Beauveria bassiana (Hypocreales: Clavicipitaceae) Volatile Organic Compounds (VOCs) Repel Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Palm Petioles Used in the Bioassays
2.2. Entomopathogenic Fungus, Beauveria bassiana
2.3. Description of Y-Tube Olfactometer Bioassay
2.4. Y-Tube Olfactometer Behavioral Bioassays
2.5. Effect of the Presence of B. bassiana Solid Formulation on the Behavioural (Y-Tube Olfactometer) Bioassays
2.6. Analysis of Volatile Organic Compounds (VOCs) from Entomopathogenic Fungus, B. bassiana 203
2.7. Behavioural (Y-tube Olfactometer) Bioassays on Candidate Chemicals
2.8. Data Assessment
3. Results
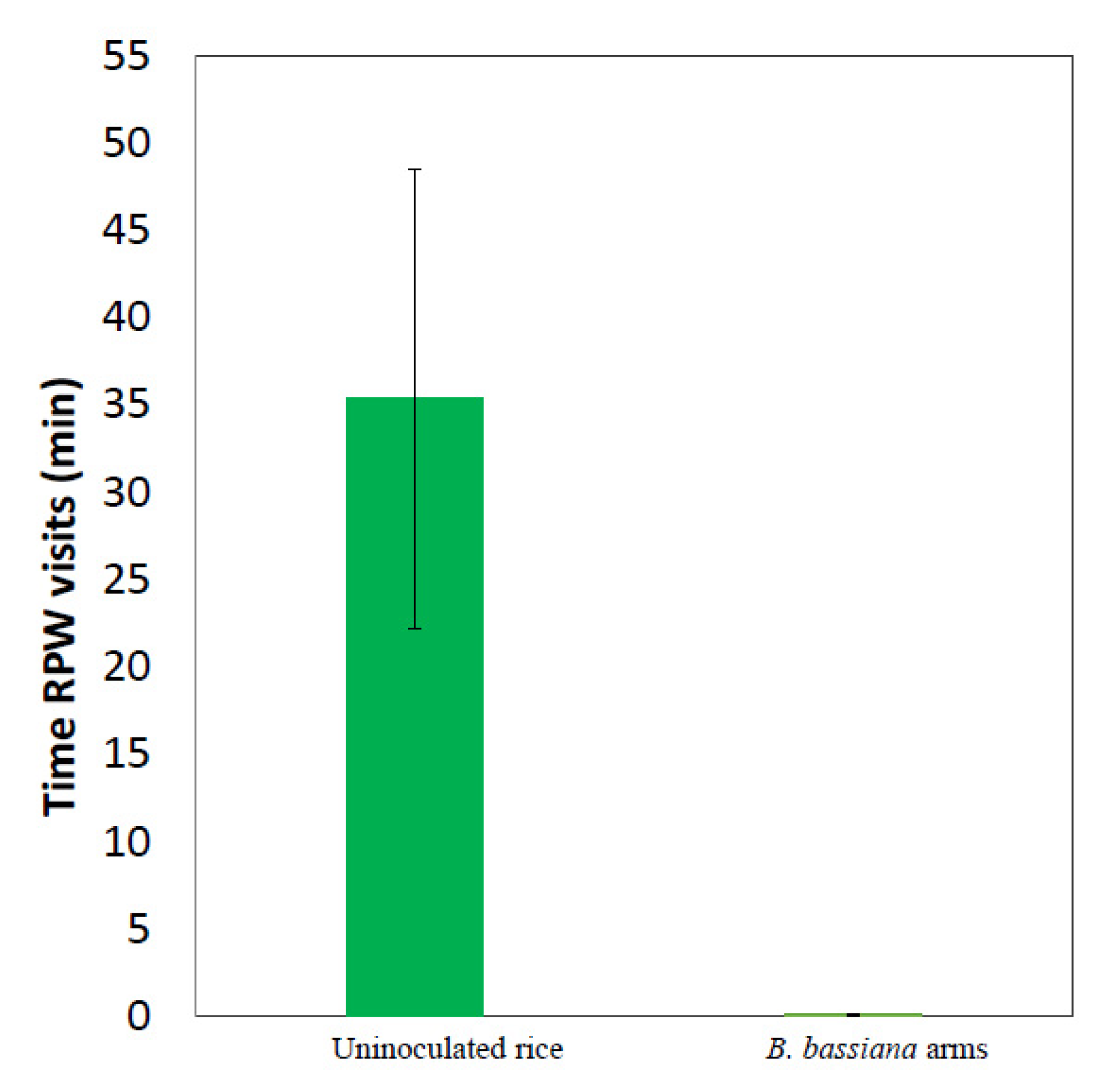
3.1. Solid Formulations of the Entomopathogenic Fungus B. bassiana Repel Red Palm Weevil Females
3.2. Identification of B. bassiana VOCs
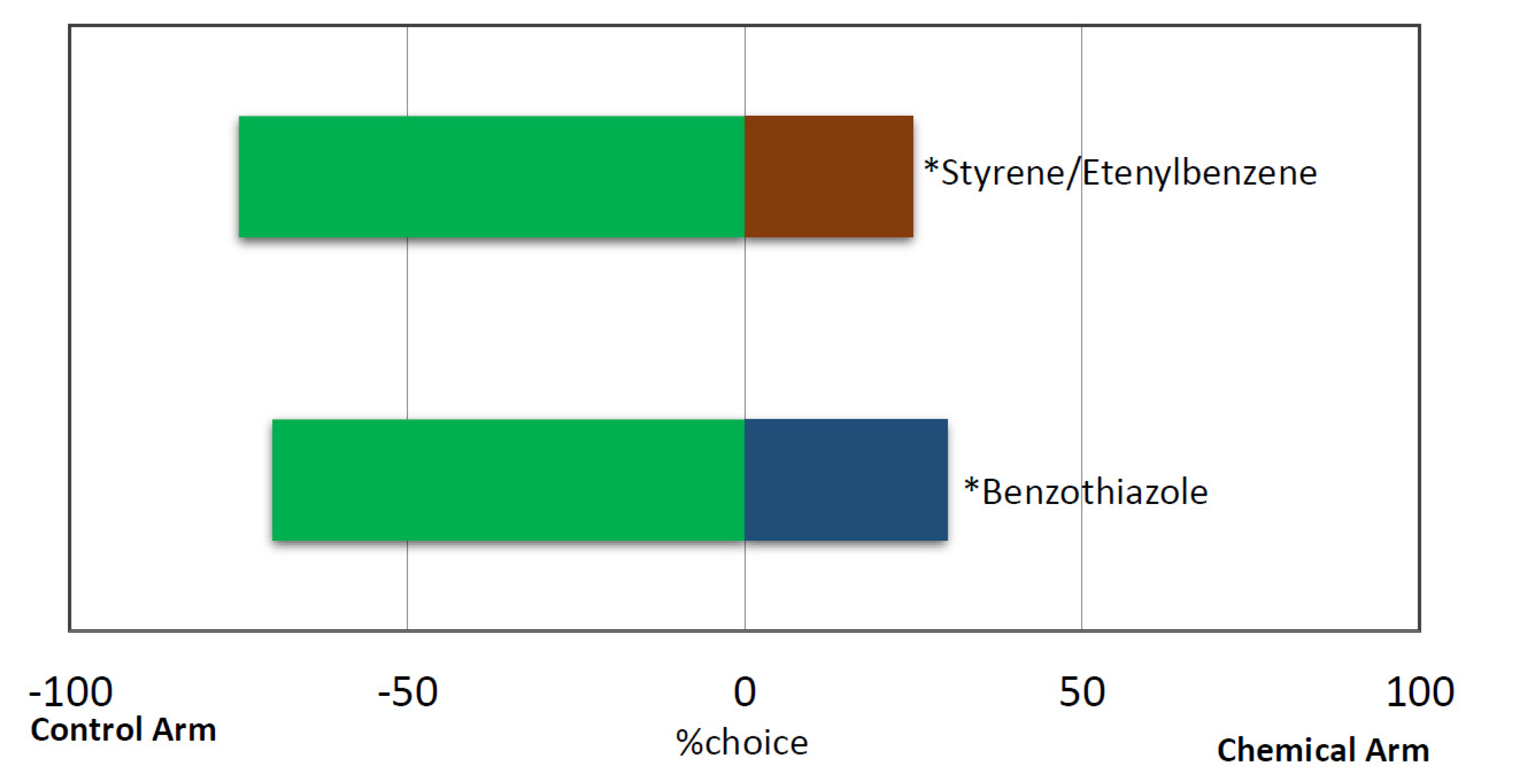
3.3. Response of Red Palm Weevil Females to Pure VOCs from B. bassiana
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferry, M.; Gómez, S.; Jimenez, E.; Navarro, J.; Ruiperez, E.; Vilella, J. The date palm grove of Elche, Spain: Research for the sustainable and preservation of a World Heritage Site. Palm 2002, 46, 139–148. [Google Scholar]
- El-Mergaway, R.A.A.M.; Al-Ajlan, A.M. Red palm weevil, Rhynchophorus ferrugineus (Oliver): Economic importance, biology, biogeography and integrated pest management. J. Agric. Sci. Technol. 2011, A1, 1–23. [Google Scholar]
- Williams, J.R.; Pillay, A.E. Metals, metalloids and toxicity in date palms: Potential environmental impact. J. Environ. Prot. 2011, 2, 592–600. [Google Scholar] [CrossRef][Green Version]
- Rahman, M.M.; Kim, E.; Kim, D.; Bhuyain, M.M.H.; Lim, U.T. Use of Aggregation Pheromone Traps Increases Infestation of Adult Riptortus pedestris (Hemiptera: Alydidae) in Soybean Fields. Pest. Manag. Sci. 2018, 74, 2578–2588. [Google Scholar] [CrossRef] [PubMed]
- Giblin-Davis, R.M.; Oehlschlager, A.C.; Perez, A.; Gries, G.; Gries, R.; Weissling, T.J.; Chinchilla, C.M.; Peña, J.E.; Hallett, R.H.; Pierce, H.D., Jr.; et al. Chemical and behavioural ecology of palm weevils (Coleoptera: Curculionidae). Fla. Entomol. 1996, 79, 153–166. [Google Scholar] [CrossRef]
- Poorjavad, N.; Goldansaz, S.H.; Avand-Faghih, A. Response of the red palm weevil Rhynchophorus ferrugineus to its aggregation pheromone under laboratory conditions. Bull. Insectology 2009, 62, 252–260. [Google Scholar]
- Rochat, D.; Malosse, C.; Lettere, M.; Ducrot, P.H.; Zagatti, P.; Renou, M.; Descoins, C. Male-produced aggregation pheromone of the American palm weevil, Rhynchophrus palmarum (L.) (Coleoptera: Curculionidae): Collection, identification, electrophysiological activity, and laboratory bioassay. J. Chem. Ecol. 1991, 17, 2127–2141. [Google Scholar] [CrossRef]
- Saïd, I.; Tauban, D.; Renou, M.; Ducrot, P.-H.; Zagatti, P.; Renou, M.; Descoins, C. Structure and function of the antennal sensilla of the palm weevil Rhynchophorus palmarum (Coleptera, Curculionidae). J. Insect. Physiol. 2003, 49, 857–872. [Google Scholar] [CrossRef]
- Ávalos, M.J.A.; Martí, C.A.; Soto, T.M.T. Study of the flying ability of Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) adults using a computer-monitored flight mill. Bull. Entomol. Res. 2014, 104, 462–467. [Google Scholar] [CrossRef]
- Pagans, E.; Font, X.; Sánchez, A. Emission of volatile organic compounds from composting of different solid wastes: Abatement by bioflitration. J. Hazard. Mater. 2006, 131, 179–186. [Google Scholar] [CrossRef]
- Korpi, A.; Järnberg, J.; Pasanen, A.L. Microbial volatile organic compounds. Crit. Rev. Toxicol. 2009, 39, 139–193. [Google Scholar] [CrossRef]
- Crespo, R.; Pedrini, N.; Juárez, M.P.; Dal Bello, G.M. Volatile organic compounds released by the entomopathogenic fungus Beauveria bassiana. Microbiol. Res. 2008, 163, 148–151. [Google Scholar] [CrossRef]
- Müller, A.; Faubert, P.; Hagen, M.; Zu Castell, W.; Polle, A.; Schnitzler, J.-P.; Rosenkranz, M. Volatile profiles of fungi-chemotyping of species and ecological functions. Fungal Genet. Biol. 2013, 54, 25–33. [Google Scholar] [CrossRef]
- Yanagawa, Y.; Yokohari, F.; Shimizu, S. Influence of fungal odor on grooming behavior of the termite Coptotermes Formos. J. Insect. Sci. 2009, 10, 141. [Google Scholar] [CrossRef]
- Herrera, J.M.; Pizzolitto, R.P.; Zunino, M.P.; Dambolena, J.S.; Zygadlo, J.A. Effect of fungal volatile organic compounds on a fungus and insect that damage stored maize. J. Stored. Prod. Res. 2015, 62, 74–80. [Google Scholar] [CrossRef]
- Daisy, B.H.; Strobel, G.A.; Castillo, U.; Ezra, D.; Sears, J.; Weaver, D.K.; Runyon, J.B. Napthalene, an insect repellent, is produced by Muscodor vitigenus, a novel endophytic fungus. Microbiology 2002, 148, 3737–3741. [Google Scholar] [CrossRef]
- Strobel, G.; Manker, D.; Mercier, J. Novel endophytic fungi and methods of use. U.S. Patent 0285543 A1, 11 November 2010. [Google Scholar]
- Ormond, E.L.; Thomas, A.P.; Pell, J.K.; Freeman, S.N.; Roy, H.E. Avoidance of a generalist entomopathogenic fungus by the ladybird, Coccinella septempunctata. FEMS Microbiol. Ecol. 2011, 77, 229–237. [Google Scholar] [CrossRef]
- Meyling, N.V.; Pell, J.K. Detection and avoidance of an entomopathogenic fungus by a generalist insect predator. Ecol. Entomol. 2006, 31, 162–171. [Google Scholar] [CrossRef]
- Thompson, S.R.; Brandenburg, R.L.; Roberson, G.T. Entomopathogenic fungi detection and avoidance by mole crickets (Orthoptera: Gryllotalpidae). Environ. Entomol. 2007, 36, 165–172. [Google Scholar] [CrossRef]
- Inamdar, A.A.; Masurekar, P.; Bennett, J.W. Neurotoxicity of fungal volatile organic compounds in Drosphilla melanogaster. Toxicol. Sci. 2010, 117, 418–426. [Google Scholar] [CrossRef]
- Bojke, A.; Tkaczuk, C.; Stepnowski, P.; Gołębiowski, M. Comparison of volatile compounds released by entomopathogenic fungi. Microbiol. Res. 2018, 214, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Morath, S.U.; Hung, R.; Bennett, J.W. Fungal volatile organic compounds: A review with emphasis on their biotechnological potential. Fungal. Biol. Rev. 2012, 26, 73. [Google Scholar] [CrossRef]
- Lozano-Soria, A.; Picciotti, U.; Lopez-Moya, F.; Lopez-Cepero, J.; Porcelli, F.; Lopez-Llorca, L. Volatile Organic Compounds from Entomopathogenic and Nematophagous Fungi, Repel Banana Black Weevil (Cosmopolites sordidus). Insects 2020, 11, 509. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-I.; Tak, J.-H.; Seo, J.; Park, S.; Kim, J.; Boo, K.-H. Repellency of Veratraldehyde (3,4-Dimethoxy Benzaldehyde) against Mosquito Females and Tick Nymphs. Appl. Sci. 2021, 11, 4861. [Google Scholar] [CrossRef]
- Wheatley, R.; Hackett, C.; Bruce, A.; Kundzewicz, A. Effect of substrate composition on production of volatile organic compounds from Trichoderma spp. Inhibitory to wood decay fungi. Int. Biodeterior. Biodegrad. 1997, 39, 199–205. [Google Scholar] [CrossRef]
- Mäki, M.; Mali, T.; Hellén, H.; Heinonsalo, J.; Lundell, T.; Bäck, J. Deadwood substrate and species-species interactions determine the release of volatile organic compounds by wood-decaying fungi. Fungal. Ecol. 2021, 54, 101106. [Google Scholar] [CrossRef]
- Ricaño, J.; Güerri-Agulló, B.; Serna-Sarriás, M.J.; Rubio-Llorca, G.; Asensio, L.; Barranco, P.; Lopez-Llorca, L.V. Evaluation of the pathogenicity of multiple isolates of Beauveria bassiana (Hypocreales: Clavicipitae) on Rhynchophorus ferrugineus (Coleopter: Dryophthoridae) for the assessment of a solid formulation under simulated field conditions. Fla. Entomol. 2013, 96, 1311–1324. [Google Scholar] [CrossRef]
- Ishak, I.; Ng, L.C.; Haris-Hussain, M.; Jalinas, J.; Idris, A.B.; Azlina, Z.; Samsudin, A.; Wahizatul, A.A. Pathogenicity of an Indigenous Strain of the Entomopathogenic Fungus Metarhizium anisopliae (Hypocreales: Clavicipitaceae) (MET-GRA4 Strain) as a Potential Biological Control Agent Against the Red Palm Weevil (Coleoptera: Dryophthoridae). J. Econ. Entomol. 2020, 113, 43–49. [Google Scholar] [CrossRef]
- Sutanto, K.D.; Husain, M.; Rasool, K.G.; Al-Qahtani, W.H.; Aldawood, A.S. Pathogenicity of local and exotic entomopathogenic fungi isolates against different life stages of red palm weevil (Rhynchophorus ferrugineus). PLoS ONE 2021, 16, e0255029. [Google Scholar] [CrossRef]
- Qayyum, M.A.; Saleem, M.A.; Saeed, S.; Wakil, W.; Ishtiaq, M.; Ashraf, W.; Ahmed, N.; Ali, M.; Ikram, R.M.; Yasin, M.; et al. Integration of entomopathogenic fungi and eco-friendly insecticides for management of red palm weevil, Rhynchophorus ferrugineus (Olivier). Saudi J. Biol. Sci. 2020, 27, 1811–1817. [Google Scholar] [CrossRef]
- Wakil, W.; Yasin, M.; Qayyum, M.A.; Ghazanfar, M.U.; Al-Sadi, A.M.; Bedford, G.O.; Kwon, Y.J. Resistance to commonly used insecticides and phosphine fumigant in red palm weevil, Rhynchophorus ferrugineus (Olivier) in Pakistan. PLoS ONE 2018, 13, e0192628. [Google Scholar] [CrossRef]
- El-Shafie, H.A.F.; Faleiro, J.R. Red Palm Weevil Rhynchophorus ferrugineus (Coleoptera: Curculionidae): Global Invasion, Current Management Options, Challenges and Future Prospects. In Invasive Species—Introduction Pathways, Economic Impact, and Possible Management Options; El-Shafie, H., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Kaakeh, W.; El-Ezaby, F.; Aboul-Nour, M.M.; Khamis, A.A. Management of the red palm weevil by a pheromone/food-based trapping system. In Proceedings of the 2nd International Conference on Date Palms, Al-Ain, United Arab Emirates, 25–27 March 2001; pp. 325–343. [Google Scholar]
- Jalinas, J.; Güerri-Agulló, B.; Mankin, R.W.; Lopez-Follana, R.; Lopez-Llorca, L.V. Acoustic assessment of Beauveria bassiana (Hypocreales:Clavicipitaceae) effects on Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) larval activity and mortality. J. Econ. Entomol. 2015, 108, 444–453. [Google Scholar] [CrossRef]
- Prabhu, S.T.; Patil, R.S. Studies on the biological aspects of red palm weevil. Rhynchophorus ferrugineus (Oliv.). Karnataka J. Agric. Sci. 2009, 22, 732–733. [Google Scholar]
- Güerri-Agulló, B.; Gómez-Vidal, S.; Asensio, L.; Barranco, P.; Lopez-Llorca, L.V. Infection of the red palm weevil (Rhynchophorus ferrugineus) by the entomopathogenic fungus Beauveria bassiana: A SEM study. Microsc. Res. Tech. 2010, 73, 714–725. [Google Scholar]
- Asensio-Berbegal, L.; Lopez-Llorca, L.V.; Carbonell, T.; Güerri-Agulló, B.; Barranco, P. Phytosanitary composition comprising an entomopathogenic fungus belonging to the species B. bassiana and triturates or fragments of dates, method of preparation and use of same. ES patent application PCT/ES2008/070010, 11 December 2008. Available online: https://patents.google.com/patent/WO2008148920A1/en (accessed on 27 July 2022).
- Rhodes, B.C.; Blair, C.P.; Takahashi, M.K.; Abrahamson, W.G. The role of olfactory cues in the sequential radiation of a gall-boring beetle, Mordellistena convicta. Ecol. Entomol. 2012, 37, 500–507. [Google Scholar] [CrossRef]
- Onagbola, E.O.; Fadamiro, H.Y. Response of Pteromalus cerealellae to conspecific odor: Evidence for female- and male-produced pheromones? J. Stored Prod. Res. 2011, 47, 393–398. [Google Scholar] [CrossRef]
- Doddala, P.R.C.; Minor, M.A.; Wang, Q.; Rogers, D.J.; Koot, E.; Trewick, S.A. Role of olfaction in host plant selection and local adaptation of a polyphagous herbivore, Eucolaspis Sharp. J. Appl. Entomol. 2016, 140, 444–452. [Google Scholar] [CrossRef]
- Zada, A.; Falach, L.; Byers, J.A. Development of sol-gel formulations for slow release of pheromones. Chemoecology 2009, 19, 37–45. [Google Scholar] [CrossRef][Green Version]
- Seidey, M.; Heydari, S.; Tork, M. Orientation of Hippodamia variegata (Coleoptera: Coccinellidae) to healthy and Beauveria bassiana-infected Aphis fabae (Hemiptera: Aphididae) in an olfactometer system. Turk. J. Zool. 2015, 39, 53–58. [Google Scholar] [CrossRef]
- Rännbäck, L.M.; Cotes, B.; Anderson, P.; Rämert, B.; Meyling, N.V. Mortality risk from entomopathogenic fungi effects oviposition behavior in the parasitoid wasp Tyrbliographa rapae. J. Invertebr. Pathol. 2015, 124, 78–86. [Google Scholar] [CrossRef]
- Azeem, M.; Rajarao, G.K.; Nordenhem, H.; Nordlander, G.; Borg-Karlson, A.K. Penicillium expansum volatiles reduce pine weevil attraction to host plants. J. Chem. Ecol. 2013, 39, 120–128. [Google Scholar] [CrossRef]
- Sever, B.; Altıntop, M.D.; Özdemir, A.; Tabanca, N.; Estep, A.S.; Becnel, J.J.; Bloomquist, J.R. Biological evaluation of a series of benzothiazole derivates as mosquitocidal agents. Open Chem. 2019, 17, 288–294. [Google Scholar] [CrossRef]
- Sever, B.; Altıntop, M.D.; Özdemir, A.; Tabanca, N.; Estep, A.S.; Becnel, J.J.; Bloomquist, J.R. Effects of benzothiazole on survival for reduced reproduction and development in Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). Pest Manag. Sci. 2020, 76, 3088–3095. [Google Scholar]
- Faleiro, J.R.; El-Shafie, H.A.F.; Oehlschlager, A.C.; Aleid, S.M.A.; Mahajan, G.R. Field evaluation of repellents against red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) through trap shutdown studies. J. Plant. Dis. Prot. 2022, 129, 791–804. [Google Scholar] [CrossRef]
- Szelényi, M.O.; Erdei, A.L.; Jósvai, J.K.; Radványi, D.; Sümegi, B.; Vétek, G.; Molnár, B.P.; Kárpáti, Z. Essential oil headspace volatiles prevent invasive box tree moth (Cydalima perspectalis) oviposition-insights from electrophysiology and behaviour. Insects 2020, 11, 465. [Google Scholar] [CrossRef]

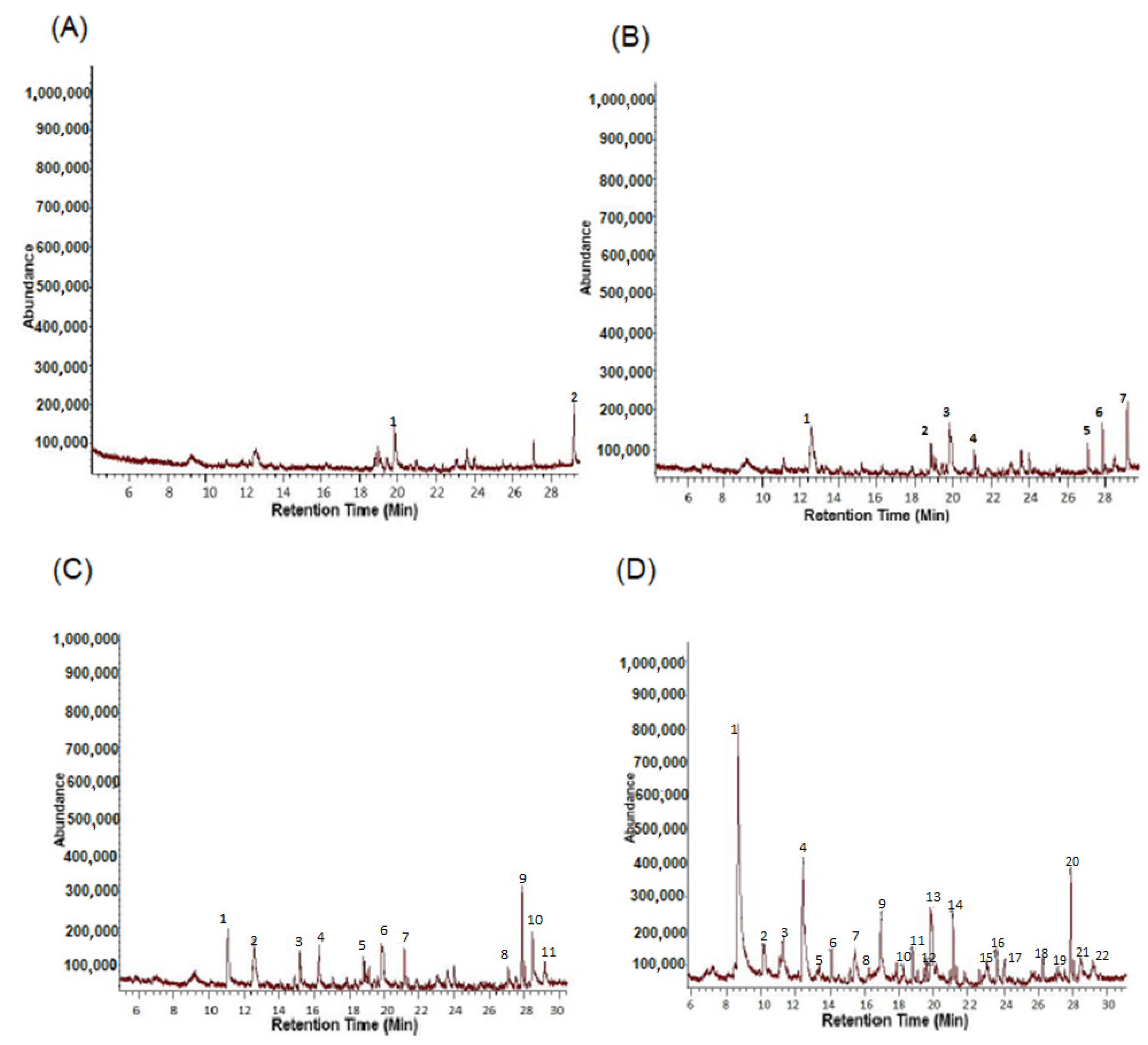

| Peak No. | R.T | Corr. Area | Quality | Compound |
|---|---|---|---|---|
| 1 | 19.839 | 6,625,068 | 93% | Benzaldehyde |
| 2 | 29.179 | 6,815,159 | 91% | 1-Decanol |
| Peak No. | R.T | Corr. Area | Quality | Compound |
|---|---|---|---|---|
| 1 | 12.564 | 14,636,750 | 86% | Cyclotrisiloxane,hexamethyl |
| 2 | 18.84 | 3,652,706 | 86% | Pentasiloxane,dodecamthyl |
| 3 | 19.843 | 10,330,336 | 93% | Benzaldehyde |
| 4 | 21.137 | 3,411,857 | 58% | Undecane, 5-methyl |
| 5 | 27.099 | 2,619,884 | 76% | Capric Aldehyde |
| 6 | 27.877 | 5,071,929 | 87% | Benzene, 1,4-bis(1,1-dimethylethyl)- |
| 7 | 29.179 | 8,110,175 | 91% | 1-Decanol |
| Peak No. | R.T | Corr. Area | Quality | Compound |
|---|---|---|---|---|
| 1 | 11.08 | 9,787,079 | 93% | Benzene, methyl- (CAS) |
| 2 | 12.564 | 12,854,506 | 86% | Cyclotrisiloxane, hexamethyl |
| 3 | 15.186 | 5,433,240 | 94% | Benzene, 1,4-dimethyl |
| 4 | 16.27 | 7,055,705 | 93% | Styrene/Benzene, Ethenyl |
| 5 | 18.84 | 4,447,065 | 86% | Pentasiloxane,dodecamethyl |
| 6 | 19.843 | 10,330,336 | 93% | Benzaldehyde |
| 7 | 21.137 | 5,086,441 | 58% | Undecane, 5-methyl |
| 8 | 27.099 | 2,619,884 | 76% | Capric Aldehyde |
| 9 | 27.877 | 9,834,548 | 87% | Benzene, 1,4-bis(1,1-dimethylethyl)- |
| 10 | 28.485 | 10,858,270 | 93% | Benzothiazole |
| 11 | 29.179 | 8,110,175 | 91% | 1-Decanol |
| Peak No. | R.T | Corr. Area | Quality | Compound |
|---|---|---|---|---|
| 1 | 8.682 | 76,630,838 | 86% | Acetic Acid |
| 2 | 10.202 | 8,126,923 | 62% | Hexane, 2,5-dimethyl |
| 3 | 11.251 | 8,753,352 | 50% | 1-Butanol, 3-methyl-(impure) |
| 4 | 12.448 | 31,223,894 | 78% | Heptane,2,4-dimethyl |
| 5 | 14.097 | 3,768,825 | 81% | Octane, 4-methyl |
| 6 | 15.186 | 5,433,240 | 94% | Benzene,1,4dimethyl |
| 7 | 15.463 | 8,189,342 | 96% | 2-Furancarboxaldehyde |
| 8 | 16.27 | 7,055,705 | 93% | Styrene/Benzene, ethenyl |
| 9 | 16.942 | 14,731,012 | 41% | Disiloxane, pentamethyl |
| 10 | 18.84 | 4,447,065 | 86% | Penatsiloxane, dodecamethyl |
| 11 | 19.63 | 2,610,757 | 46% | Undecane |
| 12 | 19.843 | 10,330,336 | 93% | Benzaldehyde |
| 12a | 19.917 | 9,968,124 | 86% | Benzaldehyde |
| 13 | 21.137 | 8,752,939 | 58% | Undercane, 5-methyl |
| 14 | 22.987 | 2342078 | 87% | Phenol |
| 15 | 23.588 | 5,138,127 | 95% | Ethanone. 1-phenyl |
| 16 | 24.01 | 3,254,487 | 64% | Cyclopentasiloxane, decamethyl |
| 17 | 26.250 | 4,020,849 | 46% | 3-Hexyn-1-ol |
| 18 | 27.099 | 2,619,884 | 76% | Capric Aldhyde |
| 19 | 27.877 | 11,251,518 | 87% | Benzene, 1,4-bis(1,1-dimethylethyl)- |
| 20 | 28.485 | 4,952,743 | 93% | Benzothiazole |
| 21 | 29.179 | 8,110,175 | 91% | 1-Decanol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalinas, J.; Lopez-Moya, F.; Marhuenda-Egea, F.C.; Lopez-Llorca, L.V. Beauveria bassiana (Hypocreales: Clavicipitaceae) Volatile Organic Compounds (VOCs) Repel Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae). J. Fungi 2022, 8, 843. https://doi.org/10.3390/jof8080843
Jalinas J, Lopez-Moya F, Marhuenda-Egea FC, Lopez-Llorca LV. Beauveria bassiana (Hypocreales: Clavicipitaceae) Volatile Organic Compounds (VOCs) Repel Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae). Journal of Fungi. 2022; 8(8):843. https://doi.org/10.3390/jof8080843
Chicago/Turabian StyleJalinas, Johari, Federico Lopez-Moya, Frutos C. Marhuenda-Egea, and Luis Vicente Lopez-Llorca. 2022. "Beauveria bassiana (Hypocreales: Clavicipitaceae) Volatile Organic Compounds (VOCs) Repel Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae)" Journal of Fungi 8, no. 8: 843. https://doi.org/10.3390/jof8080843
APA StyleJalinas, J., Lopez-Moya, F., Marhuenda-Egea, F. C., & Lopez-Llorca, L. V. (2022). Beauveria bassiana (Hypocreales: Clavicipitaceae) Volatile Organic Compounds (VOCs) Repel Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae). Journal of Fungi, 8(8), 843. https://doi.org/10.3390/jof8080843








