The Influence of Different Pretreatment Methods of Highland Barley by Solid-State Fermentation with Agaricus sinodeliciosus var. Chaidam ZJU-TP-08 on Its Nutrient Content, Functional Properties and Physicochemical Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism, Culture Media and Cultivation Conditions
2.2. Pre-Treatment of HB
2.3. Solid-State Fermentation Process
2.4. Mycelial Biomass Evaluation
2.5. Nutritional Composition Evaluation
2.6. Amino Acids Composition Detection
2.7. Total Phenolic and Flavonoid Content Detection
2.8. Antioxidant Activity Assay
2.9. Analysis of Physicochemical Characteristics
2.10. Scanning Electron Microscope (SEM) Observation Morphological Features
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effects of Different Pretreatments on Mycelial Biomass and Ergosterol Formation
3.2. Alterations in Nutritional Compositions
3.3. Total Phenolic, Flavonoid Content and Antioxidant Activity
3.4. The Description and Characterization of Physicochemical Properties
3.5. Principal Component Analysis of Nutritional Constituents of Fermented and Unfermented HB
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Kalac, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef]
- Sanchez, C. Reactive oxygen species and antioxidant properties from mushrooms. Synth. Syst. Biotechnol. 2017, 2, 13–22. [Google Scholar] [CrossRef] [PubMed]
- He, W.S.; Cui, D.D.; Li, L.L.; Tong, L.-T.; Rui, J.; Li, H.; Zhang, H.; Liu, X. Cholesterol-reducing effect of ergosterol is modulated via inhibition of cholesterol absorption and promotion of cholesterol excretion. J. Funct. Foods 2019, 57, 488–496. [Google Scholar] [CrossRef]
- Wang, Z.R.; Parra, L.A.; Callac, P.; Zhou, J.-L.; Fu, W.-J.; Dui, S.-H.; Hyde, K.D.; Zhao, R.-L. Edible species of Agaricus (Agaricaceae) from Xinjiang Province (Western China). Phytotaxa 2015, 202, 185–197. [Google Scholar] [CrossRef]
- Kuang, H.; Jiao, Y.C.; Wang, W.; Wang, F.; Chen, Q. Characterization and antioxidant activities of intracellular polysaccharides from Agaricus bitorquis (QueL.) Sacc. Chaidam ZJU-CDMA-12. Int. J. Biol. Macromol. 2020, 156, 1112–1125. [Google Scholar] [CrossRef]
- Lu, H.Y.; Jiao, Z.H.; Jiao, Y.C.; Wang, W.; Chen, Q. Phenolic Acids-Rich Fractions from Agaricus bitorguis (Quel.) Sacc. Chaidam ZJU-CDMA-12 Mycelia Modulate Hypoxic Stress on Hypoxia-Damaged PC12 Cells. Molecules 2020, 25, 4845. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Y.; Lou, H.H.; Wei, T.Y.; Liu, Z.; Jiao, Y.; Chen, Q. Ultrasound enhanced production of mycelia and exopolysaccharide by Agaricus bitorquis (Quel.) Sacc. Chaidam. Ultrason. Sonochem. 2020, 64, 105040. [Google Scholar] [CrossRef]
- Souilem, F.; Fernandes, A.; Calhelha, R.C.; Barreira, J.C.; Barros, L.; Skhiri, F.; Martins, A.; Ferreira, I.C. Wild mushrooms and their mycelia as sources of bioactive compounds: Antioxidant, anti-inflammatory and cytotoxic properties. Food Chem. 2017, 230, 40–48. [Google Scholar] [CrossRef]
- Calvo-Lerma, J.; Asensio-Grau, A.; Garcia-Hernandez, J.; Heredia, A.; Andrés, A. Exploring the Impact of Solid-State Fermentation on Macronutrient Profile and Digestibility in Chia (Salvia hispanica) and Sesame (Sesamum indicum) Seeds. Foods 2022, 11, 410. [Google Scholar] [CrossRef]
- Stoffel, F.; Santana, W.D.; Fontana, R.C.; Gregolon, J.G.N.; Kist, T.B.L.; De Siqueira, F.G.; Mendonça, S.; Camassola, M. Chemical features and bioactivity of grain flours colonized by macrofungi as a strategy for nutritional enrichment. Food Chem. 2019, 297, 124988. [Google Scholar] [CrossRef]
- Li, Y.T.; Li, T.; Liu, R.H. Bioactive compounds of highland barley and their health benefits. J. Cereal Sci. 2022, 103, 103366. [Google Scholar] [CrossRef]
- Guo, T.L.; Horvath, C.; Chen, L.; Zheng, B. Understanding the nutrient composition and nutritional functions of highland barley (Qingke): A review. Trends Food Sci. Technol. 2020, 103, 109–117. [Google Scholar] [CrossRef]
- Xiang, X.; Tan, C.; Sun, X.J.; Zhao, Y.; Zhang, J.; Zhu, Y.; Bai, J.; Dong, Y.; Zhou, X. Effects of fermentation on structural characteristics and in vitro physiological activities of barley beta-glucan. Carbohydr. Polym. 2020, 231, 115685. [Google Scholar] [CrossRef]
- Azeez, S.O.; Chinma, C.E.; Bassey, S.O.; Eze, U.R.; Makinde, A.F.; Sakariyah, A.A.; Okubanjo, S.S.; Danbaba, N.; Adebo, O.A. Impact of germination alone or in combination with solid-state fermentation on the physicochemical, antioxidant, in vitro digestibility, functional and thermal properties of brown finger millet flours. LWT-Food Sci. Technol. 2021, 154, 112734. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, K.; Ahmed, N.; Chauhan, D.; Rizvi, Q.U.E.H.; Jan, S.; Singh, T.P.; Dhaliwal, H.S. Effect of soaking and germination treatments on nutritional, anti-nutritional, and bioactive properties of amaranth (Amaranthus hypochondriacus L.), quinoa (Chenopodium quinoa L.), and buckwheat (Fagopyrum esculentum L.). Curr. Res. Food Sci. 2021, 4, 917–925. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Lu, H.; Shu, Q.; Zhang, Y.; Chen, Q. New perspectives on physiological, biochemical and bioactive components during germination of edible seeds: A review. Trends Food Sci. Technol. 2022, 123, 187–197. [Google Scholar] [CrossRef]
- Oliveira, M.E.A.S.; Coimbra, P.P.S.; Galdeano, M.C.; Carvalho, C.W.P.; Takeiti, C.Y. How does germinated rice impact starch structure, products and nutrional evidences?—A review. Trends Food Sci. Technol. 2022, 122, 13–23. [Google Scholar] [CrossRef]
- Bonto, A.P.; Tiozon, R.N.; Sreenivasulu, N.; Camacho, D.H. Impact of ultrasonic treatment on rice starch and grain functional properties: A review. Ultrason. Sonochem. 2021, 71, 105383. [Google Scholar] [CrossRef]
- Lu, H.Y.; Liu, S.Y.; Zhang, S.L.; Chen, Q. Light Irradiation Coupled with Exogenous Metal Ions to Enhance Exopolysaccharide Synthesis from Agaricus sinodeliciosus ZJU-TP-08 in Liquid Fermentation. J. Fungi 2021, 7, 992. [Google Scholar] [CrossRef]
- Nowak, R.; Nowacka-Jechalke, N.; Pietrzak, W.; Gawlik-Dziki, U. A new look at edible and medicinal mushrooms as a source of ergosterol and ergosterol peroxide-UHPLC-MS/MS analysis. Food Chem. 2022, 369, 130927. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis. In The Association of Official Analytical Chemists, 18th ed.; North Fredrick Avenue Gaithersburg: Montgomery County, MD, USA, 2015. [Google Scholar]
- Liu, B.; Yuan, D.X.; Li, Q.Y.; Zhou, X.; Wu, H.; Bao, Y.; Lu, H.; Luo, T.; Wang, J. Changes in Organic Acids, Phenolic Compounds, and Antioxidant Activities of Lemon Juice Fermented by Issatchenkia terricola. Molecules 2021, 26, 6712. [Google Scholar] [CrossRef] [PubMed]
- Watchararparpaiboon, W.; Laohakunjit, N.; Kerdchoechuen, O. An Improved Process for High Quality and Nutrition of Brown Rice Production. Food Sci. Technol. Int. 2010, 16, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Bei, Q.; Liu, Y.; Wang, L.; Chen, G.; Wu, Z. Improving free, conjugated, and bound phenolic fractions in fermented oats (Avena sativa L.) with Monascus anka and their antioxidant activity. J. Funct. Foods 2017, 32, 185–194. [Google Scholar] [CrossRef]
- Yang, X.J.; Dang, B.; Fan, M.T. Free and Bound Phenolic Compound Content and Antioxidant Activity of Different Cultivated Blue Highland Barley Varieties from the Qinghai-Tibet Plateau. Molecules 2018, 23, 879. [Google Scholar] [CrossRef]
- Wu, H.; Liu, H.N.; Ma, A.M.; Zhou, J.-Z.; Xia, X.-D. Synergetic effects of Lactobacillus plantarum and Rhizopus oryzae on physicochemical, nutritional and antioxidant properties of whole-grain oats (Avena sativa L.) during solid-state fermentation. LWT-Food Sci. Technol. 2022, 154, 112687. [Google Scholar] [CrossRef]
- Tang, Y.; Xiao, L.; Wu, X.Y.; Li, W.; Wu, T.; Zhang, P. Impact of germination pretreatment on the polyphenol profile, antioxidant activities, and physicochemical properties of three color cultivars of highland barley. J. Cereal Sci. 2021, 97, 103152. [Google Scholar] [CrossRef]
- Adebo, J.A.; Njobeh, P.B.; Gbashi, S.; Oyedeji, A.B.; Ogundele, O.M.; Oyeyinka, S.A.; Adebo, O.A. Fermentation of Cereals and Legumes: Impact on Nutritional Constituents and Nutrient Bioavailability. Fermentation 2022, 8, 63. [Google Scholar] [CrossRef]
- Espinosa-Paez, E.; Guadalupe Alanis-Guzman, M.; Hernandez-Luna, C.E.; Báez-González, J.G.; Amaya-Guerra, C.A.; Andrés-Grau, A.M. Increasing Antioxidant Activity and Protein Digestibility in Phaseolus vulgaris and Avena sativa by Fermentation with the Pleurotus ostreatus Fungus. Molecules 2017, 22, 2275. [Google Scholar] [CrossRef]
- Suarti, B.; Sukarno; Ardiansyah; Budijanto, S. Bio-active compounds, their antioxidant activities, and the physicochemical and pasting properties of both pigmented and non-pigmented fermented de-husked rice flour. AIMS Agric. Food 2021, 6, 49–64. [Google Scholar] [CrossRef]
- Ong, A.; Lee, C.L.K. Cooperative metabolism in mixed culture solid-state fermentation. LWT-Food Sci. Technol. 2021, 152, 112300. [Google Scholar] [CrossRef]
- Starzynska-Janiszewska, A.; Baczkowicz, M.; Sabat, R.; Stodolak, B.; Witkowicz, R. Quinoa Tempe as a Value-Added Food: Sensory, Nutritional, and Bioactive Parameters of Products from White, Red, and Black Seeds. Cereal Chem. 2017, 94, 491–496. [Google Scholar] [CrossRef]
- Gmoser, R.; Fristedt, R.; Larsson, K.; Undeland, I.; Taherzadeh, M.J.; Lennartsson, P.R. From stale bread and brewers spent grain to a new food source using edible filamentous fungi. Bioengineered 2020, 11, 582–598. [Google Scholar] [CrossRef]
- Xiao, X.; Li, J.Y.; Xiong, H.; Tui, W.; Zhu, Y.; Zhang, J. Effect of Extrusion or Fermentation on Physicochemical and Digestive Properties of Barley Powder. Front. Nutr. 2022, 8, 794355. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.-B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Vattem, D.A.; Shetty, K. Ellagic acid production and phenolic antioxidant activity in cranberry pomace (Vaccinium macrocarpon) mediated by Lentinus edodes using a solid-state system. Process Biochem. 2003, 39, 367–379. [Google Scholar] [CrossRef]
- Bhanja, T.; Kumari, A.; Banerjee, R. Enrichment of phenolics and free radical scavenging property of wheat koji prepared with two filamentous fungi. Bioresour. Technol. 2009, 100, 2861–2866. [Google Scholar] [CrossRef]
- Xiao, Y.; Rui, X.; Xing, G.L.; Wu, H.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Solid state fermentation with Cordyceps militaris SN-18 enhanced antioxidant capacity and DNA damage protective effect of oats (Auena sativa L.). J. Funct. Foods 2015, 16, 58–73. [Google Scholar] [CrossRef]
- Sanchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Patakova, P. Monascus secondary metabolites: Production and biological activity. J. Ind. Microbiol. Biotechnol. 2013, 40, 169–181. [Google Scholar] [CrossRef]
- Liu, W.W.; Dun, M.Q.; Liu, X.Y.; Zhang, G.; Ling, J. Effects on total phenolic and flavonoid content, antioxidant properties, and angiotensin I-converting enzyme inhibitory activity of beans by solid-state fermentation with Cordyceps militaris. Int. J. Food Prop. 2022, 25, 477–491. [Google Scholar] [CrossRef]
- Luo, J.Q.; Liu, S.Y.; Lu, H.Y.; Chen, Q.H.; Shi, Y. A comprehensive review ofmicroorganism-derived cyclic peptides: Bioactive functions and food safety applications. Compr. Rev. Food Sci. Food Saf. 2022, 11, 1–19. [Google Scholar] [CrossRef]
- Singh, H.B.; Singh, B.N.; Singh, S.P.; Nautiyal, C.S. Solid-state cultivation of Trichoderma harzianum NBRI-1055 for modulating natural antioxidants in soybean seed matrix. Bioresour. Technol. 2010, 101, 6444–6453. [Google Scholar] [CrossRef]
- Yuan, T.Z.; Liu, S.Y.; Reimer, M.; Isaak, C.; Ai, Y. Evaluation of pasting and gelling properties of commercial flours under high heating temperatures using Rapid Visco Analyzer 4800. Food Chem. 2021, 344, 128616. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Chen, M.H.; Lai, Y.S.; Chen, S.-D. Antioxidant Profile and Biosafety of White Truffle Mycelial Products Obtained by Solid-State Fermentation. Molecules 2022, 27, 109. [Google Scholar] [CrossRef]
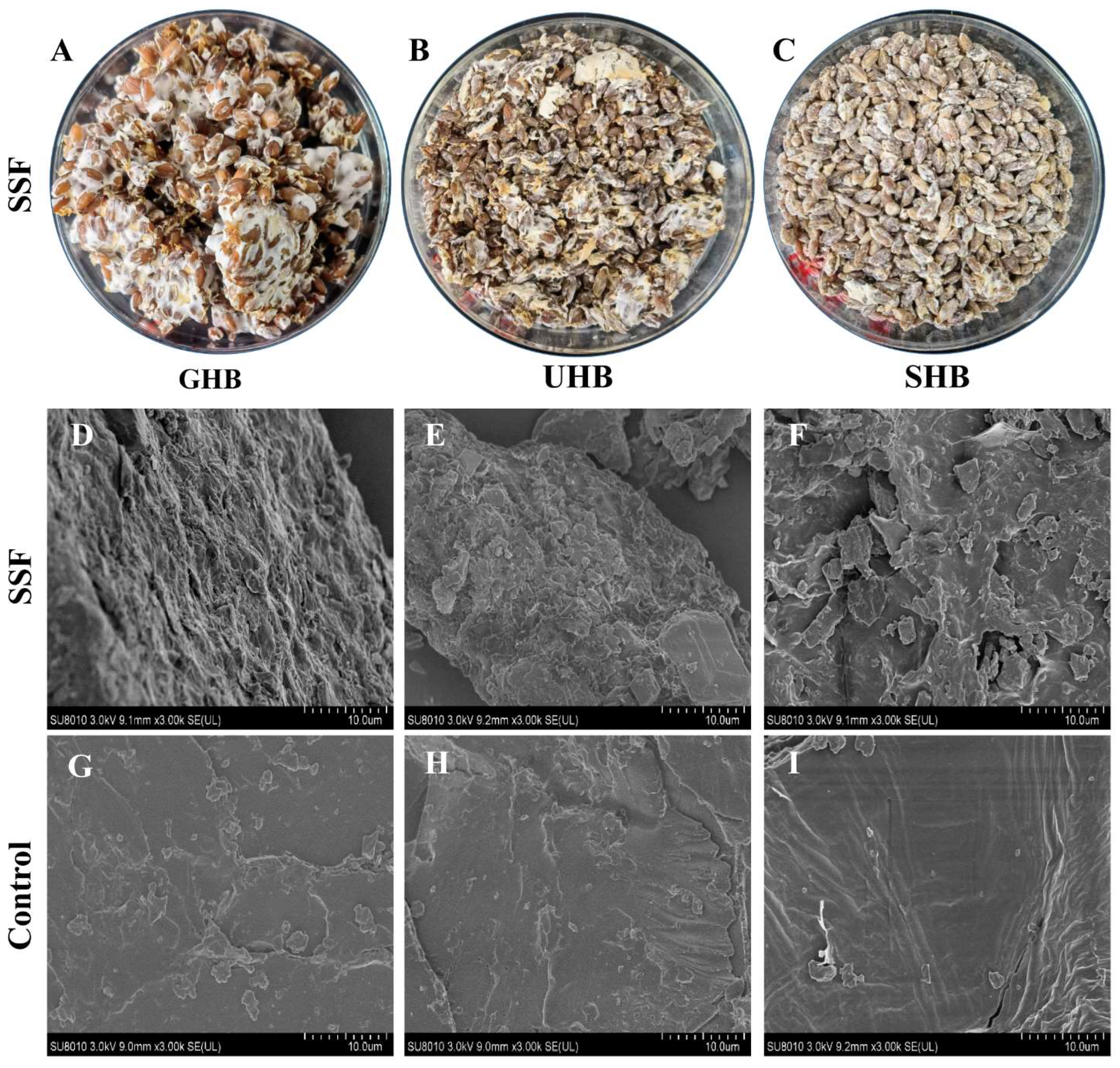
 ) SSF-GHB, (
) SSF-GHB, (  ) SSF-UHB, (
) SSF-UHB, (  ) SSF-SHB; (D): Indirect determination of mycelial biomass (mg/g) of colonized Highland barley, the different colors of legends represent different group, (
) SSF-SHB; (D): Indirect determination of mycelial biomass (mg/g) of colonized Highland barley, the different colors of legends represent different group, (  ) SSF-GHB, (
) SSF-GHB, (  ) SSF-UHB, (
) SSF-UHB, (  ) SSF-SHB. a–e: Values were determined in triplicate. Equal letters indicated that there were no significant difference at 5% (p > 0.05) in the parameter evaluated.
) SSF-SHB. a–e: Values were determined in triplicate. Equal letters indicated that there were no significant difference at 5% (p > 0.05) in the parameter evaluated.
 ) SSF-GHB, (
) SSF-GHB, (  ) SSF-UHB, (
) SSF-UHB, (  ) SSF-SHB; (D): Indirect determination of mycelial biomass (mg/g) of colonized Highland barley, the different colors of legends represent different group, (
) SSF-SHB; (D): Indirect determination of mycelial biomass (mg/g) of colonized Highland barley, the different colors of legends represent different group, (  ) SSF-GHB, (
) SSF-GHB, (  ) SSF-UHB, (
) SSF-UHB, (  ) SSF-SHB. a–e: Values were determined in triplicate. Equal letters indicated that there were no significant difference at 5% (p > 0.05) in the parameter evaluated.
) SSF-SHB. a–e: Values were determined in triplicate. Equal letters indicated that there were no significant difference at 5% (p > 0.05) in the parameter evaluated.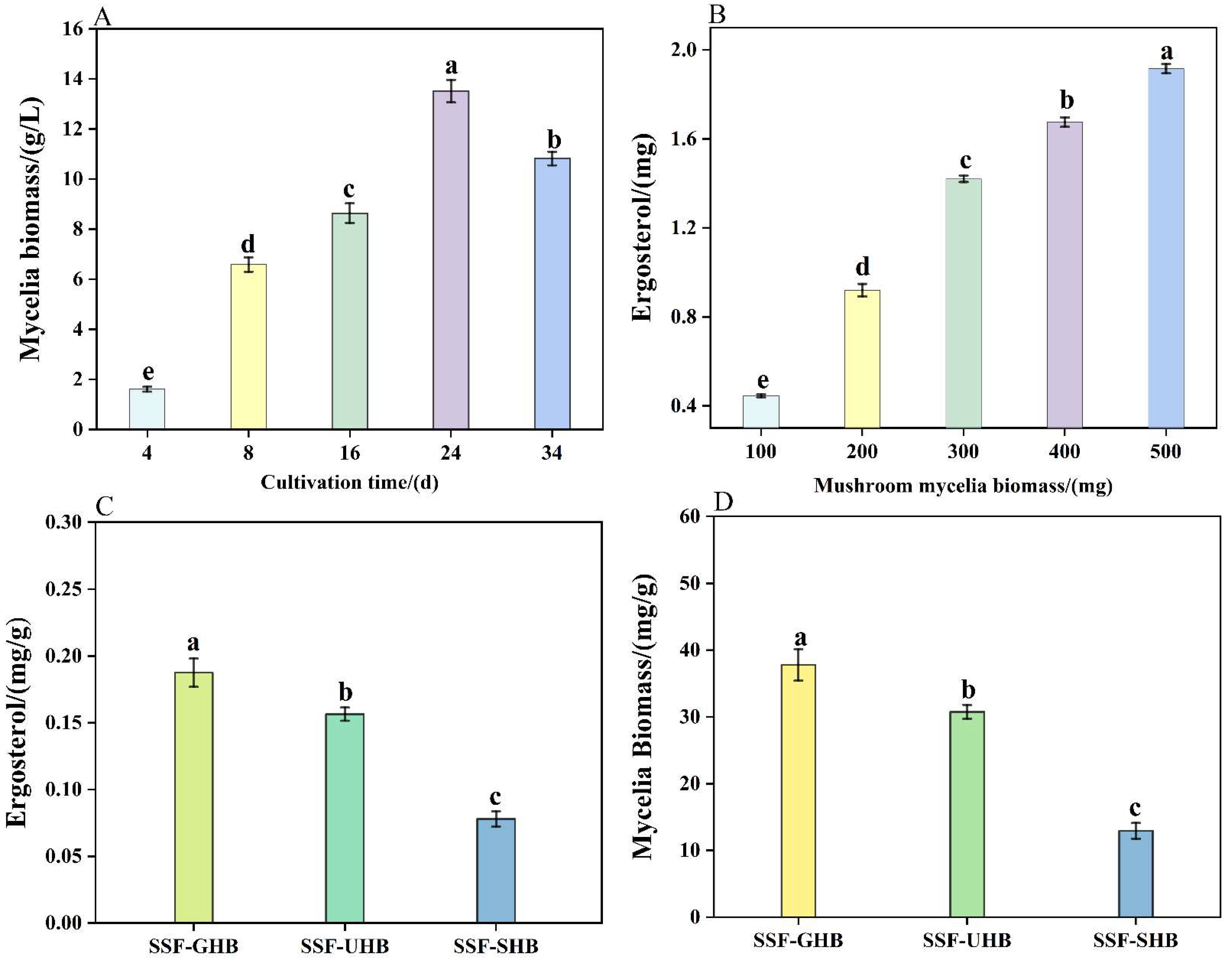

| Parameters | SSF | Control | ||||
|---|---|---|---|---|---|---|
| GHB | UHB | SHB | GHB | UHB | SHB | |
| Moisture (g/100g) | 12.38 ± 0.72 a | 12.63 ± 1.11 a | 11.57 ± 0.50 bc | 11.11 ± 1.08 bc | 10.16 ± 0.52 c | 10.06 ± 0.92 c |
| Ash (g/100g) | 4.36 ± 0.14 a | 4.03 ± 0.10 ab | 4.03 ± 0.06 ab | 4.01 ± 0.07 ab | 3.69 ± 0.19 b | 3.50 ± 0.15 b |
| Fat (g/100g) | 1.33 ± 0.05 d | 1.43 ± 0.06 cd | 1.49 ± 0.02 cd | 1.52 ± 0.04 bc | 1.68 ± 0.11 ab | 1.70 ± 0.08 a |
| Reduce sugar (mg/g) | 11.17 ± 0.68 a | 7.22 ± 0.49 b | 6.32 ± 0.26 b | 6.94 ± 0.43 b | 4.27 ± 0.29 c | 3.89 ± 0.25 c |
| Total sugar (mg/g) | 49.10 ± 1.50 a | 40.25 ± 0.31 b | 33.12 ± 0.35 c | 18.85 ± 0.57 e | 18.66 ± 0.29 e | 22.07 ± 0.78 d |
| Crude fiber (g/100g) | 2.68 ± 0.05 a | 2.51 ± 0.01 a | 2.68 ± 0.28 a | 1.78 ± 0.16 b | 1.57 ± 0.09 b | 1.94 ± 0.22 b |
| Dietary fiber (g/100g) | 3.80 ± 0.12 a | 3.23 ± 0.06 b | 3.21 ± 0.11 b | 2.47 ± 0.11 c | 2.00 ± 0.12 d | 2.13 ± 0.20 d |
| Crude protein (g/100g) | 13.02 ± 0.06 a | 12.97 ± 0.22 a | 12.95 ± 0.08 a | 12.38 ± 0.10 b | 12.30 ± 0.05 b | 12.23 ± 0.04 b |
| Beta-glucan (mg/g) | 4.72 ± 0.05 a | 4.29 ± 0.13 b | 4.35 ± 0.21 ab | 3.80 ± 0.15 c | 3.56 ± 0.24 c | 3.67 ± 0.15 c |
| GABA (mg/g) | 0.88 ± 0.15 a | 0.78 ± 0.11 a | 0.77 ± 0.12 a | 0.87 ± 0.14 a | 0.79 ± 0.08 a | 0.83 ± 0.01 a |
| Amino Acids (g/100 g) | SSF | Control | ||||
|---|---|---|---|---|---|---|
| GHB | UHB | SHB | GHB | UHB | SHB | |
| Essential amino | ||||||
| Lysine | 0.43 ± 0.00 ab | 0.47 ± 0.04 a | 0.44 ± 0.00 ab | 0.45 ± 0.02 ab | 0.40 ± 0.00 b | 0.44 ± 0.02 ab |
| Valine | 0.64 ± 0.04 a | 0.71 ± 0.06 a | 0.68 ± 0.01 a | 0.57 ± 0.19 a | 0.63 ± 0.01 a | 0.61 ± 0.03 a |
| Leucine | 0.89 ± 0.05 a | 0.89 ± 0.03 a | 0.94 ± 0.14 a | 0.92 ± 0.03 a | 0.87 ± 0.06 a | 0.84 ± 0.09 a |
| Isoleucine | 0.53 ± 0.03 a | 0.54 ± 0.01 a | 0.56 ± 0.01 a | 0.52 ± 0.03 a | 0.55 ± 0.00 a | 0.52 ± 0.01 a |
| Threonine | 0.47 ± 0.04 a | 0.47 ± 0.04 a | 0.42 ± 0.03 a | 0.47 ± 0.06 a | 0.46 ± 0.01 a | 0.47 ± 0.01 a |
| Methionine | 0.18 ± 0.03 a | 0.15 ± 0.00 abc | 0.17 ± 0.01 ab | 0.15 ± 0.00 abc | 0.14 ± 0.00 c | 0.14 ± 0.00 bc |
| Phenylalanine | 0.74 ± 0.02 a | 0.69 ± 0.05 a | 0.71 ± 0.13 a | 0.70 ± 0.02 a | 0.67 ± 0.05 a | 0.68 ± 0.04 a |
| Histidine | 0.34 ± 0.00 a | 0.34 ± 0.01 a | 0.34 ± 0.01 a | 0.34 ± 0.01 a | 0.32 ± 0.01 a | 0.31 ± 0.00 a |
| Non-essential | ||||||
| Arginine | 0.66 ± 0.01 a | 0.65 ± 0.01 a | 0.61 ± 0.04 a | 0.63 ± 0.02 a | 0.63 ± 0.02 a | 0.62 ± 0.02 a |
| Tyrosine | 0.61 ± 0.01 a | 0.61 ± 0.01 a | 0.56 ± 0.06 a | 0.63 ± 0.10 a | 0.60 ± 0.02 a | 0.62 ± 0.03 a |
| Serine | 0.30 ± 0.01 a | 0.28 ± 0.00 ab | 0.27 ± 0.01 bc | 0.26 ± 0.00 bcd | 0.25 ± 0.01 d | 0.26 ± 0.00 cd |
| Glutamic acid | 1.81 ± 0.16 a | 1.66 ± 0.07 a | 1.58 ± 0.19 a | 1.61 ± 0.05 a | 1.58 ± 0.07 a | 1.57 ± 0.13 a |
| Proline | 0.60 ± 0.01 a | 0.61 ± 0.02 a | 0.66 ± 0.06 a | 0.59 ± 0.02 a | 0.62 ± 0.08 a | 0.66 ± 0.10 a |
| Glycine | 0.70 ± 0.10 a | 0.67 ± 0.04 a | 0.69 ± 0.08 a | 0.64 ± 0.03 a | 0.63 ± 0.01 a | 0.63 ± 0.02 a |
| Alanine | 1.14 ± 0.11 a | 1.16 ± 0.08 a | 1.15 ± 0.20 a | 1.00 ± 0.11 a | 0.96 ± 0.05 a | 0.98 ± 0.06 a |
| Cystine | 0.33 ± 0.03 a | 0.33 ± 0.01 a | 0.35 ± 0.02 a | 0.32 ± 0.01 a | 0.32 ± 0.02 a | 0.31 ± 0.02 a |
| Aspartic acid | 1.64 ± 0.01 a | 1.52 ± 0.12 a | 1.62 ± 0.02 a | 1.50 ± 0.09 a | 1.51 ± 0.02 a | 1.51 ± 0.06 a |
| Total | 11.90 ± 0.10 a | 11.75 ± 0.04 a | 11.74 ± 0.17 a | 11.2 9± 0.19 b | 11.13 ± 0.02 b | 11.17 ± 0.09 b |
| Biological Activity | SSF | Control | |||||
|---|---|---|---|---|---|---|---|
| GHB | UHB | SHB | GHB | UHB | SHB | ||
| Total phenols (mg GAE/g) | Free | 3.38 ± 0.06 a | 3.10 ± 0.26 ab | 2.60 ± 0.27 bc | 3.04 ± 0.12 ab | 2.46 ± 0.30 c | 2.21 ± 0.04 c |
| Bound | 3.13 ± 0.08 a | 3.28 ± 0.21 a | 2.98 ± 0.15 a | 2.48 ± 0.10 b | 2.64 ± 0.04 b | 2.37 ± 0.03 b | |
| Total | 6.52 ± 0.22 a | 6.38 ± 0.47 a | 5.58 ± 0.12 b | 5.52 ± 0.16 b | 5.10 ± 0.35 bc | 4.58 ± 0.07 c | |
| Total flavonoids (mg RE/g) | Free | 1.17 ± 0.04 a | 0.98 ± 0.07 a | 0.69 ± 0.09 b | 0.40 ± 0.03 c | 0.53 ± 0.21 bc | 0.48 ± 0.04 bc |
| Bound | 0.94 ± 0.13 b | 0.86 ± 0.04 bc | 0.79 ± 0.03 cd | 1.09 ± 0.11 a | 0.71 ± 0.09 de | 0.67 ± 0.05 e | |
| Total | 2.15 ± 0.07 a | 2.05 ± 0.04 b | 1.87 ± 0.06 c | 1.49 ± 0.06 c | 1.24 ± 0.15 d | 1.15 ± 0.09 d | |
| Total antioxidant capacity (μmol Fe2+/g) | Free | 2.71 ± 0.06 a | 2.19 ± 0.13 c | 2.17 ± 0.09 c | 2.34 ± 0.05 b | 2.09 ± 0.06 c | 2.02 ± 0.04 c |
| Bound | 4.99 ± 0.31 a | 4.61 ± 0.15 b | 3.49 ± 0.11 d | 3.93 ± 0.12 c | 3.46 ± 0.10 d | 3.13 ± 0.08 d | |
| DPPH• radical scavenging (μmol TEAC/g) | Free | 1.19 ± 0.05 a | 1.07 ± 0.06 b | 0.75 ± 0.03 d | 0.92 ± 0.03 c | 0.99 ± 0.06 bc | 0.57 ± 0.03 e |
| Bound | 0.95 ± 0.07 a | 0.85 ± 0.03 b | 0.74 ± 0.04 c | 0.51 ± 0.04 d | 0.50 ± 0.03 d | 0.50 ± 0.05 d | |
| ABTS+• radical scavenging (μmol TEAC/g) | Free | 2.63 ± 0.06 a | 2.40 ± 0.10 b | 2.19 ± 0.14 c | 1.99 ± 0.03 d | 2.19 ± 0.04 c | 2.47 ± 0.08 ab |
| Bound | 2.24 ± 0.07 a | 2.22 ± 0.05 a | 2.13 ± 0.04 a | 1.43 ± 0.06 b | 1.25 ± 0.18 b | 1.35 ± 0.09 b | |
| Hydroxyl radical scavenging (μmol TEAC/g) | Free | 1.22 ± 0.13 a | 1.31 ± 0.16 a | 0.99 ± 0.07 b | 0.92 ± 0.07 b | 0.74 ± 0.15 c | 0.68 ± 0.03 c |
| Bound | 1.52 ± 0.09 a | 1.05 ± 0.06 b | 0.93 ± 0.05 c | 0.82 ± 0.05 d | 0.65 ± 0.06 d | 0.79 ± 0.04 e | |
| Parameters | SSF | Control | ||||
|---|---|---|---|---|---|---|
| GHB | UHB | SHB | GHB | UHB | SHB | |
| Functional properties | ||||||
| BD (g/cm3) | 0.56 ± 0.00 a | 0.55 ± 0.01 a | 0.55 ± 0.01 a | 0.54 ± 0.00 a | 0.54 ± 0.00 a | 0.54 ± 0.01 a |
| WHC (g/g) | 5.20 ± 0.18 a | 5.23 ± 0.00 a | 5.13 ± 0.35 a | 5.20 ± 0.53 a | 5.49 ± 0.10 a | 5.66 ± 0.09 a |
| WSI (%) | 26.13 ± 1.88 ab | 27.36 ± 0.26 a | 22.10 ± 1.05 abc | 21.41 ± 4.54 bc | 18.91 ± 1.01 c | 10.92 ± 1.38 d |
| Pasting properties | ||||||
| PV (cP) | 293.00 ± 1.41 d | 140.50 ± 0.77 f | 209.50 ± 3.54 e | 652.00 ± 1.77 c | 829.00 ± 2.33 b | 1169.25 ± 1.06 a |
| TV (cP) | 285.00 ± 5.66 d | 140.00 ± 0.00 f | 205.00 ± 2.83 e | 608.00 ± 1.49 c | 775.00 ± 8.48 b | 1129.00 ± 5.66 a |
| BV (cP) | 5.50 ± 0.51 d | 0.50 ± 0.71 e | 6.50 ± 2.15 d | 44.00 ± 0.61 b | 54.00 ± 1.41 a | 41.00 ± 0.53 c |
| FV (cP) | 527.00 ± 0.71 d | 270.00 ± 2.02 f | 405.00 ± 20.51 e | 1003.00 ± 2.83 c | 1247.00 ± 17.68 b | 1698.00 ± 16.97 a |
| SV (cP) | 237.00 ± 2.73 d | 130.50 ± 2.09 f | 200.50 ± 17.68 e | 396.00 ± 4.24 c | 478.00 ± 26.16 b | 561.00 ± 11.31 a |
| PT (°C) | n.d. | n.d. | n.d. | 92.50 ± 2.12 a | 93.39 ± 1.51 a | 87.59 ± 1.90 b |
| Peak time (min) | 6.12 ± 0.11 b | 5.75 ± 0.09 c | 5.37 ± 0.04 d | 6.95 ± 0.07 a | 7.02 ± 0.02 a | 6.94 ± 0.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Lu, H.; Shu, Q.; Chen, Q.; Wang, J. The Influence of Different Pretreatment Methods of Highland Barley by Solid-State Fermentation with Agaricus sinodeliciosus var. Chaidam ZJU-TP-08 on Its Nutrient Content, Functional Properties and Physicochemical Characteristics. J. Fungi 2022, 8, 940. https://doi.org/10.3390/jof8090940
Liu B, Lu H, Shu Q, Chen Q, Wang J. The Influence of Different Pretreatment Methods of Highland Barley by Solid-State Fermentation with Agaricus sinodeliciosus var. Chaidam ZJU-TP-08 on Its Nutrient Content, Functional Properties and Physicochemical Characteristics. Journal of Fungi. 2022; 8(9):940. https://doi.org/10.3390/jof8090940
Chicago/Turabian StyleLiu, Biao, Hongyun Lu, Qin Shu, Qihe Chen, and Jinling Wang. 2022. "The Influence of Different Pretreatment Methods of Highland Barley by Solid-State Fermentation with Agaricus sinodeliciosus var. Chaidam ZJU-TP-08 on Its Nutrient Content, Functional Properties and Physicochemical Characteristics" Journal of Fungi 8, no. 9: 940. https://doi.org/10.3390/jof8090940
APA StyleLiu, B., Lu, H., Shu, Q., Chen, Q., & Wang, J. (2022). The Influence of Different Pretreatment Methods of Highland Barley by Solid-State Fermentation with Agaricus sinodeliciosus var. Chaidam ZJU-TP-08 on Its Nutrient Content, Functional Properties and Physicochemical Characteristics. Journal of Fungi, 8(9), 940. https://doi.org/10.3390/jof8090940






