB and/or TB treatment failure in Nigerians. Int. J. Tuberc. Lung Dis. 2017, 21, 1056–1061. [Google Scholar] [CrossRef]
- Namusobya, M.; Bongomin, F.; Mukisa, J.; Olwit, W.K.; Batte, C.; Mukashyaka, C.; Mande, E.; Kwizera, R.; Denning, D.W.; Rhein, J.; et al. Chronic pulmonary aspergillosis in patients with active pulmonary tuberculosis with persisting symptoms in Uganda. Mycoses 2022, 65, 625–634. [Google Scholar] [CrossRef]
- Hedayati, M.T.; Azimi, Y.; Droudinia, A.; Mousavi, B.; Khalilian, A.; Hedayati, N.; Denning, D.W. Prevalence of chronic pulmonary aspergillosis in patients with tuberculosis from Iran. Eur. J. Clin. Microbiol. 2015, 34, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Rozaliyani, A.; Rosianawati, H.; Handayani, D.; Agustin, H.; Zaini, J.; Syam, R.; Adawiyah, R.; Tugiran, M.; Setianingrum, F.; Burhan, E.; et al. Chronic Pulmonary Aspergillosis in Post Tuberculosis Patients in Indonesia and the Role of LDBio Aspergillus ICT as Part of the Diagnosis Scheme. J. Fungi 2020, 6, 318. [Google Scholar] [CrossRef]
- Nguyen, N.T.B.; Le Ngoc, H.; Nguyen, N.V.; Dinh, L.V.; Nguyen, H.V.; Nguyen, H.T.; Denning, D.W. Chronic Pulmonary Aspergillosis Situation among Post Tuberculosis Patients in Vietnam: An Observational Study. J. Fungi 2021, 7, 532. [Google Scholar] [CrossRef]
- Setianingrum, F.; Rozaliyani, A.; Adawiyah, R.; Syam, R.; Tugiran, M.; Sari, C.Y.I.; Nandipinto, F.; Ramnath, J.; Arifin, A.R.; Handayani, D.; et al. A prospective longitudinal study of chronic pulmonary aspergillosis in pulmonary tuberculosis in Indonesia (APICAL). Thorax 2021, 77, 821–828. [Google Scholar] [CrossRef]
- Volpe-Chaves, C.E.; Venturini, J.; Castilho, S.B.; Fonseca, S.S.O.; Nunes, T.F.; Cunha, E.A.T.; Lima, G.M.E.; Nunes, M.O.; Vicentini, A.P.; Oliveira, S.M.V.L.; et al. Prevalence of chronic pulmonary aspergillosis regarding time of tuberculosis diagnosis in Brazil. Mycoses 2022, 65, 715–723. [Google Scholar] [CrossRef]
- Baluku, J.B.; Nuwagira, E.; Bongomin, F.; Denning, D.W. Pulmonary TB and chronic pulmonary aspergillosis: Clinical differences and similarities. Int. J. Tuberc. Lung Dis. 2021, 25, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Cadranel, J.; Beigelman-Aubry, C.; Ader, F.; Chakrabarti, A.; Blot, S.; Ullmann, A.J.; Dimopoulos, G.; Lange, C.; Dimopoulos, Christoph Lange on behalf of the European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: Rationale and clinical guidelines for diagnosis and management. Eur. Respir. J. 2016, 47, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Moon, J.-W.; Park, Y.-B.; Ko, Y. Serological Changes in Anti-Aspergillus IgG Antibody and Development of Chronic Pulmonary Aspergillosis in Patients Treated for Pulmonary Tuberculosis. J. Fungi 2022, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Tiseo, G.; Falcone, M.; Menichetti, F. Pulmonary Aspergillosis: An Evolving Challenge for Diagnosis and Treatment. Infect. Dis. Ther. 2020, 9, 511–524. [Google Scholar] [CrossRef]
- Sapienza, L.G.; Gomes, M.J.L.; Maliska, C.; Norberg, A.N. Hemoptysis due to fungus ball after tuberculosis: A series of 21 cases treated with hemostatic radiotherapy. BMC Infect. Dis. 2015, 15, 546. [Google Scholar] [CrossRef]
- Ocansey, B.K.; Otoo, B.; Adjei, A.; Gbadamosi, H.; Kotey, F.C.N.; Kosmidis, C.; Afriyie-Mensah, J.S.; Denning, D.W.; Opintan, J.A. Chronic Pulmonary Aspergillosis is Common among Patients with Presumed Tuberculosis Relapse in Ghana. Med. Mycol. 2022, 60, myac063. [Google Scholar] [CrossRef]
- Al-Shair, K.; Atherton, G.T.; Kennedy, D.; Powell, G.; Denning, D.W.; Caress, A. Validity and reliability of the St. George’s Respiratory Questionnaire in assessing health status in patients with chronic pulmonary aspergillosis. Chest 2013, 144, 623–631. [Google Scholar] [CrossRef]
- Pasipanodya, J.G.; Miller, T.L.; Vecino, M.; Munguia, G.; Bae, S.; Drewyer, G.; Weis, S.E. Using the St. George Respiratory Questionnaire to Ascertain Health Quality in Persons with Treated Pulmonary Tuberculosis. Chest 2007, 132, 1591–1598. [Google Scholar] [CrossRef]
- Denning, D.W.; Page, I.D.; Chakaya, J.; Jabeen, K.; Jude, C.M.; Cornet, M.; Alastruey-Izquierdo, A.; Bongomin, F.; Bowyer, P.; Chakrabarti, A.; et al. Case Definition of Chronic Pulmonary Aspergillosis in Resource-Constrained Settings. Emerg. Infect. Dis. 2018, 24, 8. [Google Scholar] [CrossRef]
- Sprute, R.; Van Braeckel, E.; Flick, H.; Hoenigl, M.; Kosmidis, C.; Agarwal, R.; Seidel, D. EQUAL CPA Score 2022: A tool to measure guideline adherence for chronic pulmonary aspergillosis. J. Antimicrob. Chemother. 2022, dkac378. [Google Scholar] [CrossRef]
- Page, I.D.; Richardson, M.; Denning, D.W. Antibody testing in aspergillosis—Quo vadis? Med. Mycol. 2015, 53, 417–439. [Google Scholar] [CrossRef] [PubMed]
- Volpe Chaves, C.E.; do Valle Leone de Oliveira, S.M.; Venturini, J.; Grande, A.J.; Sylvestre, T.F.; Poncio Mendes, R.; Mello Miranda Paniago, A. Accuracy of serological tests for diagnosis of chronic pulmonary aspergillosis: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0222738. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Choudhary, H.; Agnihotri, S.; Sehgal, I.S.; Agarwal, R.; Kaur, H.; Ghosh, A.; Chakrabarti, A.; Rudramurthy, S.M. LDBio Aspergillus immunochromatographic test lateral flow assay for IgG/IgM antibody detection in chronic pulmonary aspergillosis: Single-centre evaluation and meta-analysis. Indian J. Med. Microbiol. 2022, 40, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Hunter, E.S.; Richardson, M.D.; Denning, D.W. Evaluation of LD Bio Aspergillus ICT lateral flow assay for IgG and IgM antibody detection in chronic pulmonary aspergillosis. J. Clin. Microbiol. 2019, 57, e00538-19. [Google Scholar]
- Ray, A.; Chowdhury, M.; Sachdev, J.; Sethi, P.; Meena, V.P.; Singh, G.; Xess, I.; Vyas, S.; Khan, M.A.; Sinha, S.; et al. Efficacy of LD Bio Aspergillus ICT Lateral Flow Assay for Serodiagnosis of Chronic Pulmonary Aspergillosis. J. Fungi 2022, 8, 400. [Google Scholar] [CrossRef]
- Kwizera, R.; Katende, A.; Teu, A.; Apolot, D.; Worodria, W.; Kirenga, B.J.; Bongomin, F. Algorithm-aided diagnosis of chronic pulmonary aspergillosis in low- and middle-income countries by use of a lateral flow device. Eur. J. Clin. Microbiol. 2019, 39, 1–3. [Google Scholar] [CrossRef]
- Rozaliyani, A.; Setianingrum, F.; Azahra, S.; Abdullah, A.; Fatril, A.; Rosianawati, H.; Burhan, E.; Handayani, D.; Arifin, A.; Zaini, J.; et al. Performance of LDBio Aspergillus WB and ICT Antibody Detection in Chronic Pulmonary Aspergillosis. J. Fungi 2021, 7, 311. [Google Scholar] [CrossRef]
- Hunter, E.S.; Wilopo, B.; Richardson, M.D.; Kosmidis, C.; Denning, D.W. Effect of patient immunodeficiencies on the diagnostic performance of serological assays to detect Aspergillus-specific antibodies in chronic pulmonary aspergillosis. Respir. Med. 2021, 178, 106290. [Google Scholar] [CrossRef]
- Singla, R.; Singhal, R.; Rathore, R.; Gupta, A.; Sethi, P.; Myneedu, V.P.; Kumar, V. Risk factors for chronic pulmonary aspergillosis in post-TB patients. Int. J. Tuberc. Lung Dis. 2021, 25, 324–326. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).