Arbuscular Mycorrhizal Fungi Reduce Cadmium Leaching from Sand Columns by Reducing Availability and Enhancing Uptake by Maize Roots
Abstract
:1. Introduction
2. Material and Methods
2.1. Test Materials
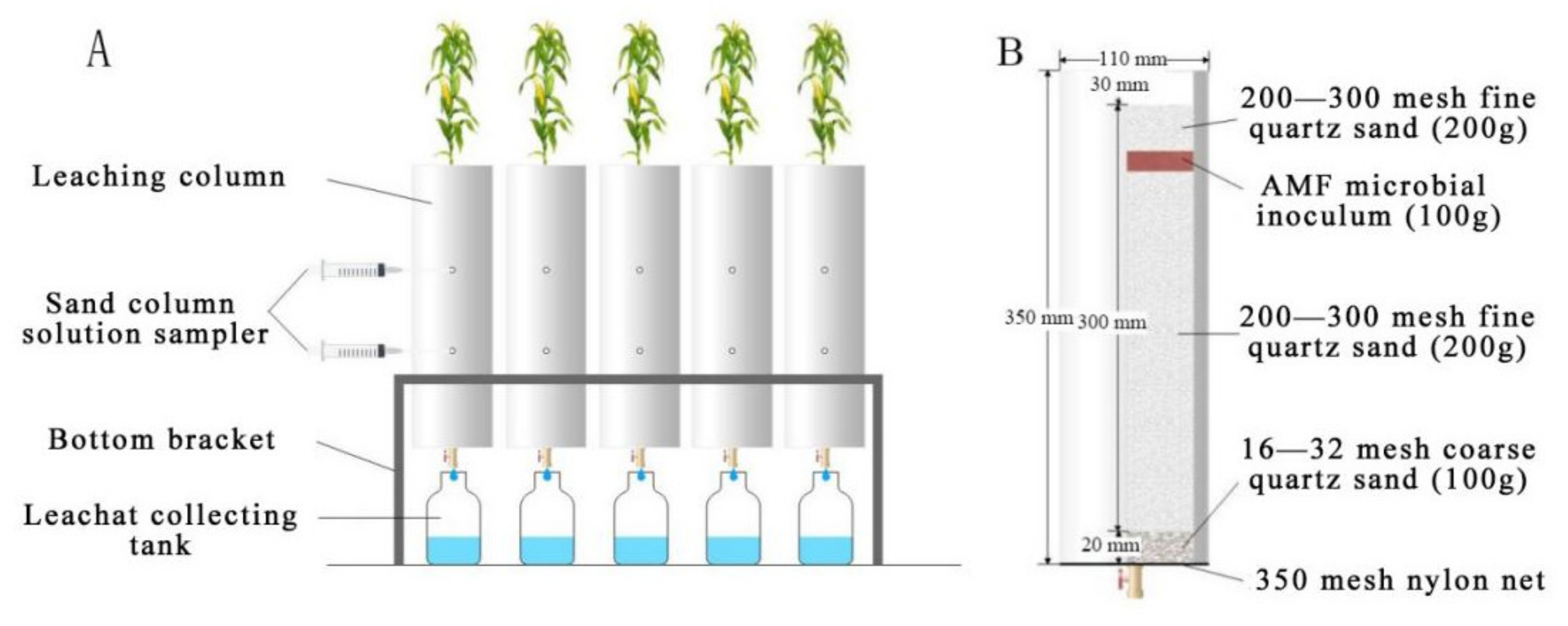
2.2. Sand Column Preparation and Leaching Experiment
2.3. Determination of AMF Colonization
2.4. Determination of Root Exudates, Root Morphology and Plant Biomass
2.5. Determination of Cadmium Concentration, Uptake by Plants and Leaching Loss
2.6. Determination of Cadmium Adsorption by Inner Wall of Column and Sand
2.7. Data Processing and Statistical Analysis
3. Results
3.1. Colonization of Arbuscular Mycorrhizal Fungi in the Maize Rhizosphere
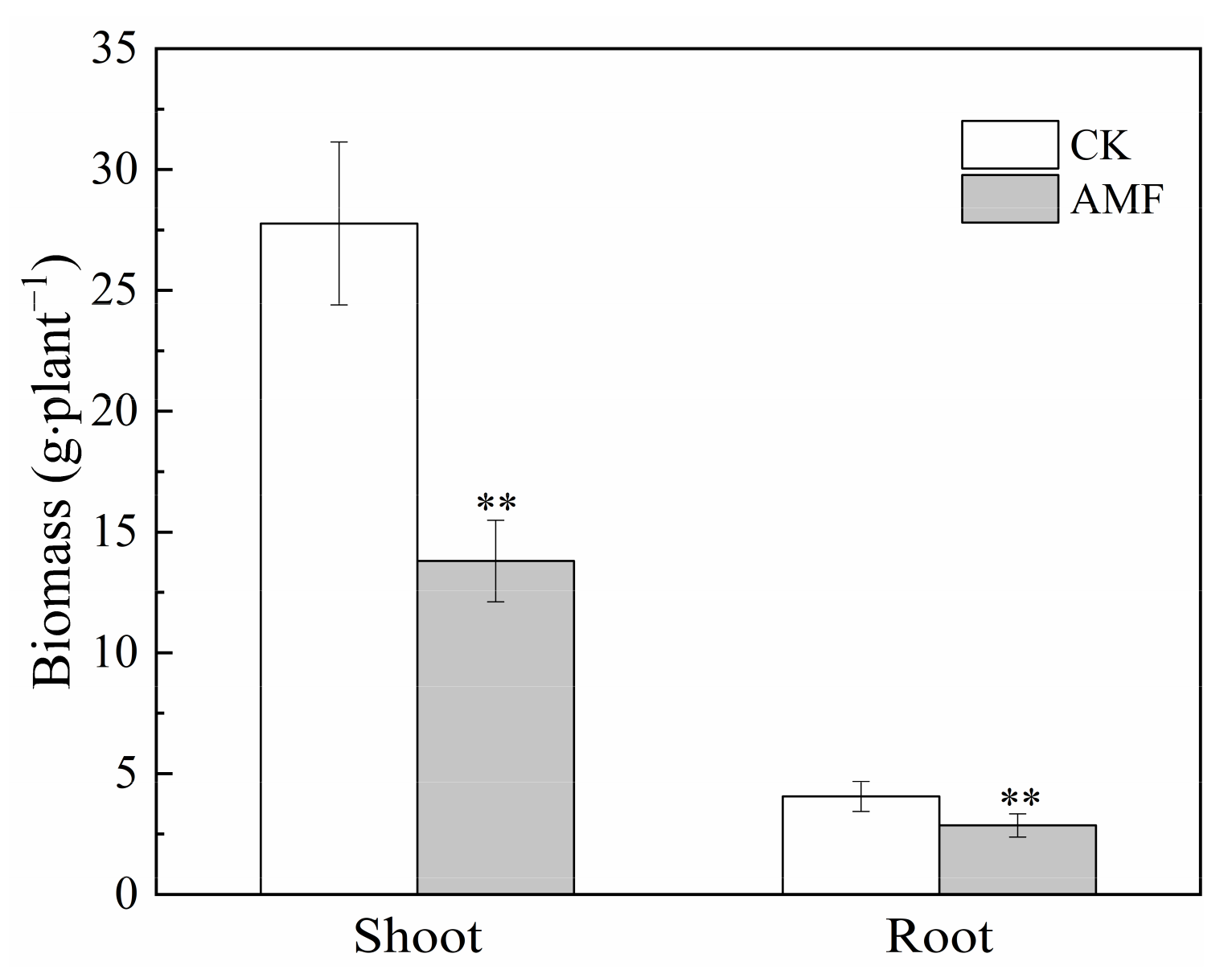
3.2. Effect of Arbuscular Mycorrhizal Fungi on Maize Biomass, Root Morphology and Root Exudate Content
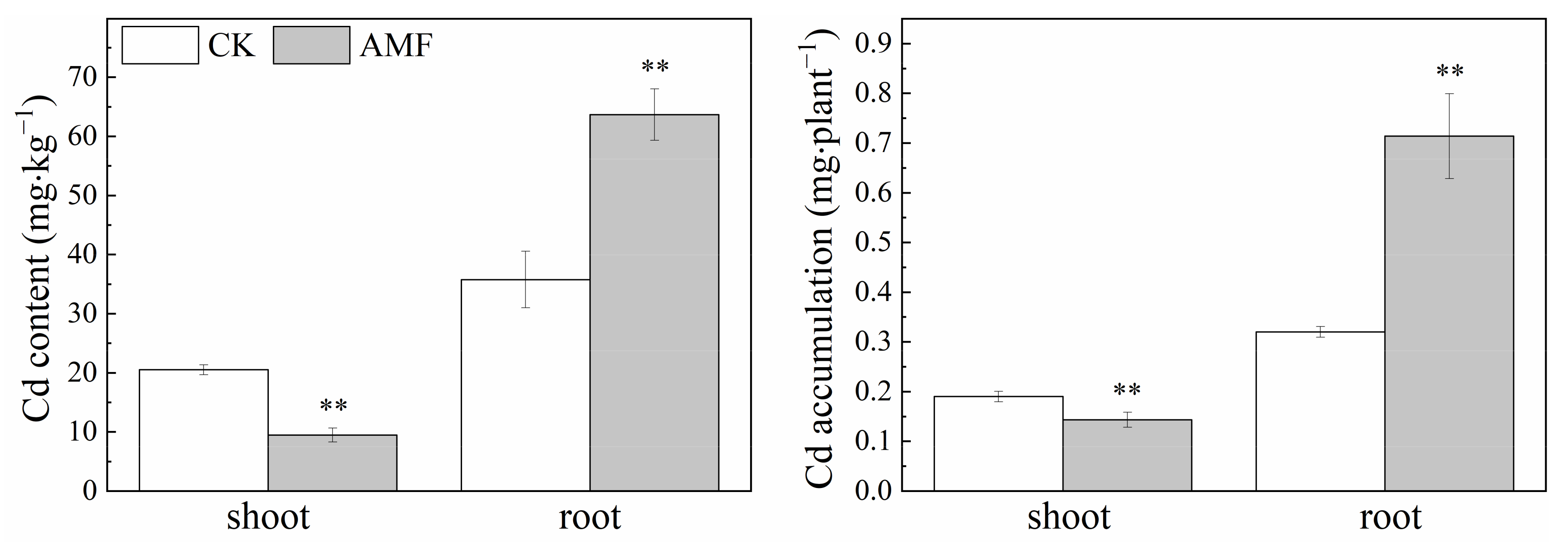
3.3. Effect of Arbuscular Mycorrhizal Fungi on Cadmium Content and Uptake by Maize
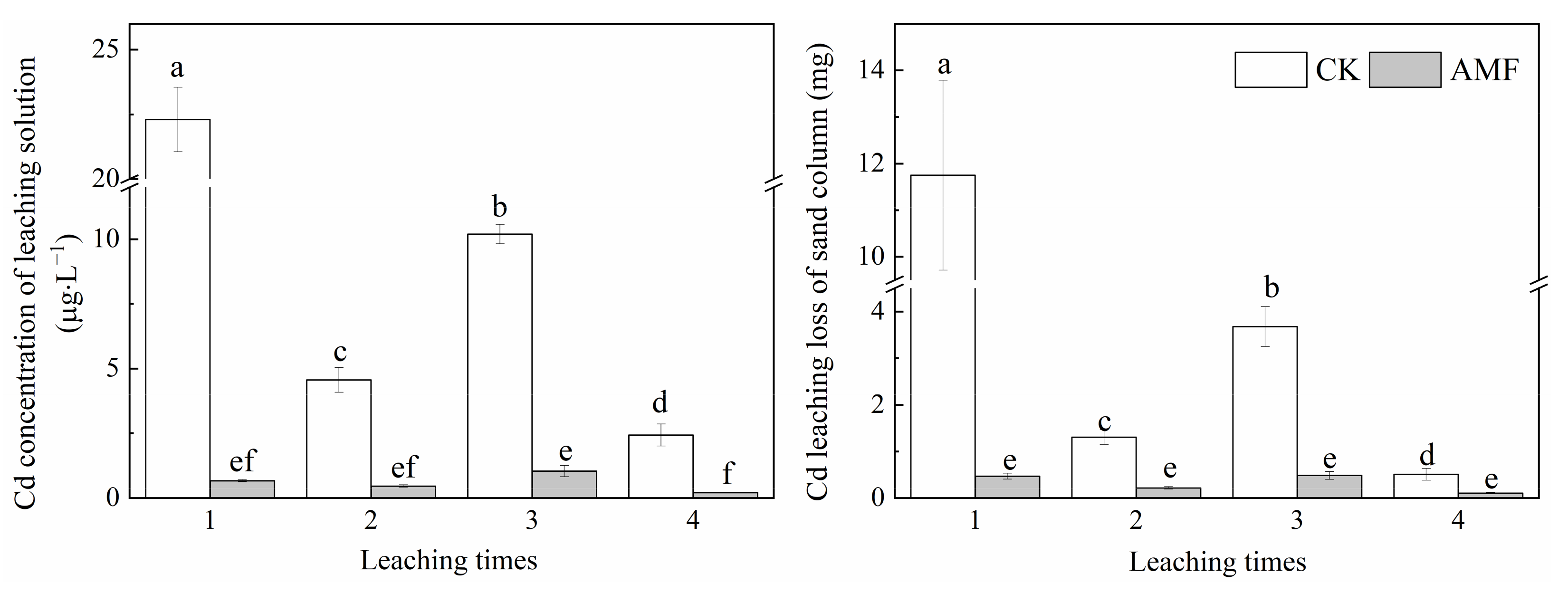
3.4. Effect of Arbuscular Mycorrhizal Fungi on Cd Concentration in Sand Solution and Cd Leaching Loss
3.5. Effect of Arbuscular Mycorrhizal Fungi on Cd Adsorption by Sand
3.6. Effect of Arbuscular Mycorrhizal Fungi on Cadmium Distribution in Sand Column-Maize System
3.7. Correlation Analysis
4. Discussion
4.1. Effect of Arbuscular Mycorrhizal Fungi on Cadmium Adsorption by Sand
4.2. Effect of Arbuscular Mycorrhizal Fungi on Plant Growth and Cadmium Uptake
4.3. Effect of Arbuscular Mycorrhizal Fungi on Cadmium Leaching Loss
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, W.; Wang, D.; Xu, Z.; Liao, G.; Du, Z. Effects of cadmium pollution on the safety of rice and fish in a rice-fish coculture system. Environ. Int. 2020, 143, 105898. [Google Scholar] [CrossRef] [PubMed]
- Matthias, W.; Moritz, B.; Martin, I.; Armin, K.; Mark, R.; Wolfgang, W.; Emmanuel, F. Using isotopes to trace freshly applied cadmium through mineral phosphorus fertilization in soil-fertilizer-plant systems. Sci. Total Environ. 2018, 648, 779–786. [Google Scholar]
- Lai, C.H.; Li, D.Q.; Qin, J.H.; Li, J.; Yan, Z.G.; Chen, G.K.; Li, H.S. The migration of cadmium and lead in soil columns and their bioaccumulation in a multi-species soil system. Chemosphere 2021, 262, 127718. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.W.; Monaghan, R.M.; Orchiston, T.; Laurenson, S.; Cavanagh, J.A. Cadmium losses in overland flow from an agricultural soil. Environ. Sci. Pollut. Res. Int. 2017, 24, 24046–24053. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Su, Y.; Lu, S.G. Predicting accumulation of Cd in rice (Oryza sativa L.) and soil threshold concentration of Cd for rice safe production. Sci. Total Environ. 2020, 738, 139805. [Google Scholar] [CrossRef] [PubMed]
- Landre, A.L.; Watmough, S.A.; Dillon, P.J. Metal pools, fluxes, and budgets in an acidified forested catchment on the precambrian shield, central Ontario, Canada. Water Air Soil Pollut. 2010, 209, 209–228. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, Z.; Wu, X.; Liu, Z.; Zou, J.; Chen, Y.; Su, N.; Cui, J. Lower cadmium accumulation and higher antioxidative capacity in edible parts of Brassica campestris L. seedlings applied with glutathione under cadmium toxicity. Environ. Sci. Pollut. Res. 2019, 26, 12235–13245. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, Y.; Liu, T.; Huang, S.P.; Chang, Y.F. Elevated CO2 increases glomalin-related soil protein (GRSP) in the rhizosphere of Robinia pseudoacacia L. seedlings in Pb- and Cd-contaminated soils. Environ. Pollut. 2016, 218, 349–357. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.W.; Wong, M.H. Arbuscular mycorrhizal fungi reduced the ratios of inorganic / organic arsenic in rice grains. Chemosphere 2016, 145, 224–230. [Google Scholar] [CrossRef]
- Janoušková, M.; Pavlíková, D. Cadmium immobilization in the rhizosphere of arbuscular mycorrhizal plants by the fungal extraradical mycelium. Plant Soil 2010, 332, 511–520. [Google Scholar] [CrossRef]
- Wu, Z.; McGrouther, K.; Huang, J.; Wu, P.; Wu, W.; Wang, H. Decomposition and the contribution of glomalin-related soil protein (GRSP) in heavy metal sequestration: Field experiment. Soil Biol. Biochem. 2014, 68, 283–290. [Google Scholar] [CrossRef]
- Zhu, X.R.; Li, X.T.; Xing, F.; Chen, C.; Huang, G.H.; Gao, Y. Interaction Between Root Exudates of the Poisonous Plant Stellera chamaejasme L. and Arbuscular Mycorrhizal Fungi on the Growth of Leymus Chinensis (Trin.) Tzvel. Microorganisms 2020, 8, 364. [Google Scholar] [CrossRef]
- Wu, J.T.; Wang, L.; Zhao, L.; Huang, X.C.; Ma, F. Arbuscular mycorrhizal fungi effect growth and photosynthesis of Phragmites australis (Cav.) Trin ex. Steudel under copper stress. Plant Biol. 2020, 22, 62–69. [Google Scholar] [CrossRef]
- Pichardo, S.T.; Su, Y.; Han, F.X. The Potential Effects of Arbuscular Mycorrhizae (AM) on the Uptake of Heavy Metals by Plants from Contaminated Soils. J. Bioremediation Biodegrad. 2012, 10, e124. [Google Scholar] [CrossRef]
- Yin, Z.P.; Zhang, Y.; Hu, N.; Shi, Y.C.; Li, T.; Zhao, Z.W. Differential responses of 23 maize cultivar seedlings to an arbuscular mycorrhizal fungus when grown in a metal-polluted soil. Sci. Total Environ. 2021, 789, 148015. [Google Scholar] [CrossRef]
- Huang, X.; Ho, S.H.; Zhu, S.S.; Ma, F.J.; Wu, W.J.; Yang, J.X.; Wang, L. Adaptive response of arbuscular mycorrhizal symbiosis to accumulation of elements and translocation in Phragmites australis affected by cadmium stress. Environ. Manag. 2017, 197, 448–455. [Google Scholar] [CrossRef]
- Gu, H.; Zhou, Z.; Gao, Y.; Yuan, X.T.; Ai, Y.J.; Zhang, J.Y.; Zuo, W.Z.; Taylor, A.A.; Nan, S.Q.; Li, F. The influences of arbuscular mycorrhizal fungus on phytostabilization of lead/zinc tailings using four plant species. Int. J. Phytoremediation 2017, 19, 739–745. [Google Scholar] [CrossRef]
- Zhang, F.G.; Liu, M.H.; Li, Y.; Che, Y.Y.; Xiao, Y. Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Sci. Total Environ. 2019, 655, 1150–1158. [Google Scholar] [CrossRef]
- Yu, Z.H.; Zhao, X.L.; Su, L.; Yan, K.; Li, B.; He, Y.M.; Zhan, F.D. Effect of an arbuscular mycorrhizal fungus on maize growth and cadmium migration in a sand column. Ecotoxicol. Environ. Saf. 2021, 225, 112782. [Google Scholar] [CrossRef]
- Amir, H.B.; Forough, A.; Reza, J. Soil-indigenous arbuscular mycorrhizal fungi and zeolite addition to soil synergistically increase grain yield and reduce cadmium uptake of bread wheat (through improved nitrogen and phosphorus nutrition and immobilization of Cd in roots). Environ. Sci. Pollut. Res. 2019, 26, 30794–30807. [Google Scholar]
- Khaleghi, M.; Rowshanzamir, M.A. Biologic improvement of a sandy soil using single and mixed cultures: A comparison study. Soil Tillage Res. 2019, 186, 112–119. [Google Scholar] [CrossRef]
- Zhang, X.F.; Hu, Z.H.; Yan, T.X.; Lu, R.R.; Peng, C.; Li, S.S.; Jing, Y.X. Arbuscular mycorrhizal fungi alleviate Cd phytotoxicity by altering Cd subcellular distribution and chemical forms in Zea mays. Ecotoxicol. Environ. Saf. 2019, 171, 352–360. [Google Scholar] [CrossRef]
- Chen, J.J.; Yu, W.; Zu, Y.Q.; LI, Y. Variety Difference of Cd Accumulation and Translocation in Zea mays. Ecol. Environ. Sci. 2014, 23, 1671–1676. [Google Scholar]
- Chen, J.X.; Guo, J.W.; Li, Z.R.; Liang, X.R.; You, Y.; Li, M.; He, Y.M.; Zhan, F. Effects of an Arbuscular Mycorrhizal Fungus on the Growthof and Cadmium Uptake in Maize Grown on Polluted Wasteland, Farmland and Slopeland Soils in a Lead-Zinc Mining Area. Toxics 2022, 10, 359. [Google Scholar] [CrossRef]
- Wang, L.; Fan, W.G.; Ma, K.X.; Liu, J.; Chen, S.; Du, M.Y.; Qiang, W.; Yang, H.Q. Change of Mineral Elements and Amino Acids in Malus hupehensis var. pingyiensis Leaves under Cd Treatment. Agric. Sci. 2018, 9, 221–227. [Google Scholar]
- Fountouli, T.V.; Chrysikopoulos, C.V.; Tsanis, I.K. Effect of salinity on formaldehyde interaction with quartz sand and kaolinite colloid particles: Batch and column experiments. Environ. Earth Sci. 2019, 78, 152–163. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–164. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Wright, S.F.; Franke-Snyder, M.; Morton, J.B. Time-course study and partial characterization of a protein on hyphae of arbuscular mycorrhizal fungi during active colonization of roots. Plant Soil 1996, 181, 193–203. [Google Scholar] [CrossRef]
- Jakobsen, I.; Abbott, L.K.; Robosen, A.D. External hyphae of vesiculararbuscular mycorrhizal fungi associated with Trifolium subterraneum L. New Phytol. 1992, 120, 371–380. [Google Scholar] [CrossRef]
- Keerthisinghe, G.; Hocking, P.J.; Ryan, P.R.; Delhaize, E. Effect of phosphorus supply on the formation and function of proteoid roots of white lupin (Lupinus albus L.). Plant Cell Environ. 2010, 21, 467–478. [Google Scholar] [CrossRef]
- Tolra, R.P.; Poschenrieder, C.; Barcelo, J. Zinc hyperaccumulation in Thlaspi caerulescens. II. Influence on organic acids. J. Plant Nutr. 1996, 19, 1541–1550. [Google Scholar] [CrossRef]
- Kachova, V.G.; Atanassova, I.D. Heavy metal pools in urban soils from city parks of Sofia, Bulgaria. Agric. Sci. Technol. 2017, 9, 144–150. [Google Scholar] [CrossRef]
- Jing, F.; Chen, X.; Yang, Z.; Guo, B. Heavy metals status, transport mechanisms, sources, and factors affecting their mobility in Chinese agricultural soils. Environ. Earth Sci. 2018, 77, 104. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Mallik, A.; Zhang, J.C.; Huang, Y.Q.; Zhou, L.W. Effects of arbuscular mycorrhizal fungi on inoculated seedling growth and rhizosphere soil aggregates. Soil Tillage Res. 2019, 194, 104340. [Google Scholar] [CrossRef]
- Chen, B.D.; Sun, Y.Q.; Zhang, X.; Wu, S.L. Underlying Mechanisms of the Heavy Metal Tolerance of Mycorrhizal Fungi. Environ. Sci. 2015, 36, 1123–1132. [Google Scholar]
- Zhang, X.; Ren, B.H.; Wu, S.L.; Sun, Y.Q.; Ge, L.; Chen, B.D. Arbuscular mycorrhizal symbiosis influences arsenic accumulation and speciation in Medicago truncatula L. in arsenic-contaminated soil. Chemosphere 2015, 119, 224–230. [Google Scholar] [CrossRef]
- Hu, Q.Q.; Li, Z.A.; Huang, H.X.; Hou, M.F.; Zhang, J.X.; Duan, J. Effects of citric acid on the desorption of Cd from soil. Ecol. Environ. Sci. 2011, 20, 1338–1342. [Google Scholar]
- Teng, Q.M.; Zhang, Z.F.; Li, H.Y.; Xu, G.P.; Zhou, L.B.; Huang, Y.Q. Effects of Arbuscular Mycorrhizal Fungi on Growth, Photosynthesis Characteristics and Mineral Nutrition of Arundo donax Under Cd Stress. Soils 2020, 52, 1212–1221. [Google Scholar]
- Liu, Y.; Wei, X.L. Dark Septate Endophyte Improves Drought Tolerance of Ormosia hosiei Hemsley & E. H. Wilson by Modulating Root Morphology, Ultrastructure, and the Ratio of Root Hormones. Forests 2019, 10, 830. [Google Scholar]
- Camenzind, T.; Homeier, J.; Dietrich, K.; Hempel, S.; Hertel, D.; Krohn, A.; Leuschner, C.; Oelmann, Y.; Olsson, P.A.; Suarez, J.P.; et al. Opposing effects of nitrogen versus phosphorus additions on mycorrhizal fungal abundance along an elevational gradient in tropical montane forests. Soil Biol. Biochem. 2018, 94, 37–47. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Q.; Wang, Q.; Wang, X.; Gange, A. Mycorrhizal-induced growth depression in plants. Symbiosis 2018, 72, 81–88. [Google Scholar] [CrossRef]
- Cartmill, A.D.; Valdez-Aguilar, L.A.; Cartmill, D.L.; Volder, A.; Alarcó, A. Arbuscular mycorrhizal colonization does not alleviate sodium chloride-salinity stress in vinca [Catharanthus roseus (L.) G. don]. J. Plant Nutr. 2013, 36, 164–178. [Google Scholar] [CrossRef]
- González-Guerrero, M.; Benabdellah, K.; Valderas, A.; Azcón-Aguilar, C.; Ferrol, N. GintABC1 encodes a putative ABC transporter of the MRP subfamily induced by Cu, Cd, and oxidative stress in Glomus intraradices. Mycorrhiza 2010, 20, 137–146. [Google Scholar] [CrossRef]
- Chen, B.; Nayuki, K.; Kuga, Y.; Zhang, X.; Wu, S.; Ohtomo, R. Uptake and Intraradical Immobilization of Cadmium by Arbuscular Mycorrhizal Fungi as Revealed by a Stable Isotope Tracer and Synchrotron Radiation μX-ray Fluorescence Analysis. Microbes Environ. 2018, 33, 257–263. [Google Scholar] [CrossRef]
- Peng, X.; Hong-Bin, H.E.; Cheng, J.K.; Xin, G.R. Effects of Arbuscular Mycorrhizal Fungi and Potting Media on Water and Fertilizer Loss of Zoysia japonica. J. Fungal Res. 2017, 15, 21–26. [Google Scholar]
- Liu, L.Z.; Gong, Z.Q.; Zhang, Y.L.; Jun, P. Growth, Cadmium Accumulation and Physiology of Marigold (Tagetes erecta L.) as Affected by Arbuscular Mycorrhizal Fungi. Pedosphere 2011, 21, 319–327. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J.; Xue, W.; Zhao, J.; Wang, J.; Liu, X. Effect of the size of variable charge soil particles on cadmium accumulation and adsorption. J. Soils Sediments 2017, 7, 2810–2821. [Google Scholar] [CrossRef]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- González-Chávez, M.C.; Carrillo-González, R.; Wright, S.F.; Nichols, K.A. The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environ. Pollut. 2004, 130, 317–323. [Google Scholar] [CrossRef]

| Treatment | Root Length (mm) | Root Surface Area (cm2) | Average Root Diameter (mm) | Root Volume (cm3) | Root Tip Number (n) | Root Crossings Number (n) |
|---|---|---|---|---|---|---|
| CK | 798.8 ± 45.4 | 38.0 ± 4.7 | 0.74 ± 0.05 | 9.4 ± 0.47 | 51,177 ± 1862 | 6419 ± 258 |
| AMF | 665.3 ± 40.2 ** | 30.4 ± 2.1 ** | 0.60 ± 0.10 | 7.8 ± 0.63 ** | 45,823 ± 1773 ** | 4081 ± 366 ** |
| Treatment | Malonic Acid Content (mg·L−1) | Oxalic Acid Content (mg·L−1) | Succinic Acid Content (mg·L−1) | Malic Acid Content (mg·L−1) | Acetic Acid Content (mg·L−1) | Citric Acid Content (mg·L−1) |
|---|---|---|---|---|---|---|
| CK | 4.89 ± 0.22 | 0.48 ± 0.04 | 208.2 ± 14.6 | 19.0 ± 2.7 | 17.2 ± 2.2 | 33.9 ± 3.6 |
| AMF | 12.43 ± 0.8 ** | 1.01 ± 0.04 ** | 320.6 ± 21.7 ** | 23.4 ± 3.6 | 0.78 ± 0.11 ** | 13.6 ± 1.36 ** |
| Treatment | Cd Adsorption by Sand (mg) | Cd Uptake in Maize (mg) | Cd Leaching Loss (mg) | Cd Adsorption by the Inner Wall (mg) |
|---|---|---|---|---|
| CK | 36.9 ± 0.5 (92.3%) | 2.35 ± 0.55 (5.89%) | 0.71 ± 0.09 (1.78%) | 0.026 ± 0.001 (0.066%) |
| AMF | 39.2 ± 0.6 ** (98.0%) | 0.49 ± 0.58 (1.22%) | 0.32 ± 0.01 ** (0.80%) | 0.004 ± 0.000 ** (0.010%) |
| Index | Total Root Length | Root Surface Area | Average Root Diameter | Root Volume | Root Tip Number | Root Crossing Number |
|---|---|---|---|---|---|---|
| EE-GRSP | −0.811 ** | −0.583 | 0.059 | −0.626 | −0.738 * | −0.820 ** |
| T-GRSP | −0.900 ** | −0.722 * | 0.291 | −0.749 * | −0.820 ** | −0.863 ** |
| Cd content of shoot | 0.914 ** | 0.754 * | −0.309 | 0.694 * | 0.852 ** | 0.934 ** |
| Cd content of root | 0.921 ** | 0.791 ** | −0.337 | 0.683 * | 0.832 ** | 0.907 ** |
| Cd uptake by maize shoot | −0.920 ** | −0.758 * | 0.268 | −0.672 * | −0.844 ** | −0.860 ** |
| Cd uptake by maize root | −0.848 ** | −0.748 * | 0.167 | −0.702 * | −0.884 ** | −0.857 ** |
| Cd leaching loss | 0.907 ** | 0.749 * | −0.322 | 0.732 * | 0.866 ** | 0.946 ** |
| Index | Oxalic Acid | Succinic Acid | Malic Acid | Malonate | Acetic Acid | Citric Acid |
|---|---|---|---|---|---|---|
| EE-GRSP | 0.924 ** | 0.916 ** | 0.586 | 0.923 ** | −0.892 ** | −0.893 ** |
| T-GRSP | 0.916 ** | 0.904 ** | 0.432 | 0.912 ** | −0.902 ** | −0.914 ** |
| Cd adsorption by sand | 0.781 ** | 0.573 | 0.243 | 0.735 * | −0.753 * | −0.745 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Zhao, X.; Liang, X.; Li, Z.; Wang, L.; He, Y.; Zhan, F. Arbuscular Mycorrhizal Fungi Reduce Cadmium Leaching from Sand Columns by Reducing Availability and Enhancing Uptake by Maize Roots. J. Fungi 2022, 8, 866. https://doi.org/10.3390/jof8080866
Yu Z, Zhao X, Liang X, Li Z, Wang L, He Y, Zhan F. Arbuscular Mycorrhizal Fungi Reduce Cadmium Leaching from Sand Columns by Reducing Availability and Enhancing Uptake by Maize Roots. Journal of Fungi. 2022; 8(8):866. https://doi.org/10.3390/jof8080866
Chicago/Turabian StyleYu, Zihao, Xiaoling Zhao, Xinran Liang, Zuran Li, Lei Wang, Yongmei He, and Fangdong Zhan. 2022. "Arbuscular Mycorrhizal Fungi Reduce Cadmium Leaching from Sand Columns by Reducing Availability and Enhancing Uptake by Maize Roots" Journal of Fungi 8, no. 8: 866. https://doi.org/10.3390/jof8080866
APA StyleYu, Z., Zhao, X., Liang, X., Li, Z., Wang, L., He, Y., & Zhan, F. (2022). Arbuscular Mycorrhizal Fungi Reduce Cadmium Leaching from Sand Columns by Reducing Availability and Enhancing Uptake by Maize Roots. Journal of Fungi, 8(8), 866. https://doi.org/10.3390/jof8080866






