Abstract
Zearalenone (ZEA) is known as a Fusarium-produced mycotoxin, representing a risk to cereal food safety with repercussions for economies and worldwide trade. Recent studies have reported the co-occurrence of ZEA and masked ZEA in a variety of cereals and cereal-based products, which may exert adverse effects on public health due to additive/synergistic interactions. However, the co-contamination of ZEA and masked ZEA has received little attention. In order to minimize the threats of co-contamination by ZEA and masked ZEA, it is necessary to recognize the occurrence and formation of ZEA and masked ZEA. This review focuses on the characteristics, incidence, and detection of ZEA and its masked forms. Additionally, the fate of ZEA and masked ZEA during the processing of bread, cake, biscuits, pasta, and beer, as well as the ZEA limit, are discussed. The incidence of masked ZEA is lower than that of ZEA, and the mean level of masked ZEA varies greatly between cereal samples. Published data showed a considerable degree of heterogeneity in the destiny of ZEA during cereal-based food processing, mostly as a result of the varying contamination levels and complicated food processing methods. Knowledge of the fate of ZEA and masked ZEA throughout cereal-based food processing may reduce the likelihood of severe detrimental market and trade ramifications. The revision of legislative limits of masked ZEA may become a challenge in the future.
1. Introduction
Cereal and cereal-based products constitute the majority of the world’s dietary energy and nutrients, accounting for more than half of the average per capita caloric consumption and nearly half of the average per capita protein intake [1,2]. Cereal grains have a long history in the brewing industry as raw ingredients. They can also be processed into flours that are utilized in the production of bread, pasta, cake, and biscuits [3,4]. Additionally, cereal by-products play a crucial function in animal feeding because they supply the required energy and protein for the growth of livestock. It was estimated that approximately 2799 million tons of cereal will be produced in 2021, and cereal production has a significant impact on global food security [5].
However, cereals are easily contaminated with fungi during planting, resulting in mycotoxin production [6]. Mycotoxins are the toxic secondary metabolites of fungi associated with adverse effects on animals, humans, and crops, leading to health issues and economic losses [7]. The major mycotoxins with agro-economic importance are aflatoxin, fumonisins, ochratoxin, zearalenone (ZEA), and trichothecenes, and ZEA is one of the most widespread mycotoxins in cereal [7,8]. ZEA is generated by Fusarium genera such as F. graminearum (Gibberella zeae), F. culmorum, F. crookwellense, F. poae, F. semitectum, and F. equiseti [9,10]. After plant and fungal conversion, animal metabolism, and matrix effects and reactions during food processing, ZEA can be transformed to masked ZEA in cereal and cereal-derived commodities [11,12,13]. Concerns about the safety of contaminated products have been further heightened by these masked ZEA. Due to analytical challenges, masked ZEA may not be detectable in cereals, posing potential threats in vivo for their retained toxicity.
Given the prevalence of ZEA and masked ZEA in cereals, consumption of contaminated cereal and cereal-derived products may comprise a significant source of ZEA exposure in humans [14]. In implementing preventative strategies to lower the intake risks of ZEA and masked ZEA, it is of utmost significance to not only know the characteristics of ZEA and masked ZEA in cereals and final commodities but primarily the fate of ZEA in typical cereal-derived food processing, such as bread, cake, biscuit, pasta, and beer.
2. Characteristics and Biosynthesis of ZEA and Masked ZEA
2.1. ZEA
ZEA, C18H22O5 (Figure 1), with a molar mass of 318 g/mol, existing as white crystals, can dissolve in alkaline aqueous solutions and some organic solvents such as acetone and ethanol, but is insoluble in water [15]. ZEA exhibits high thermal stability (it is stable up to 150 °C) [16]. ZEA in cereals is produced by Fusarium species, particularly Fusarium graminearum and Fusarium culmorum. Synthesis of ZEA in cereal foods relies on several factors, such as water activity (aw), temperature, incubation time, the composition of storage atmosphere, cultural practices, pH, food substrate, mold abundance, and the effect of anti-fungal agents [17,18]. These factors have a complicated influence on ZEA production, making it difficult to obtain general conclusions about optimal conditions for ZEA production that can be applied to different fungal strains to control ZEA production [18]. Further studies are needed to illustrate the relationship between fungal growth and ZEA production and its molecular mechanisms under the impact of several factors.
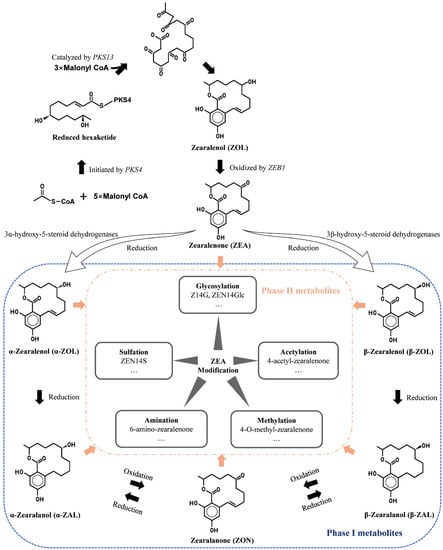
Figure 1.
An overview of ZEA and masked ZEA formation.
Years of research have figured out the synthesis pathways of ZEA [19,20,21,22,23]. Generally, polyketide ZEA is from the oxidation of zearalenol (ZOL), which acts as the precursor deriving from the head-to-tail condensation of nine acetate units via the acetate–polymalonate pathway. Polyketide synthases (PKSs), ZEB genes, and other clustered regulatory genes participate in synthesizing ZEA. A scheme for ZEA synthesis is presented in Figure 1. PKS4 plays a part in initiating ZEA biosynthesis by encoding an enzyme to drive carbon condensation from CoA to form hexaketide. The non-reducing polyketide synthase gene PKS13 catalyzes malonyl-CoA’s connection to the pre-formed reduced hexaketide and folding of non-reduced ketide units. ZEB1 was reported to take part in ZOL oxidation to ZEA via encoding isoamyl alcohol oxidase, and ZEB2 regulated transcription of other genes, which was primarily affected by nutrient and pH conditions [19]. Gaffoor et al. [20] also confirmed that ZEA1 and ZEA2 were indispensable in ZEA production, mainly responsible for carbon addition. Though various PKSs of Fusarium species have been identified, studies on their functions are still scarce.
2.2. Masked ZEA
Masked ZEA refers to zearalenone derivatives produced through a variety of processes. They can be precursors, metabolites, or degradation products of the “parent” (or free) form of the ZEA, or they can be the results of a biotic or chemical reaction between ZEA and the matrix [24,25]. Fusarium graminearum is capable of producing ZEA-sulfate, α-zearalenol (α-ZOL), and β-zearalenol (β-ZOL) [26]. Fusarium and other fungal co-contaminations enhance the likelihood of masked ZEA incidence. In addition, microbial biotransformation, plant response, in vivo metabolism, and food processing are common methods of masked ZEA formation. Rhizopus arrhizus could transform ZEA to ZEA-4-O-sulfate [27]. When co-incubated with Aspergillus and Rhizopus, ZEA was found to convert to several conjugated forms, such as ZEA-14-sulfate (ZEA14S), ZEA-O-14-glucoside (ZEA14Glc), ZEA-O-16-glucoside (ZEA16Glc), α-ZOL, and α-ZOL-sulfate [28]. Plant responses to ZEA modification are considered their defense against xenobiotics during growth. In plants, the modified ZEA (biologically modified) would typically undergo compartmentalization into the vacuole and the cell wall [29]. Diverse plant defenses may enhance the unpredictability of food processing and oral consumption. In vivo metabolism of ZEA in animals can be regarded as a detoxification process, through which ZEA is modified and finally eliminated through urine and feces [30]. ZEA in food can be converted to masked ZEA for complex chemical and biological transformation in food processing.
Although masked ZEA can be generated in multiple ways, the modified ZEA is generally classified into two categories, phase I and phase II metabolites (Figure 1). Phase I conversion means that free ZEA is oxidized, reduced, or hydrolyzed, such as α/β-ZOL, α/β-zearalanol (α/β-ZAL), and zearalanone (ZON). It is usually deemed as a transforming process to higher toxicity for ZEA because of the more estrogen-like structure alteration. Phase II conversion, during which ZEA or phase I metabolites are conjugated with endogenous molecules such as sugars, amino acid, acetic, and sulfate, is regarded as a detoxification process. During these courses, more hydrophilic compounds are synthesized, facilitating the elimination of mycotoxins.
3. Incidence of ZEA and Masked ZEA in Cereal and Cereal-Based Food
ZEA contamination in cereal and cereal-based food has been a long-lasting safety issue. ZEA can be considered as a widespread mycotoxin contaminant, despite its relatively low concentration in the majority of cereals. Research for thirty samples of maize, wheat, oats, and other cereal-derived food revealed an 80% incidence of ZEA [31]. The World Health Organization [32] studied ZEA incidence in crops around the globe and reported that approximately 30–40% of crops are contaminated with ZEA. It was proposed that the rate of ZEA contamination ranked second in cereals and cereal-based food samples, with about 46% of barley as well as 24% of wheat products being contaminated with ZEA [33]. A survey from the EFSA Panel on Contaminants in the Food Chain [34] showed that the frequency of ZEA occurrence in maize reached 33%, with the mean level reaching 15 μg/kg. ZEA occurrence in maize germ oil occupied a higher percentage of 86%, and the mean content was as high as 72 μg/kg. Lee et al. [35] compiled the global occurrence data over the past 10 years and presented that the incidences and maximum levels of ZEA in raw cereal were 46% and 3049 μg/kg, respectively.
The prevalence of ZEA contamination in cereals depends on several factors, e.g., the sample differences, weather fluctuations, and processing [36]. According to published works, grains and animal feed are the items most frequently exposed to ZEA [37,38]. Temperatures that are mild or low are more favorable for ZEA production. Previous work demonstrated that fungi stressed between 8–25 °C were able to produce ZEA [39,40], while ZEA was not generated over 37 °C [41]. The effects of humidity on the development of fungi and mycotoxin were shown to be larger than that of temperature. Fusarium species typically produced ZEA under conditions with a water activity greater than 0.9 [42]. A 10-year global survey suggested that ZEA concentration was positively correlated with precipitation proportion [43]. Stanciu et al. [44] found that ZEA registered the highest frequency with medium-to-high precipitation and moderate temperatures. In addition, a higher CO2 concentration in the atmosphere increased the pathogenicity of fungi and the susceptibility of crops to pathogens [45]. Some pre-harvest and post-harvest procedures, such as rotation, weeding, drying, and hulling, affected the ZEA level in cereals. The persistence of fungi on leftover debris resulted in successive infestation during rotation [46]. Weeds could be alternative hosts for the Fusarium species complex, causing ZEA contamination in cereals [47]. The practices that influenced ZEA existence during processing are discussed in detail in Section 5.
Masked ZEA was also present in cereal and cereal-based foods, although it was not as prevalent as ZEA. Table 1 provides an overview of the incidence of masked ZEA in cereals and cereal-based food. The incidence and mean level of masked ZEA vary greatly among different cereal samples. The phase I metabolites, primarily β-ZOL and α-ZOL, were broadly distributed and rather abundant in cereals. Comparatively, a variety of phase II metabolites were found in several cereals, including maize, wheat, oats, and barley. It is quite probable that cereals contain additional phase II metabolites. It is important to note that some masked ZEA was present in cereal-based food stuffs with high concentrations (e.g., β-ZOL and α-ZOL in bread reached 79 µg/kg and 64 µg/kg, respectively [31]). In light of the possible toxicity of masked ZEA, more data on masked ZEA occurrences in cereal and cereal-based food should be collected to facilitate risk assessment and hazard control of masked ZEA.

Table 1.
Occurrence of masked ZEA in cereals and cereal-based food.
4. Methods of Detection
Chromatographic, immunochemical, and electrochemical detection methods, such as liquid chromatography–tandem mass spectrometry, enzyme-linked immunosorbent assay, and electrochemical biosensors, are extensively employed for the detection and quantification of ZEA [50,51,52,53,54,55].
LC-MS/MS is the most used method for ZEA and masked ZEA detection. The instrumental manner typically includes sample preparation, extraction, sample pretreatment, separation, and determination. Vendl et al. [56] developed an LC-MS/MS method with recoveries above 70% for monitoring the levels of ZEA, zearalenone-4-glucoside, α-ZOL, β-ZOL, α-zearalenol-4-glucoside, and β-zearalenol-4-glucoside in four cereal-based food matrices. This method performs well in terms of accuracy, precision, and repeatability, but has limits for large-scale and on-site rapid analysis due to its complex analytical methodologies, high cost, and need for analysts. Moreover, despite the fact that several masked ZEAs, such as α/β-ZOL, zearalenone-4/14/16-glucoside, and zearalenone-4/14-sulfate, were found in cereal samples using LC-MS/MS, analysis for masked ZEA remains challenging because of the occurrence of several unknown forms of masked ZEA and the lack of analytical standards and accurate LC-MS/MS methods [56].
Immunoassay can evaluate multiple food samples concurrently for masked ZEA detection, which is considerably quicker but less accurate than LC-MS/MS. Preparation of the sample, extraction, hydrolysis, and determination are typically the steps in the procedure. Beloglazova et al. [57] first established an immunochemical approach for ZEA and zearalenone-4-glucoside detection in cereal samples. Hou et al. [58] developed a spherical colloidal gold-based immunochromatographic test strip that could rapidly and precisely monitor the presence of ZEA in grains with a visual detection limit of 6 ng/mL. Immunoassay is quick, high-throughput, practical, and inexpensive. However, it can only provide qualitative or (semi-)quantitative results, necessitating a follow-up confirmatory analysis [59].
Electrochemical biosensors, characterized by high sensitivity, selectivity, cost-effectiveness, and simplicity, are extensively applied for ZEA detection. In addition to direct identification, the recognition elements of electrochemical approaches frequently include antibody, aptamer, and dsDNA, with antibodies and aptamers being the most common receptors [55,60,61,62]. The majority of electrochemical ZEA biosensors are electrochemical immunosensors based on high affinity interactions between antigens and antibodies [63]. The electrochemical immunosensors were applied to multiple cereals and cereal-based samples, including maize, wheat, and others, spanning a broad range of detection from ng/mL to mg/mL. Aptamers are chosen over antibodies for ZEA detection due to their superior sensitivity and specificity, as well as their ease of synthesis, regeneration, and chemical modification [55]. Ji et al. [61] proved that an aptamer-based electrochemical biosensor has a detection range of 1 fg/mL to 100 ng/mL. Currently, there are two aptamers for ZEA determination, and interference from complex dietary matrices poses the greatest challenge for molecular diagnostic components [55]. With additional advancements in surface immobilization and aptamer-based biosensors, electrochemical biosensors are projected to become a more reliable analytical platform for ZEA and masked ZEA detection.
5. Fate of ZEA in Cereal-Based Food Processing
In addition to field and storage contamination, food processing plays a crucial role in ZEA transformation and content fluctuation. This section focuses primarily on the fate of ZEA throughout the processing of various typical cereal-based foods, including bread, biscuits, cake, pasta, and beer, in order to provide a thorough explanation of ZEA alterations and a reference for maintaining the safety of cereal-based foods.
5.1. Bread
After the mixed dough has been prepared, the remaining steps in bread processing include kneading, fermentation, and baking (Figure 2). Existing literature has proved that fermentation and baking are the key determinants of ZEA content changes during the bread-making process. To replicate the natural contamination of ZEA, artificially contaminated flour is commonly employed. The majority of studies confirmed that fermentation induced ZEA reduction in bread production. A meta-analysis that compiled research from 1983 to 2017 revealed that dough fermentation could reduce ZEA level by around 3% [64]. Heidari et al. [65] similarly discovered lower ZEA levels in flour following fermentation, with a higher decrease in the first proof due to the faster growth of Saccharomyces cerevisiae (S. cerevisiae). Yeasts involved in fermentation may create an acid environment that promotes the transformation and degradation of ZEA. Therefore, the type of yeast used during fermentation is crucial for ZEA reduction. Compressed yeast was considerably more effective than instant dry yeast under identical processing circumstances [65]. The higher destruction of yeast may be due to two aspects. The first is that it can produce more CO2, resulting in a reduction in pH, the development of various destructive substances, such as acids, alcohols, and enzymes, and a prolonged fermentation period. The second is the performance of yeast cell wall adsorption. It was proven that the adsorption capacity of yeast depends on the mycotoxin concentrations [66]. This concentration-dependent relationship was also observed in the bread-making process, where the initial ZEA concentration in the dough was proportional to the decrease efficiency of S. cerevisiae [67]. However, Cano-Sancho et al. [68] reported no statistical difference in ZEA levels between the dough and fermented dough at 25 °C in wheat bread production, which may be attributable to the initially low ZEA contaminated level of wheat flour (0.319 ± 0.281 μg/g).
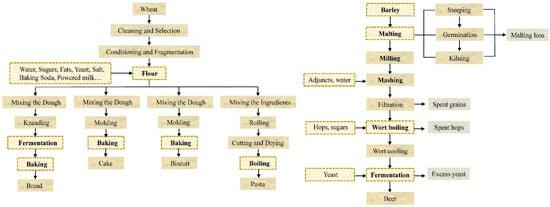
Figure 2.
Steps of wheat and beer processing. Steps affecting zearalenone content during food processing were marked with dotted line.
Concerning the baking process in bread production, changes in ZEA content are controversial. Bol et al. [69] examined ZEA levels in flour and bakery products after thermal processing and pointed out that ZEA decreased by about 89% after baking at 220 °C for 35 min, indicating that heat treatment was effective for ZEA reduction. Heidari [65] declared temperature and processing time as the most important factors in declining ZEA, provided that ZEA conjugates were formed during bread production. Gilbert et al. [70] suggested that around 40% of ZEA was lost after bread baking when the dough was contaminated with a high level of ZEA. However, other researchers presented that heat treating failed to reduce ZEA concentration, and even increased it [12,71]. Cano-Sancho et al. [68] determined the constant ZEA concentration after 20 min of baking at 200 °C. Results of the maize bread baking experiment by Numanoglu et al. [72] showed inconsistent results: baking at 250 °C for 70 min generated a little increase in ZEA (3.3%) in the crumb, but thermal treatment reduced ZEA by 13% in the crust. This result was also consistent with their previous research on ZEA content fluctuations in traditional Turkish maize bread [73]. Since ZEA is somewhat heat stable, it cannot be anticipated to degrade significantly after moderate thermal processing in the absence of a reaction that would produce masked ZEA. Numerous factors, such as the initial ZEA concentration of contaminated flour, baking temperature and duration, and masked ZEA formation and degradation, are hypothesized to influence ZEA concentration during the baking process. Increased masked ZEA content in bread is possible as a result of decreased ZEA level after heat treatment. Several masked ZEAs, including β-ZOL, α-ZOL, ZEA-4-sulfate, and ZEA-4-glucoside, have been identified in bread (Table 1). Alternatively, masked ZEA in flour can break down and release free ZEA when heated. Bryla et al. [74] approved that Z14G and Z14S concentrations were reduced by 42% and 48%, respectively, during fermentation and baking of malt loaf production. However, the cooccurrence of ZEA and masked ZEA, as well as their changes during food processing, remain understudied, hence increasing food safety issues. To improve hazard control, it is vital to monitor the changes in ZEA and masked ZEA at various production steps.
5.2. Biscuit and Cake
The ingredients used to make biscuits and cake are almost identical to those used to make bread, but unlike bread, fermentation is not a stage that is required to make biscuits and cake (Figure 2). Therefore, research on changes in ZEA concentration during the process considers the effects of ingredients and baking.
Scudamore et al. [75] studied the impact of flour content on ZEA levels in two biscuits throughout processing. It was discovered that the ZEA content of semisweet biscuits comprising 70% flour decreased by 27.5% when fat and sugar were added, whereas the ZEA content of crackers having nearly 90% flour remained unchanged after baking [75]. Following 15 min of baking at 190 to 200 °C with 3% baking powder added to the dough, ZEA levels decreased by between 16% and 27% [76]. It was stated that the dilution effects were the likely mechanisms of ZEA reduction caused by component addition [69].
Another key factor in the production of cakes and biscuits that affects changes in ZEA level is baking. A previous study proved that baking led to 93% and 84% ZEA loss during the production of cake and biscuits, respectively, and that a higher baking temperature of 270 °C was more conducive to ZEA reduction than a lower temperature of 170 °C [69]. However, ZEA reduction cannot be achieved alone with heating treatment. In a different study conducted by Bol et al. [69], the most substantial drop of ZEA occurred in cakes with a high flour proportion of 75%, and it was found that liposoluble ZEA was probably easier to solubilize in cakes with a high oil content and low amount of water, hence promoting ZEA decomposition in baking. Furthermore, the addition of either ammonium persulfate (0.03%) or kansui (1% potassium carbonate) considerably accelerated the degradation of ZEA during heating [76].
In conclusion, these results suggested that biscuit and cake ingredients play a significant influence in ZEA level variations, and that baking led to ZEA reduction in biscuit and cake processing. It is still unclear, though, how adding substances such as oil, sugar, and baking powder reduced the ZEA concentration. The thermal treatment of bread, biscuits, and cake had comparable effects on ZEA concentrations. Although no study has reported the presence of masked ZEA in biscuit and cake, it is probable that during the baking process, ZEA reacts with other components to generate masked ZEA in dough. To further reduce masked ZEA contamination, understanding of masked ZEA occurrence in biscuit and cake processing is necessary.
5.3. Pasta
The preparatory stages for producing pasta resemble those for preparing bread, cake, and biscuits (Figure 2). Before being brought to market, the mixed dough is rolled, cut, and dried. The operations of rolling and cutting influence the shape of pasta, while drying reduces its moisture content. While boiling was discovered to promote ZEA reduction in pasta, no studies have shown that rolling or drying during the pasta-making process influences the level of ZEA. Therefore, this section focuses mostly on the impact of boiling on ZEA alterations of pasta.
Bol et al. [69] found that ZEA significantly decreased by 75% after 15 min of cooking, with around 10% leaching into the cooking water. Adding 1% potassium carbonate to instant noodles spiked with 1 and 20 μg/kg ZEA and heating at 100 °C for 3 min, followed by 140–150 °C for 2 min, degraded ZEA by approximately 48% and 62%, respectively [76]. Boiling is effective for ZEA reduction, and the decomposition efficiency appears to be dependent on the initial ZEA level and temperature. Similar to the bread-making process, pasta with a higher initial ZEA contamination concentration may see more toxin reduction after boiling. However, compared to bread, biscuit, and cake, pasta requires a different heating mode and temperature. Cooking pasta in boiling water required a temperature between 85 and 98 °C, whereas baking bread in an oven required a much higher temperature, such as 220 °C. Considering the thermal stability of ZEA, it is possible that the mycotoxin is more stable in boiling than in baking. To maintain the safety of pasta, it is important to ensure the quality of the ingredients, which is the primary source of ZEA contamination. In addition, more research is required to better understand the mechanisms underlying ZEA level variations and masked ZEA incidence during the drying and boiling processes.
5.4. Beer
The primary ingredient in beer is barley, along with water, malt, hops, and various additives such as maize, oats, and sorghum. ZEA can easily contaminate the raw materials of beer, hence increasing the likelihood of beer contamination. Recent studies have shown that some brewing processes affect ZEA and masked ZEA levels. Figure 2 illustrates the various steps involved in brewing beer.
The malting process includes germination and kilning. To encourage germination, barley is steeped for 36–52 h at 12–20 °C, immersed in aerated water, and exposed to humified air to increase the moisture content to approximately 45%. A further kilning is intended to reduce the moisture content of germinating barley to roughly 4~5%. Plentiful chemical reactions occur during the kilning step, which has a significant impact on the color, odor, flavor, and texture of the beer. Piacentini et al. [77] found that ZEA concentrations on the third day of germination were greater than those on the first day of steeping, indicating that ZEA production was greater during germination. Due to ZEA conversion to α-zearalenol, β-zearalenol, and other masked toxins, the level of ZEA in the malting process was reduced by around 79% [77]. Pascari et al. [78] discovered that following a 40% loss of ZEA in the first steeping water, the ZEA level in barley increased during the kilning process, leading to a constant ZEA concentration in the malting stages. Similarly, another study demonstrated the same variations in ZEA levels during the steeping process, but the ZEA content rose during germination and kilning [79]. El-Banna [80] found that malting might severely degrade ZEA, although Wall-Martínez et al. [81] asserted that malting had no effect on ZEA. Since barley is vulnerable to scab and is easily contaminated with ZEA, the initial ZEA concentration controls the volatility of ZEA level throughout malting. Tabuc et al. [82] investigated ZEA concentration in 21 malting barleys in the southeastern part of Romania and found that ZEA contaminated nearly 71.4% of barleys, with an average concentration of 132.7 μg/kg. ZEA in contaminated barley would likely be released during the malting process, resulting in an increase in ZEA concentration. Alternatively, ZEA may change into α-zearalenol, β-zearalenol, and other masked toxins during malting, resulting in a decrease in ZEA.
The malted barley was then milled and mashed, and some additive adjuncts were added (Figure 2). After filtration, the wort was boiled at a high temperature, and most ZEA decreased during the process. More than 89% of ZEA was shown to be removed by mashing and boiling [81]. According to a study by Pascari et al. [83], 30 min of boiling resulted in a 100% reduction in ZEA. Longer boiling time promoted ZEA degradation but may diminish nutritional quality [84]. High heat tolerance of ZEA and the inclusion of adjuncts such hops, corn, wheat, and sorghum might result in stable or increased ZEA content after boiling [77,85]. Changes in the ZEA concentration during fermentation are likewise contentious. GILBER [70] proposed that ZEA remained constant during fermentation, whereas Wall-Martínez et al. [86] found that 30~70% of ZEA was degraded following fermentation in commercial beer due to yeast cell adsorption. A study revealed that ZEA in wort varied from 26 to 285 μg/L and from 20 to 201 μg/L in beer, with a carry-over ranging from 23 to 403% [87]. The high carry-over could be ascribed to the addition of unfermented wort after fermentation, leading to an increased ZEA level. In addition, the transformation of ZEA to masked ZEA during fermentation may contribute to the decrease in ZEA concentration. Scott et al. [88] confirmed that in the first 1–2 days of fermentation, Saccharomyces cerevisiae metabolized 69% of ZEA to β-zearalenol and 8.1% of ZEA to α-zearalenol. It was observed that 85.9% of ZEA was converted to β-zearalenol in artificially ZEA-contaminated fermented wort [89]. Chilaka et al. [90] found that α-zearalenol and β-zearalenol were present in beer at concentrations of 22 ± 18 and 31 ± 16 μg/kg, respectively, although masked ZEA did not contaminate raw sorghum.
In conclusion, these results suggested that four steps (adding raw material and adjuncts, malting, boiling and fermentation) in the beer-making process significantly affect the occurrence of ZEA and masked ZEA in beer. The most important factor is the level of ZEA contamination in the raw materials and adjuncts, which determines the presence of ZEA and masked ZEA in the final product. Malting and fermentation promoted ZEA transformation to masked forms. However, during these processes, complex biochemical reactions occur, resulting in the controversial statement regarding ZEA content fluctuations. Due to the thermal stability of ZEA and the addition of adjuncts, variations in ZEA concentration during boiling are unexpected. To minimize masked ZEA development as well as nutritional and flavor loss, it is necessary to conduct additional research on the fate of ZEA in the beer-making process.
6. Allowable Limit of ZEA in Cereal and Cereal-Based Food
The maximum limit of ZEA in cereal and cereal-based food has been established in various countries (Table 2). The allowable residue levels of ZEA vary greatly from country to country. The European Union set the maximum residue levels for wheat at 100 µg/kg, however, in Armenia, Colombia, Russia, and other countries, the maximum residue levels reached 1000 µg/kg. The difference may be attributed to the availability of toxicological data, information on dietary exposure, the distribution of mycotoxins across products, the regulations of other nations with which there are trade contacts, and the availability of analytical methods [91]. However, several nations have neither specified maximum levels nor guidance limits for ZEA in cereals and cereal-based food. In addition, the presence of masked ZEA was excluded from the regulation. Considering the incidence of both ZEA and masked ZEA, it is suggested that future legislation incorporate masked ZEA.

Table 2.
Maximum limits for zearalenone in cereals and cereal products in various countries.
7. Conclusions
In conclusion, ZEA is a prevalent mycotoxin in cereals, but little data have reported the presence of masked ZEA (particularly phase II metabolites) to date. The co-occurrence of ZEA and masked ZEA in cereals has received little attention. During the processing of cereal-based foods, certain operations resulted in ZEA level variations and masked ZEA formation. Co-contamination of ZEA and masked ZEA is of particular concern due to potential additive or synergistic health effects. Regarding this topic, the primary future challenge includes: (1) increasing the number of analyzed masked ZEA in cereals; (2) improving knowledge on the effects of cereal-based food processing on the incidence of ZEA and masked ZEA; (3) developing rapid and precise methods for simultaneous detection of ZEA and masked ZEA; (4) risk assessments for the ZEA and masked ZEA that are ordinarily regarded as negligible; (5) revising the legislative limits of masked ZEA to ensure the greatest protection of human health.
Author Contributions
Conceptualization: H.Y.; Writing—original draft preparation and editing: H.Y., J.Z. (Junhui Zhang), Y.C.; Project administration: J.Z. (Jiajin Zhu). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fast, R.B.; Caldwell, E.F. Breakfast Cereals, and How They Are Made; American Association of Cereal Chemists: Eagan, MN, USA, 2000. [Google Scholar]
- Nematollahi, A.; Kamankesh, M.; Hosseini, H.; Ghasemi, J.; Hosseini-Esfahani, F.; Mohammadi, A. Investigation and determination of acrylamide in the main group of cereal products using advanced microextraction method coupled with gas chromatography-mass spectrometry. J. Cereal Sci. 2019, 87, 157–164. [Google Scholar] [CrossRef]
- Faltermaier, A.; Waters, D.; Becker, T.; Arendt, E.; Gastl, M. Common wheat (Triticum aestivum L.) and its use as a brewing cereal—A review. J. Inst. Brew. 2014, 120, 1–15. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Martins, L.M.; von Hertwig, A.M.; Bertoldo, R.; Sant’Ana, A.S. Deoxynivalenol and its masked forms: Characteristics, incidence, control and fate during wheat and wheat based products processing—A review. Trends Food Sci. Technol. 2018, 71, 13–24. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Global Cereal Trade and Utilization in 2021/22 Revised Down. 2022. Available online: https://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 9 April 2022).
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajslova, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Imran, M.; Cao, S.; Wan, S.; Chen, Z.; Saleemi, M.K.; Wang, N.; Naseem, M.; Munawar, J. Mycotoxins–a global one health concern: A review. Agrobiol. Rec. 2020, 2, 1–16. [Google Scholar] [CrossRef]
- Alborch, L.; Bragulat, M.R.; Castella, G.; Abarca, M.L.; Cabanes, F.J. Mycobiota and mycotoxin contamination of maize flours and popcorn kernels for human consumption commercialized in Spain. Food Microbiol. 2012, 32, 97–103. [Google Scholar] [CrossRef]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef]
- Waskiewicz, A.; Gromadzka, K.; Wisniewska, H.; Golinski, P. Accumulation of zearalenone in genotypes of spring wheat after inoculation with Fusarium culmorum. Cereal Res. Commun. 2008, 36, 401–403. [Google Scholar]
- De Boevre, M.; Landschoot, S.; Audenaert, K.; Maene, P.; Di Mavungu, J.D.; Eeckhout, M.; Haesaert, G.; De Saeger, S. Occurrence and within field variability of Fusarium mycotoxins and their masked forms in maize crops in Belgium. World Mycotoxin J. 2014, 7, 91–102. [Google Scholar] [CrossRef]
- Paris, M.P.K.; Schweiger, W.; Hametner, C.; Stuckler, R.; Muehlbauer, G.J.; Varga, E.; Krska, R.; Berthiller, F.; Adam, G. Zearalenone-16-O-glucoside: A New Masked Mycotoxin. J. Agric. Food Chem. 2014, 62, 1181–1189. [Google Scholar] [CrossRef]
- Gratz, S.W.; Dinesh, R.; Yoshinari, T.; Holtrop, G.; Richardson, A.J.; Duncan, G.; MacDonald, S.; Lloyd, A.; Tarbin, J. Masked trichothecene and zearalenone mycotoxins withstand digestion and absorption in the upper GI tract but are efficiently hydrolyzed by human gut microbiota in vitro. Mol. Nutr. Food Res. 2017, 61, 10. [Google Scholar] [CrossRef] [PubMed]
- Olopade, B.K.; Oranusi, S.U.; Nwinyi, O.C.; Gbashi, S.; Njobeh, P.B. Occurrences of deoxynivalenol, zearalenone and some of their masked forms in selected cereals from Southwest Nigeria. NFS J. 2021, 23, 24–29. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Appropriateness to set a group health-based guidance value for zearalenone and its modified forms. EFSA J. 2016, 14, 4425. [Google Scholar]
- Poor, M.; Kunsagi-Mate, S.; Balint, M.; Hetenyi, C.; Gerner, Z.; Lemli, B. Interaction of mycotoxin zearalenone with human serum albumin. J. Photochem. Photobiol. B-Biol. 2017, 170, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Malekinejad, H.; Maas-Bakker, R.F.; Fink-Gremmels, J. Enzyme kinetics of zearalenone biotransformation: pH and cofactor effects. Arch. Toxicol. 2005, 79, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Lahouar, A.; Marin, S.; Crespo-Sempere, A.; Said, S.; Sanchis, V. Influence of temperature, water activity and incubation time on fungal growth and production of ochratoxin A and zearalenone by toxigenic Aspergillus tubingensis and Fusarium incarnatum isolates in sorghum seeds. Int. J. Food Microbiol. 2017, 242, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Lee, Y.R.; Jin, J.M.; Han, K.H.; Kim, H.; Kim, J.C.; Lee, T.; Yun, S.H.; Lee, Y.W. Two different polyketide synthase genes are required for synthesis of zearalenone in Gibberella zeae. Mol. Microbiol. 2005, 58, 1102–1113. [Google Scholar] [CrossRef]
- Gaffoor, I.; Trail, F. Characterization of two polyketide synthase genes involved in zearalenone biosynthesis in Gibberella zeae. Appl. Environ. Microbiol. 2006, 72, 1793–1799. [Google Scholar] [CrossRef]
- Lysoe, E.; Bone, K.R.; Klemsdal, S.S. Real-Time Quantitative Expression Studies of the Zearalenone Biosynthetic Gene Cluster in Fusarium graminearum. Phytopathology 2009, 99, 176–184. [Google Scholar] [CrossRef]
- Lysoe, E.; Bone, K.R.; Klemsdal, S.S. Identification of up-regulated genes during zearalenone biosynthesis in Fusarium. Eur. J. Plant Pathol. 2008, 122, 505–516. [Google Scholar] [CrossRef]
- Nahle, S.; El Khoury, A.; Atoui, A. Current status on the molecular biology of zearalenone: Its biosynthesis and molecular detection of zearalenone producing Fusarium species. Eur. J. Plant Pathol. 2021, 159, 247–258. [Google Scholar] [CrossRef]
- DallAsta, C.; Berthiller, F. Masked mycotoxins in food: Formation, occurrence and toxicological relevance. R. Soc. Chem. 2016, 24, 1–13. [Google Scholar]
- Kovac, M.; Subaric, D.; Bulaic, M.; Kovac, T.; Sarkanj, B. Yesterday masked, today modified; what do mycotoxins bring next? Arh. Hig. Rada. Toksikol. 2018, 69, 196–214. [Google Scholar] [CrossRef] [PubMed]
- Yerkovich, N.; Palazzini, J.M.; Sulyok, M.; Chulze, S.N. Trichothecene genotypes, chemotypes and zearalenone production by Fusarium graminearum species complex strains causing Fusarium head blight in Argentina during an epidemic and non-epidemic season. Trop. Plant Pathol. 2017, 42, 190–196. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef]
- Brodehl, A.; Moller, A.; Kunte, H.J.; Koch, M.; Maul, R. Biotransformation of the mycotoxin zearalenone by fungi of the genera Rhizopus and Aspergillus. FEMS Microbiol. Lett. 2014, 359, 124–130. [Google Scholar] [CrossRef]
- Mahato, D.K.; Devi, S.; Pandhi, S.; Sharma, B.; Maurya, K.K.; Mishra, S.; Dhawan, K.; Selvakumar, R.; Kamle, M.; Mishra, A.K.; et al. Occurrence, Impact on Agriculture, Human Health, and Management Strategies of Zearalenone in Food and Feed: A Review. Toxins 2021, 13, 92. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- De Boevre, M.; Di Mavungu, J.D.; Maene, P.; Audenaert, K.; Deforce, D.; Haesaert, G.; Eeckhout, M.; Callebaut, A.; Berthiller, F.; Van Peteghem, C.; et al. Development and validation of an LC-MS/MS method for the simultaneous determination of deoxynivalenol, zearalenone, T-2-toxin and some masked metabolites in different cereals and cereal-derived food. Food Addit. Contam. Part A-Chem. 2012, 29, 819–835. [Google Scholar] [CrossRef]
- World Health Organization. Evaluation of Certain Food Additives and Contaminants; WHO Technical Report Series; WHO: Geneva, Switzerland, 2001; 896p. [Google Scholar]
- Kirincic, S.; Skrjanc, B.; Kos, N.; Kozolc, B.; Pirnat, N.; Tavcar-Kalcher, G. Mycotoxins in cereals and cereal products in Slovenia—Official control of foods in the years 2008–2012. Food Control 2015, 50, 157–165. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin Contamination in the EU Feed Supply Chain: A Focus on Cereal Byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef]
- Ropejko, K.; Twaruzek, M. Zearalenone and Its Metabolites-General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef]
- Colovic, R.; Puvaca, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Duragic, O.; Kos, J.; Pinotti, L. Decontamination of Mycotoxin-Contaminated Feedstuffs and Compound Feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef]
- Gupta, R.C.; Mostrom, M.S.; Evans, T.J. Zearalenone. In Veterinary Toxicology; Academic Press: Cambrdige, MA, USA, 2018; pp. 1055–1063. [Google Scholar]
- Manstretta, V.; Rossi, V. Effects of Temperature and Moisture on Development of Fusarium graminearum Perithecia in Maize Stalk Residues. Appl. Environ. Microbiol. 2016, 82, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Aggarwal, A.; Paul, S.; Singh, V.; Singh, P.K.; Sharma, D.; Shaharan, M.S. Effect of temperature and pH on the growth of Fusarium spp. causing Fusarium head blight (FHB) in wheat. South Asian J. Exp. Biol 2016, 6, 186–193. [Google Scholar] [CrossRef]
- Edwards, S.G. Zearalenone risk in European wheat. World Mycotoxin J. 2011, 4, 433–438. [Google Scholar] [CrossRef]
- Han, X.; Huangfu, B.X.; Xu, T.X.; Xu, W.T.; Asakiya, C.; Huang, K.L.; He, X.Y. Research Progress of Safety of Zearalenone: A Review. Toxins 2022, 14, 386. [Google Scholar] [CrossRef]
- Stanciu, O.; Juan, C.; Berrada, H.; Miere, D.; Loghin, F.; Manes, J. Study on Trichothecene and Zearalenone Presence in Romanian Wheat Relative to Weather Conditions. Toxins 2019, 11, 163. [Google Scholar] [CrossRef]
- Marroquin-Cardona, A.G.; Johnson, N.M.; Phillips, T.D.; Hayes, A.W. Mycotoxins in a changing global environment—A review. Food Chem. Toxicol. 2014, 69, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.; Erbs, M.; Forrer, H.R.; Vogelgsang, S.; Wettstein, F.E.; Schwarzenbach, R.P.; Bucheli, T.D. Occurrence of zearalenone on Fusarium graminearum infected wheat and maize fields in crop organs, soil, and drainage water. Environ. Sci. Technol. 2008, 42, 5455–5460. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Xu, J.H.; Zhang, X.; Wang, S.F.; Xing, Y.J.; Mokoena, M.P.; Olaniran, A.O.; Shi, J.R. Gramineous weeds near paddy fields are alternative hosts for the Fusarium graminearum species complex that causes fusarium head blight in rice. Plant Pathol. 2020, 69, 433–441. [Google Scholar] [CrossRef]
- Nathanail, A.V.; Syvahuoko, J.; Malachova, A.; Jestoi, M.; Varga, E.; Michlmayr, H.; Adam, G.; Sievilainen, E.; Berthiller, F.; Peltonen, K. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal. Bioanal. Chem. 2015, 407, 4745–4755. [Google Scholar] [CrossRef] [PubMed]
- Bryla, M.; Waskiewicz, A.; Podolska, G.; Szymczyk, K.; Jedrzejczak, R.; Damaziak, K.; Sulek, A. Occurrence of 26 Mycotoxins in the Grain of Cereals Cultivated in Poland. Toxins 2016, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Majerus, P.; Graf, N.; Kramer, M. Rapid determination of zearalenone in edible oils by HPLC with fluorescence detection. Mycotoxin Res. 2009, 25, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Pallaroni, L.; Bjorklund, E.; von Holst, C. Optimization of atmospheric pressure chemical ionization interface parameters for the simultaneous determination of deoxynivalenol and zearalenone using HPLC/MS. J. Liq. Chromatogr. Relat. Technol. 2002, 25, 913–926. [Google Scholar] [CrossRef]
- Biselli, S.; Wegner, H.; Hummert, C. A multicomponent method for Fusarium toxins in cereal based food and feed samples using HPLC-MS/MS. Mycotoxin Res. 2005, 21, 18–22. [Google Scholar] [CrossRef]
- Changwa, R.; Abia, W.; Msagati, T.; Nyoni, H.; Ndleve, K.; Njobeh, P. Multi-Mycotoxin Occurrence in Dairy Cattle Feeds from the Gauteng Province of South Africa: A Pilot Study Using UHPLC-QTOF-MS/MS. Toxins 2018, 10, 294. [Google Scholar] [CrossRef]
- Burmistrova, N.A.; Goryacheva, I.Y.; Basova, E.Y.; Franki, A.S.; Elewaut, D.; Van Beneden, K.; Deforce, D.; Van Peteghem, C.; De Saeger, S. Application of a new anti-zearalenone monoclonal antibody in different immunoassay formats. Anal. Bioanal. Chem. 2009, 395, 1301–1307. [Google Scholar] [CrossRef]
- Caglayan, M.O.; Sahin, S.; Ustundag, Z. Detection Strategies of Zearalenone for Food Safety: A Review. Crit. Rev. Anal. Chem. 2022, 52, 294–313. [Google Scholar] [CrossRef] [PubMed]
- Vendl, O.; Berthiller, F.; Crews, C.; Krska, R. Simultaneous determination of deoxynivalenol, zearalenone, and their major masked metabolites in cereal-based food by LC-MS-MS. Anal. Bioanal. Chem. 2009, 395, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Beloglazova, N.V.; De Boevre, M.; Goryacheva, I.Y.; Werbrouck, S.; Guo, Y.; De Saeger, S. Immunochemical approach for zearalenone-4-glucoside determination. Talanta 2013, 106, 422–430. [Google Scholar] [CrossRef]
- Hou, S.L.; Ma, J.J.; Cheng, Y.Q.; Wang, H.G.; Sun, J.H.; Yan, Y.X. One-step rapid detection of fumonisin B-1, dexyonivalenol and zearalenone in grains. Food Control 2020, 117, 7. [Google Scholar] [CrossRef]
- De Rycke, E.; Foubert, A.; Dubruel, P.; Bol’hakov, O.I.; De Saeger, S.; Beloglazova, N. Recent advances in electrochemical monitoring of zearalenone in diverse matrices. Food Chem. 2021, 353, 8. [Google Scholar] [CrossRef]
- Riberi, W.I.; Tarditto, L.V.; Zon, M.A.; Arevalo, F.J.; Fernandez, H. Development of an electrochemical immunosensor to determine zearalenone in maize using carbon screen printed electrodes modified with multi-walled carbon nanotubes/polyethyleneimine dispersions. Sens. Actuator B-Chem. 2018, 254, 1271–1277. [Google Scholar] [CrossRef]
- Ji, X.D.; Yu, C.; Wen, Y.L.; Chen, J.; Yu, Y.J.; Zhang, C.L.; Gao, R.F.; Mu, X.Y.; He, J.L. Fabrication of pioneering 3D sakura-shaped metal-organic coordination polymers Cu@L-Glu phenomenal for signal amplification in highly sensitive detection of zearalenone. Biosens. Bioelectron. 2019, 129, 139–146. [Google Scholar] [CrossRef]
- Sadrabadi, N.R.; Ensafi, A.A.; Heydari-Bafrooei, E.; Fazilati, M. Screening of Food Samples for Zearalenone Toxin Using an Electrochemical Bioassay Based on DNA-Zearalenone Interaction. Food Anal. Meth. 2016, 9, 2463–2470. [Google Scholar] [CrossRef]
- Afzali, D.; Padash, M.; Mostafavi, A. Determination of trace amounts of zearalenone in beverage samples with an electrochemical sensor. Mycotoxin Res. 2015, 31, 203–208. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Fakhri, Y.; Sant’Ana, A.S. Impact of unit operations during processing of cereal-based products on the levels of deoxynivalenol, total aflatoxin, ochratoxin A, and zearalenone: A systematic review and meta-analysis. Food Chem. 2018, 268, 611–624. [Google Scholar] [CrossRef]
- Heidari, S.; Milani, J.; Nazari, S. Effect of the bread-making process on zearalenone levels. Food Addit. Contam. Part A-Chem. 2014, 31, 2047–2054. [Google Scholar] [CrossRef] [PubMed]
- Joannis-Cassan, C.; Tozlovanu, M.; Hadjeba-Medjdoub, K.; Ballet, N.; Pfohl-Leszkowicz, A. Binding of Zearalenone, Aflatoxin B-1, and Ochratoxin A by Yeast-Based Products: A Method for Quantification of Adsorption Performance. J. Food Prot. 2011, 74, 1175–1185. [Google Scholar] [CrossRef]
- El-Desouky, T.A.; Amer, M.M.; Naguib, K. Effects of pan bread making on zearalenone levels in artificial contaminated wheat flour. J. Agroaliment. Process. Technol. 2014, 20, 269–274. [Google Scholar]
- Cano-Sancho, G.; Sanchis, V.; Ramos, A.J.; Marin, S. Effect of food processing on exposure assessment studies with mycotoxins. Food Addit. Contam. Part A-Chem. 2013, 30, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Bol, E.K.; Araujo, L.; Veras, F.F.; Welke, J.E. Estimated exposure to zearalenone, ochratoxin A and aflatoxin B1 through the consume of bakery products and pasta considering effects of food processing. Food Chem. Toxicol. 2016, 89, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J. Current views on the occurrence and significance of Fusarium toxins. J. Appl. Bacteriol. Symp. Suppl. 1989, 67, 89S–98S. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Fakhri, Y.; Raeisi, S.; Armoon, B.; Sant’Ana, A.S. Prevalence and concentration of ochratoxin A, zearalenone, deoxynivalenol and total aflatoxin in cereal-based products: A systematic review and meta-analysis. Food Chem. Toxicol. 2018, 118, 830–848. [Google Scholar] [CrossRef]
- Numanoglu, E.; Yener, S.; Gokmen, V.; Uygun, U.; Koksel, H. Modelling thermal degradation of zearalenone in maize bread during baking. Food Addit. Contam. Part A-Chem. 2013, 30, 528–533. [Google Scholar] [CrossRef]
- Numanoglu, E.; Uygun, U.; Koksel, H.; Solfrizzo, M. Stability of Fusarium toxins during traditional Turkish maize bread production. Qual. Assur. Saf. Crop. Foods 2010, 2, 84–92. [Google Scholar] [CrossRef]
- Bryla, M.; Ksieniewicz-Wozniak, E.; Waskiewicz, A.; Yoshinari, T.; Szymczyk, K.; Podolska, G.; Gwiazdowski, R.; Kubiak, K. Transformations of Selected Fusarium Toxins and Their Modified Forms During Malt Loaf Production. Toxins 2020, 12, 385. [Google Scholar] [CrossRef]
- Scudamore, K.A.; Hazel, C.M.; Patel, S.; Scriven, F. Deoxynivalenol and other Fusarium mycotoxins in bread, cake, and biscuits produced from UK-grown wheat under commercial and pilot scale conditions. Food Addit. Contam. Part A-Chem. 2009, 26, 1191–1198. [Google Scholar] [CrossRef]
- Matsuura, Y.; Yoshizawa, T.; Morooka, N. Effects of food additives and heating on the decomposition of zearalenone in wheat flour. J. Food Hyg. Soc. Jpn. Shokuhin Eiseigaku Zasshi 1981, 22, 293–298. [Google Scholar] [CrossRef]
- Piacentini, K.C.; Belakova, S.; Benesova, K.; Pernica, M.; Savi, G.D.; Rocha, L.O.; Hartman, I.; Caslavsky, J.; Correa, B. Fusarium Mycotoxins Stability during the Malting and Brewing Processes. Toxins 2019, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Pascari, X.; Gil-Samarra, S.; Marin, S.; Ramos, A.J.; Sanchis, V. Fate of zearalenone, deoxynivalenol and deoxynivalenol-3-glucoside during malting process. LWT-Food Sci. Technol. 2019, 99, 540–546. [Google Scholar] [CrossRef]
- Schwarz, P.B.; Casper, H.H.; Beattie, S. Fate and Development of Naturally Occurring Fusarium Mycotoxins During Malting and Brewing. J. Am. Soc. Brew. Chem. 1995, 53, 121–127. [Google Scholar]
- El-Banna, A.A. Stability of citrinin and deoxynivalenol during germination process of barley. Mycotoxin Res. 1987, 3, 37–41. [Google Scholar] [CrossRef]
- Wall-Martinez, H.A.; Pascari, X.; Bigorda, A.; Ramos, A.J.; Marin, S.; Sanchis, V. The fate of Fusarium mycotoxins (deoxynivalenol and zearalenone) through wort fermenting by Saccharomyces yeasts (S. cerevisiae and S. pastorianus). Food Res. Int. 2019, 126, 8. [Google Scholar] [CrossRef]
- Tabuc, C.; Marin, D.; Guerre, P.; Sesan, T.; Bailly, J.D. Molds and Mycotoxin Content of Cereals in Southeastern Romania. J. Food Prot. 2009, 72, 662–665. [Google Scholar] [CrossRef]
- Pascari, X.; Rodriguez-Carrasco, Y.; Juan, C.; Manes, J.; Marin, S.; Ramos, A.J.; Sanchis, V. Transfer of Fusarium mycotoxins from malt to boiled wort. Food Chem. 2019, 278, 700–710. [Google Scholar] [CrossRef]
- Chilaka, C.A.; De Boevre, M.; Atanda, O.O.; De Saeger, S. Stability of fumonisin B-1, deoxynivalenol, zearalenone, and T-2 toxin during processing of traditional Nigerian beer and spices. Mycotoxin Res. 2018, 34, 229–239. [Google Scholar] [CrossRef]
- Pascari, X.; Ramos, A.J.; Marin, S.; Sanchis, V. Mycotoxins and beer. Impact of beer production process on mycotoxin contamination. A review. Food Res. Int. 2018, 103, 121–129. [Google Scholar] [CrossRef]
- Wall-Martinez, H.A.; Pascari, X.; Ramos, A.J.; Marin, S.; Sanchis, V. Fate of the mycotoxins in the wort and yeast during ale and lager fermentation and their evaluation under different technological parameters. LWT-Food Sci. Technol. 2020, 132, 7. [Google Scholar] [CrossRef]
- Nkwe, D.O.; Taylor, J.E.; Siame, B.A. Fungi, aflatoxins, fumonisin B-1 and zearalenone contaminating sorghum-based traditional malt, wort and beer in Botswana. Mycopathologia 2005, 160, 177–186. [Google Scholar] [CrossRef]
- Scott, P.M.; Kanhere, S.R.; Daley, E.F.; Farber, J.M. Fermentation of wort containing deoxynivalenol and zearalenone. Mycotoxin Res. 1992, 8, 58–66. [Google Scholar] [CrossRef]
- Mizutani, K.; Nagatomi, Y.; Mochizuki, N. Metabolism of Zearalenone in the Course of Beer Fermentation. Toxins 2011, 3, 134–141. [Google Scholar] [CrossRef]
- Chilaka, C.A.; De Boevre, M.; Atanda, O.O.; De Saeger, S. Fate of Fusarium mycotoxins during processing of Nigerian traditional infant foods (ogi and soybean powder). Food Res. Int. 2019, 116, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Vanegmond, H.P. Rationale for regulatory programmes for mycotoxins in human foods and animal feeds. Food Addit. Contam. 1993, 10, 29–36. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Correa, A.N.R.; Ferreira, C.D. Mycotoxins in Grains and Cereals Intended for Human Consumption: Brazilian Legislation, Occurrence Above Maximum Levels and Co-Occurrence. Food Rev. Int. 2022, 1–14. [Google Scholar] [CrossRef]
- Ji, F.; Xu, J.H.; Liu, X.; Yin, X.C.; Shi, J.R. Natural occurrence of deoxynivalenol and zearalenone in wheat from Jiangsu province. China Food Chem. 2014, 157, 393–397. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Worldwide Regulations for Mycotoxins in Food and Feed in 2003. 2003. Available online: https://www.fao.org/3/y5499e/y5499e0d.htm (accessed on 9 April 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).