Invasive Fungal Infection Caused by Magnusiomyces capitatus in an Immunocompromised Pediatric Patient with Acute Lymphoblastic Leukemia in Mexico City: A Case Report
Abstract
:1. Introduction
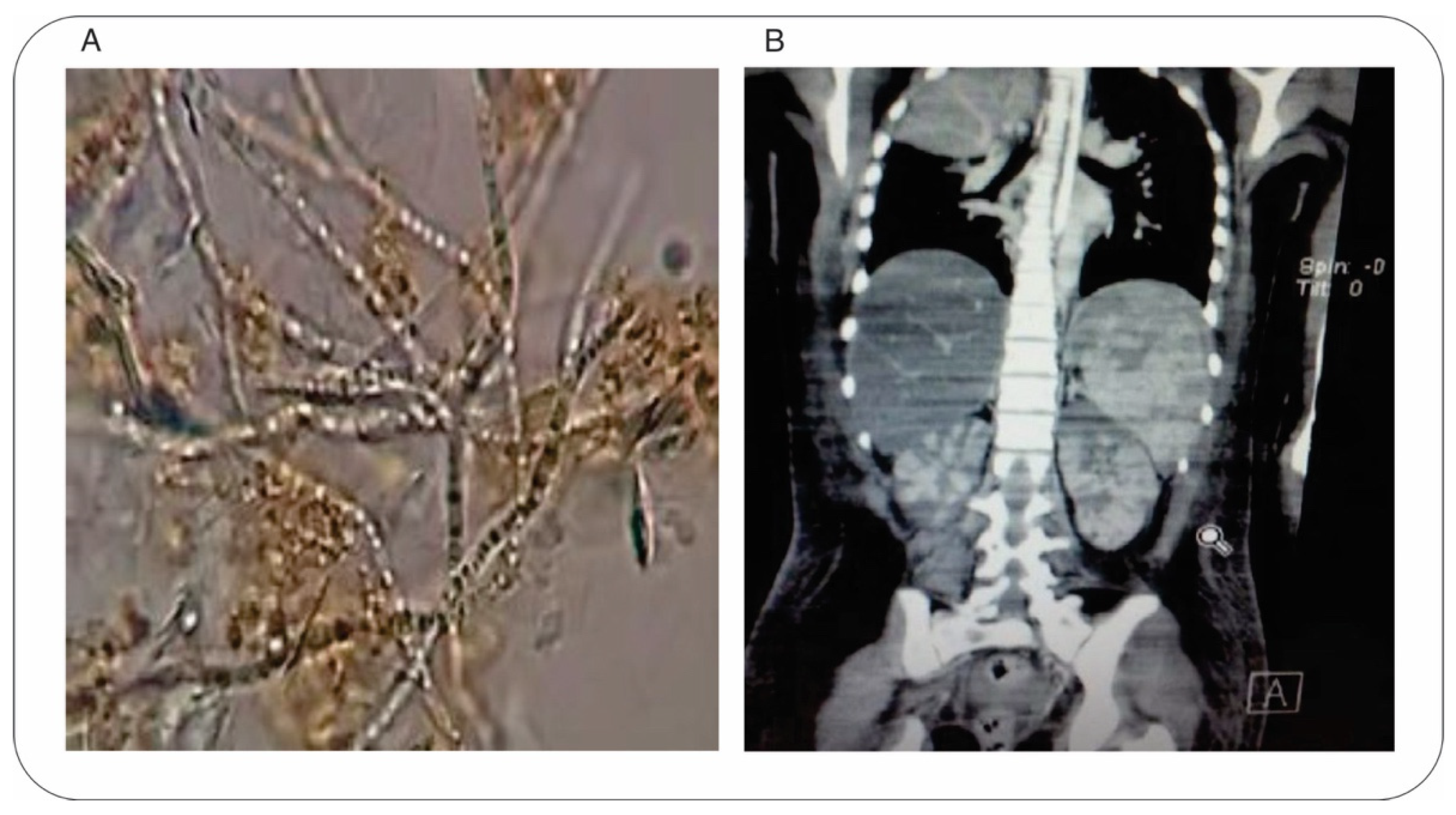
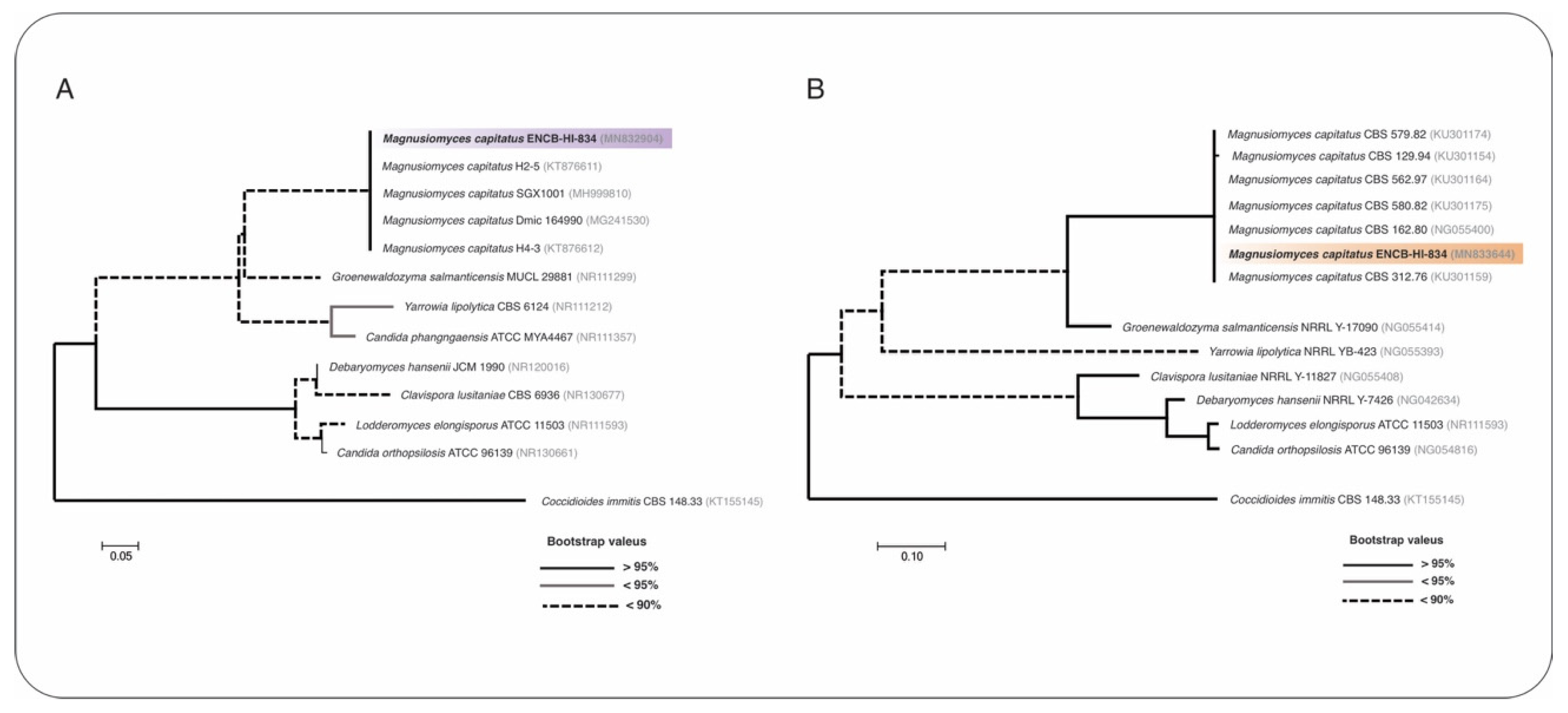
2. Case Presentation
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouza, E.; Muñoz, P. Invasive infections caused by Blastoschizomyces capitatus and Scedosporium spp. Clin. Microbiol. Infect. 2004, 10 (Suppl. S1), 76–85. [Google Scholar] [CrossRef]
- Gadea, I.; Cuenca-Estrella, M.; Prieto, E.; Diaz-Guerra, T.M.; Garcia-Cia, J.I.; Mellado, E.; Tomas, J.F.; Rodriguez-Tudela, J.L. Genotyping and antifungal susceptibility profile of Dipodascus capitatus isolates causing disseminated infection in seven hematological patients of a tertiary hospital. J. Clin. Microbiol. 2004, 42, 1832–1836. [Google Scholar] [CrossRef]
- Miceli, M.H.; Díaz, J.A.; Lee, S.A. Emerging opportunistic yeast infections. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef]
- Buchta, V.; Bolehovská, R.; Hovorková, E.; Cornely, O.A.; Seidel, D.; Žák, P. Saprochaete clavata Invasive Infections—A New Threat to Hematological-Oncological Patients. Front. Microbiol. 2019, 10, 2196. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, J.C.; López-Soria, L.; Olazábal, I.; Amutio, E.; Arrieta-Aguirre, I.; Velasco-Benito, V.; Pontón, J.; Moragues, M.D. Invasive infections caused by Saprochaete capitata in patients with haematological malignancies: Report of five cases and review of the antifungal therapy. Rev. Iberoam. Micol. 2013, 30, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Odabasi, Z.; Paetznick, V.L.; Rodriguez, J.R.; Chen, E.; McGinnis, M.R.; Ostrosky-Zeichner, L. Differences in beta-glucan levels in culture supernatants of a variety of fungi. Med. Mycol. 2006, 44, 267–272. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Boekhout, T.; Akova, M.; Meis, J.F.; Cornely, O.A.; Lortholary, O.; Arikan-Akdagli, S.; Cuenca-Estrella, M.; Dannaoui, E.; van Diepeningen, A.D.; et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin. Microbiol. Infect. 2014, 20 (Suppl. S3), 76–98. [Google Scholar] [CrossRef] [PubMed]
- Desnos-Ollivier, M.; Blanc, C.; Garcia-Hermoso, D.; Hoinard, D.; Alanio, A.; Dromer, F. Misidentification of Saprochaete clavata as Magnusiomyces capitatus in clinical isolates: Utility of internal transcribed spacer sequencing and matrix-assisted laser desorption ionization-time of flight mass spectrometry and importance of reliable databas. J. Clin. Microbiol. 2014, 52, 2196–2198. [Google Scholar] [CrossRef]
- Alobaid, K.; Abdullah, A.A.; Ahmad, S.; Joseph, L.; Khan, Z. Magnusiomyces capitatus fungemia: The value of direct microscopy in early diagnosis. Med. Mycol. Case Rep. 2019, 25, 32–34. [Google Scholar] [CrossRef]
- El Zein, S.; Hindy, J.R.; Kanj, S.S. Invasive Saprochaete Infections: An Emerging Threat to Immunocompromised Patients. Pathogens 2020, 9, 922. [Google Scholar] [CrossRef]
- Hoffman, C.S.; Winston, F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 1987, 57, 267–272. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes form phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press Inc.: London, UK, 1990; pp. 315–322. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Pei, D.; Wolf, J.; Howard, S.C.; Hayden, R.T.; Go, M.; Varechtchouk, O.; Hahn, T.; Buaboonnam, J.; Metzger, M.L.; et al. Infection-related complications during treatment for childhood acute lymphoblastic leukemia. Ann. Oncol. 2017, 28, 386–392. [Google Scholar] [CrossRef]
- Martino, R.; Salavert, M.; Parody, R.; Tomás, J.F.; De La Cámara, R.; Vázquez, L.; Jarque, I.; Prieto, E.; Sastre, J.L.; Gadea, I.; et al. Blastoschizomyces capitatus infection in patients with leukemia: Report of 26 cases. Clin. Infect. Dis. 2004, 38, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Girmenia, C.; Pagano, L.; Martino, B.; D’Antonio, D.; Fanci, R.; Specchia, G.; Melillo, L.; Buelli, M.; Pizzarelli, G.; Venditti, M.; et al. Invasive infections caused by Trichosporon species and Geotrichum capitatum in patients with hematological malignancies: A retrospective multicenter study from Italy and review of the literature. J. Clin. Microbiol. 2005, 43, 1818–1828. [Google Scholar] [CrossRef]
- Gao, G.X.; Tang, H.L.; Zhang, X.; Xin, X.L.; Feng, J.; Chen, X.Q. Invasive fungal infection caused by Geotrichum capitatum in patients with acute lymphoblastic leukemia: A case study and literature review. Int. J. Clin. Exp. Med. 2015, 8, 14228. [Google Scholar] [PubMed]
- Birrenbach, T.; Bertschy, S.; Aebersold, F.; Mueller, N.J.; Achermann, Y.; Muehlethaler, K.; Zimmerli, S. Emergence of Blastoschizomyces capitatus yeast infections, Central Europe. Emerg. Infect. Dis. 2012, 18, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Subramanya Supram, H.; Gokhale, S.; Chakrabarti, A.; Rudramurthy, S.M.; Gupta, S.; Honnavar, P. Emergence of Magnusiomyces capitatus infections in Western Nepal. Med. Mycol. 2016, 54, 103–110. [Google Scholar] [CrossRef]
- D’Assumpcao, C.; Lee, B.; Heidari, A. A Case of Magnusiomyces capitatus Peritonitis Without Underlying Malignancies. J. Investig. Med. High Impact Case Rep. 2018, 6, 2324709618795268. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.; Al-Hatmi, A.M.S.; Ilkit, M.; Gerrits van den Ende, A.H.G.; Hagen, F.; Meis, J.F.; Sybren De Hoog, G. Molecular Diagnostics of Arthroconidial Yeasts, Frequent Pulmonary Opportunists. J. Clin. Microbiol. 2017, 56, e01427-17. [Google Scholar] [CrossRef] [PubMed]
- Tanuskova, D.; Horakova, J.; Svec, P.; Bodova, I.; Lengerova, M.; Bezdicek, M.; Poczova, M.; Koppl, J.; Kolenova, A. First case of invasive Magnusiomyces capitatus infection in Slovakia. Med. Mycol. Case Rep. 2017, 16, 12–15. [Google Scholar] [CrossRef]
- Menu, E.; Criscuolo, A.; Desnos-Ollivier, M.; Cassagne, C.; D’Incan, E.; Furst, S.; Ranque, S.; Berger, P.; Dromer, F. Saprochaete clavata Outbreak Infecting Cancer Center through Dishwasher. Emerg. Infect. Dis. 2020, 26, 2031–2038. [Google Scholar] [CrossRef]
- Leoni, M.; Riccardi, N.; Rotulo, G.A.; Godano, E.; Faraci, M.; Bandettini, R.; Esposto, M.C.; Castagnola, E. Magnusiomyces clavatus infection in a child after allogeneic hematotopoietic stem cell transplantation: Diagnostic and therapeutic implications. Med. Mycol. Case Rep. 2018, 21, 65–67. [Google Scholar]
- O’Connor, D.; Bate, J.; Wade, R.; Clack, R.; Dhir, S.; Hough, R.; Vora, A.; Goulden, N.; Samarasinghe, S. Infection-related mortality in children with acute lymphoblastic leukemia: An analysis of infectious deaths on UKALL2003. Blood 2014, 124, 1056–1061. [Google Scholar] [CrossRef]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 2022, 29, 557–571. [Google Scholar] [CrossRef]
- Wiederhold, N.P. Emerging Fungal Infections: New Species, New Names, and Antifungal Resistance. Clin. Chem. 2021, 68, 83–90. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Álvarez, J.; Reséndiz-Sánchez, J.; Juárez-Montiel, M.; Hernández-García, J.A.; Vázquez-Guerrero, E.; Hernández-Rodríguez, C.; Villa-Tanaca, L. Invasive Fungal Infection Caused by Magnusiomyces capitatus in an Immunocompromised Pediatric Patient with Acute Lymphoblastic Leukemia in Mexico City: A Case Report. J. Fungi 2022, 8, 851. https://doi.org/10.3390/jof8080851
Ortiz-Álvarez J, Reséndiz-Sánchez J, Juárez-Montiel M, Hernández-García JA, Vázquez-Guerrero E, Hernández-Rodríguez C, Villa-Tanaca L. Invasive Fungal Infection Caused by Magnusiomyces capitatus in an Immunocompromised Pediatric Patient with Acute Lymphoblastic Leukemia in Mexico City: A Case Report. Journal of Fungi. 2022; 8(8):851. https://doi.org/10.3390/jof8080851
Chicago/Turabian StyleOrtiz-Álvarez, Jossue, Jesús Reséndiz-Sánchez, Margarita Juárez-Montiel, Juan Alfredo Hernández-García, Edwin Vázquez-Guerrero, César Hernández-Rodríguez, and Lourdes Villa-Tanaca. 2022. "Invasive Fungal Infection Caused by Magnusiomyces capitatus in an Immunocompromised Pediatric Patient with Acute Lymphoblastic Leukemia in Mexico City: A Case Report" Journal of Fungi 8, no. 8: 851. https://doi.org/10.3390/jof8080851
APA StyleOrtiz-Álvarez, J., Reséndiz-Sánchez, J., Juárez-Montiel, M., Hernández-García, J. A., Vázquez-Guerrero, E., Hernández-Rodríguez, C., & Villa-Tanaca, L. (2022). Invasive Fungal Infection Caused by Magnusiomyces capitatus in an Immunocompromised Pediatric Patient with Acute Lymphoblastic Leukemia in Mexico City: A Case Report. Journal of Fungi, 8(8), 851. https://doi.org/10.3390/jof8080851






