Inhibitory Effects and Mechanism of Action of Elsinochrome A on Candida albicans and Its Biofilm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Antifungal Susceptibility Testing
2.3. Effect of PACT on the Growth Kinetics of C. albicans
2.4. Effect of PACT on Biofilm Formation
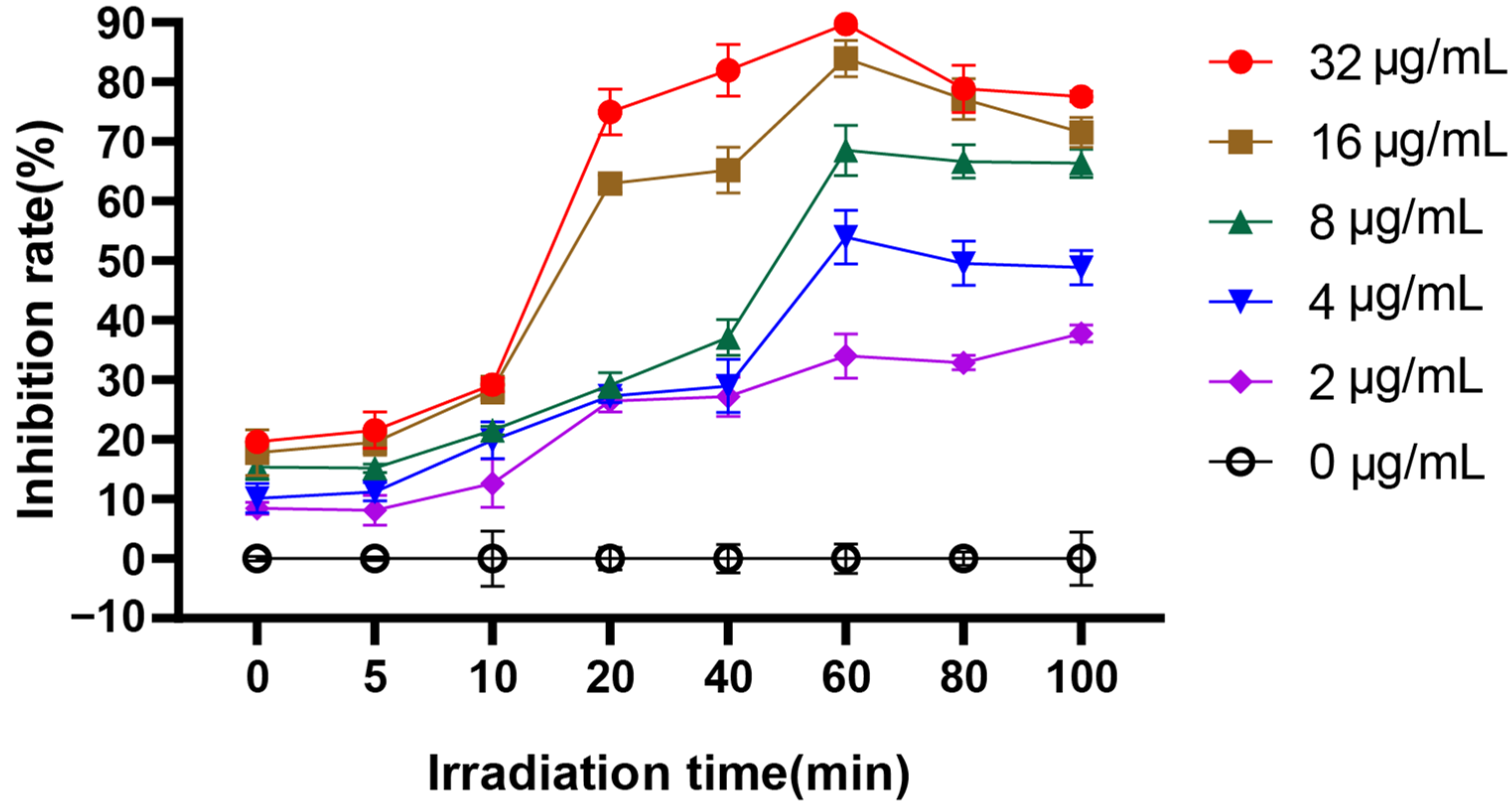
2.5. Time-Dependent Photoinactivation Effect
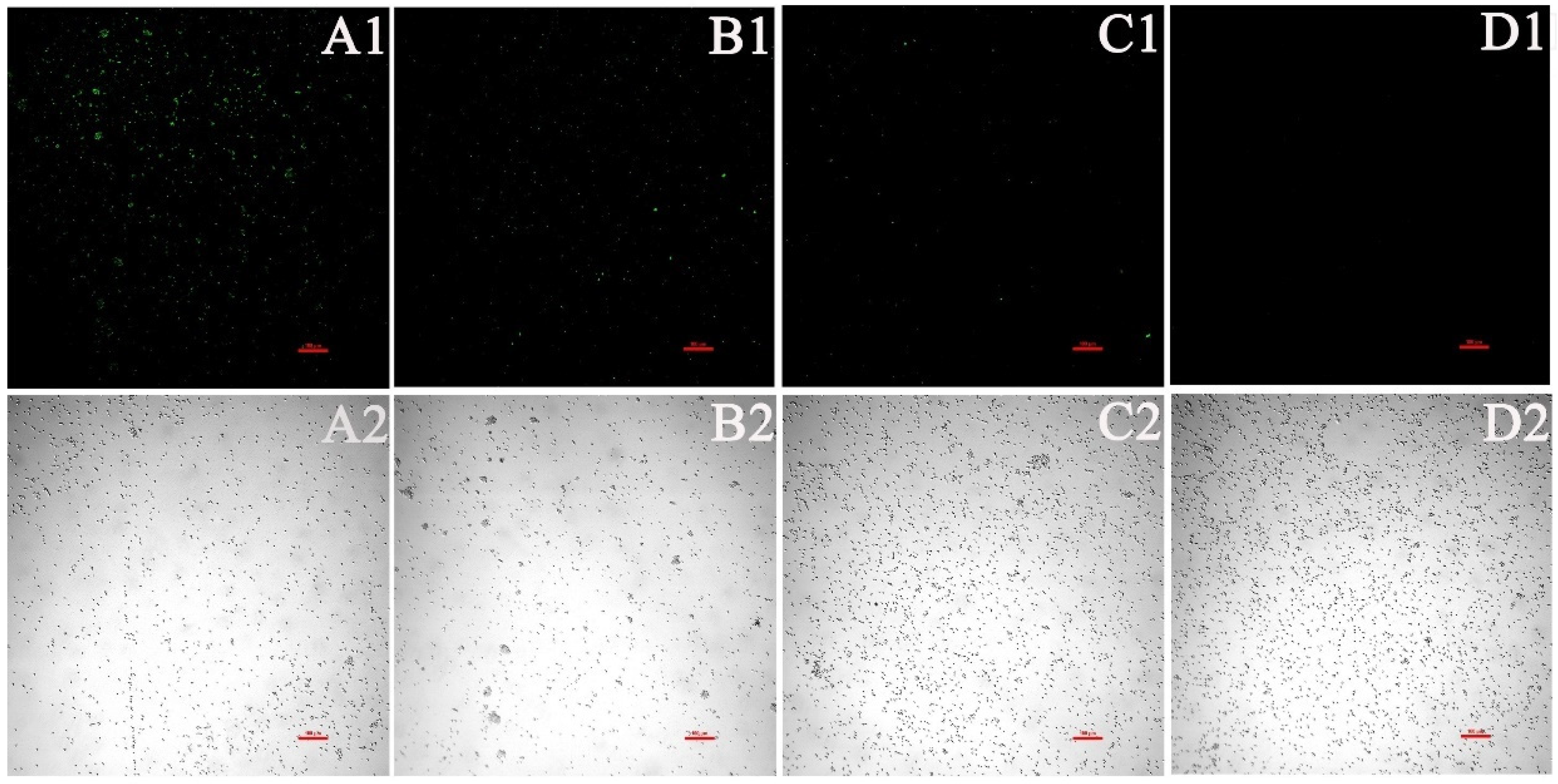
2.6. Biofilm Initial Cell Attachment Assay
2.6.1. XTT Assay for the Effect of EA on the Adhesion of C. albicans
2.6.2. Adhesion-Related Gene Transcription Analysis by qRT-PCR Assays
2.7. Biofilm Eradication Assay
2.8. Effect of PACT on Cell Permeability
2.9. Effect of PACT on Relative Electric Conductivity of Cell Membrane
2.10. Effect of PACT on Plasma Membrane Dynamics
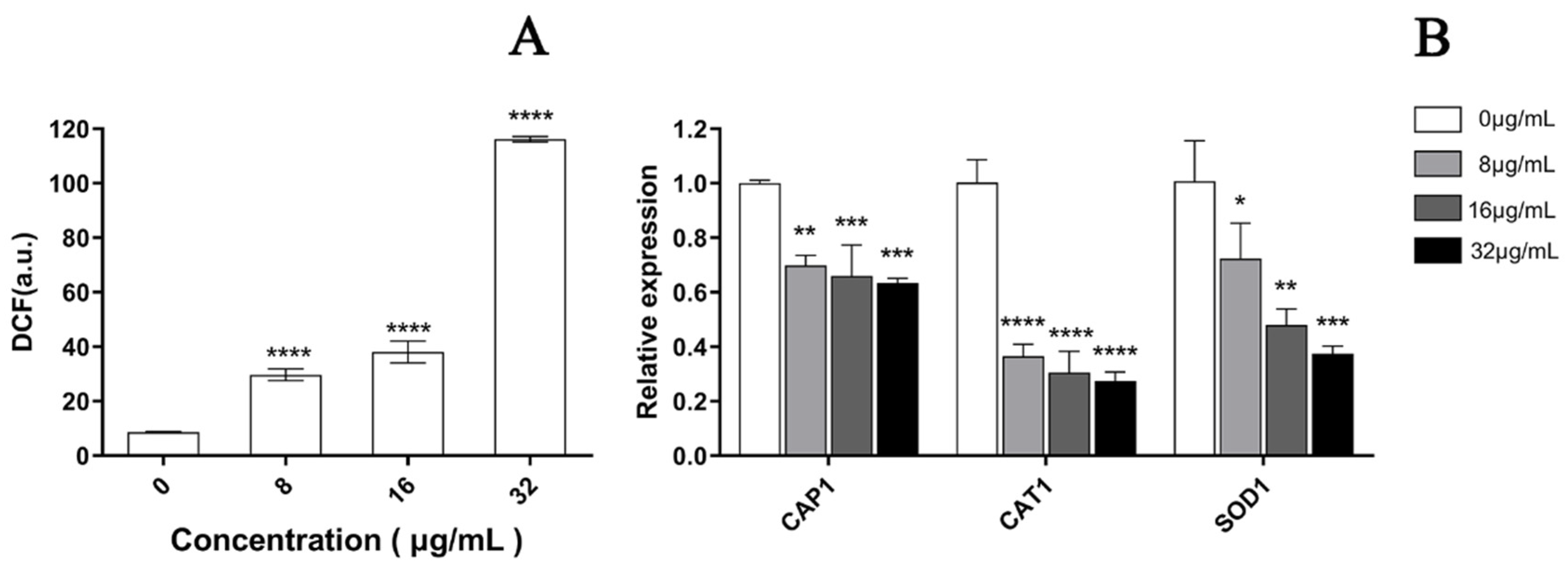
2.11. Effect of PACT on ROS Production
2.11.1. Determination of ROS Production
2.11.2. Oxidative-Stress-Related Genes Transcription Analysis by qRT-PCR Assays
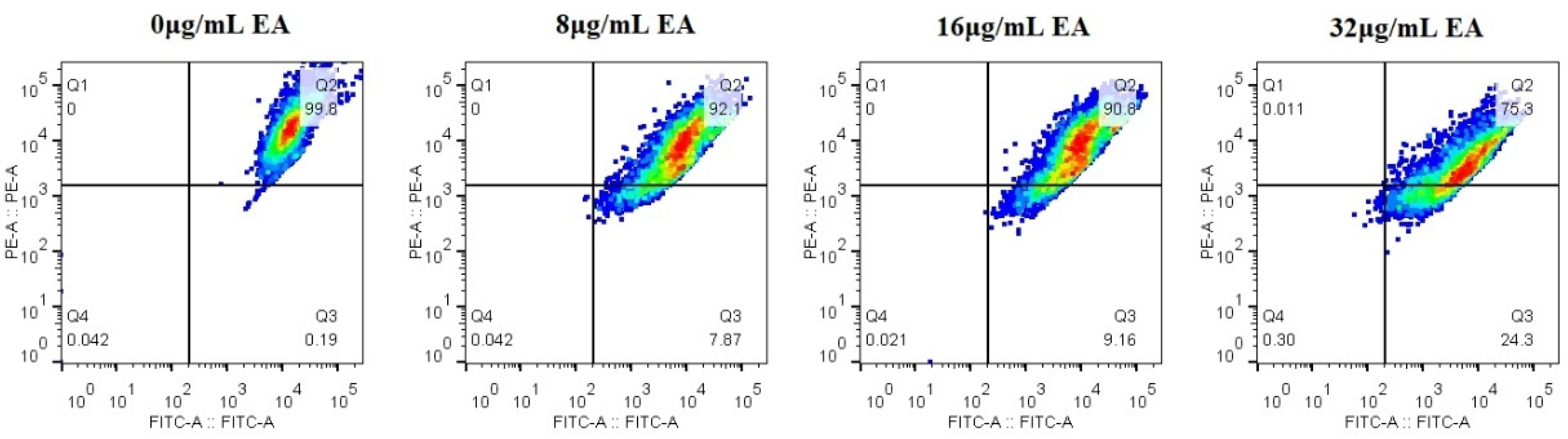
2.12. Mitochondrial Membrane Potential
2.13. Detection of DNA Fragmentation
| Primers | Sequence | References |
|---|---|---|
| MP65-F | TCAACACTGAACCACCTC | [31] |
| MP65-R | ATACCTTTAGCACCACCA | [31] |
| ALS1-F | CATCATTGACTCAGTTGT | [32] |
| ALS1-R | CAGTGGAAGTAGATTGTG | [32] |
| CAP1-F | AGTCAATTCAATGTTCAAG | [32] |
| CAP1-R | AATGGTAATGTCCTCAAG | [32] |
| CAT1-F | GACTGCTTACATTCAAAC | [32] |
| CAT1-R | AACTTACCAAATCTTCTCA | [32] |
| SOD1-F | TTGAACAAGAATCCGAATCC | [33] |
| SOD1-R | AGCCAATGACACCACAAGCAG | [33] |
| ACT1-F | TTGATTTGGCTGGTAGAG | [34] |
| ACT1-R | ATGGCAGAAGATTGAGAA | [34] |
2.14. Statistical Analysis
3. Results
3.1. EA-Mediated PACT Decreases C. albicans Survival
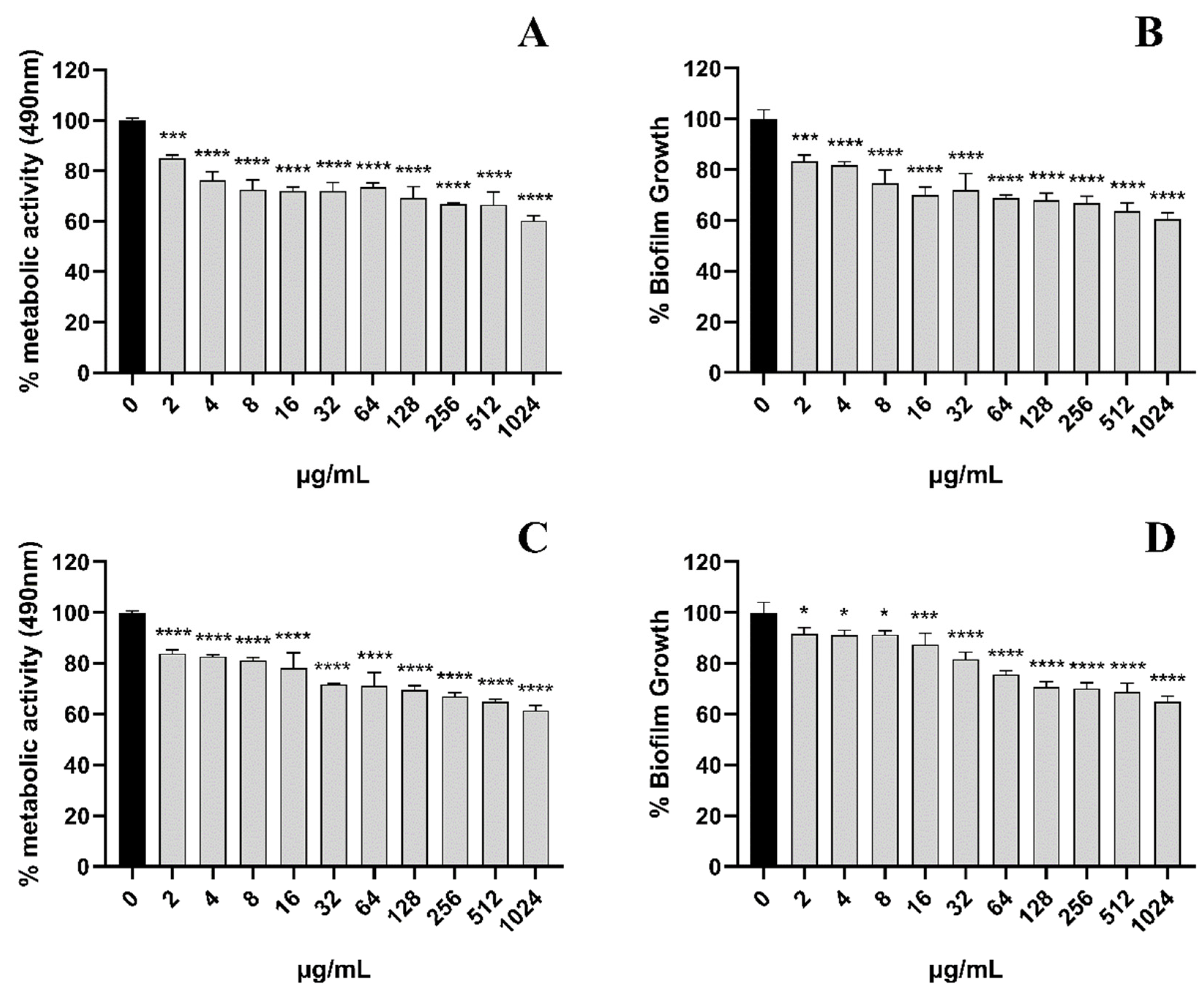
3.2. EA-Mediated PACT Inhibits C. albicans Biofilm Formation
3.3. EA-Mediated PACT Has Anti-Biofilm Activity toward Two Phases of C. albicans Biofilm Maturation
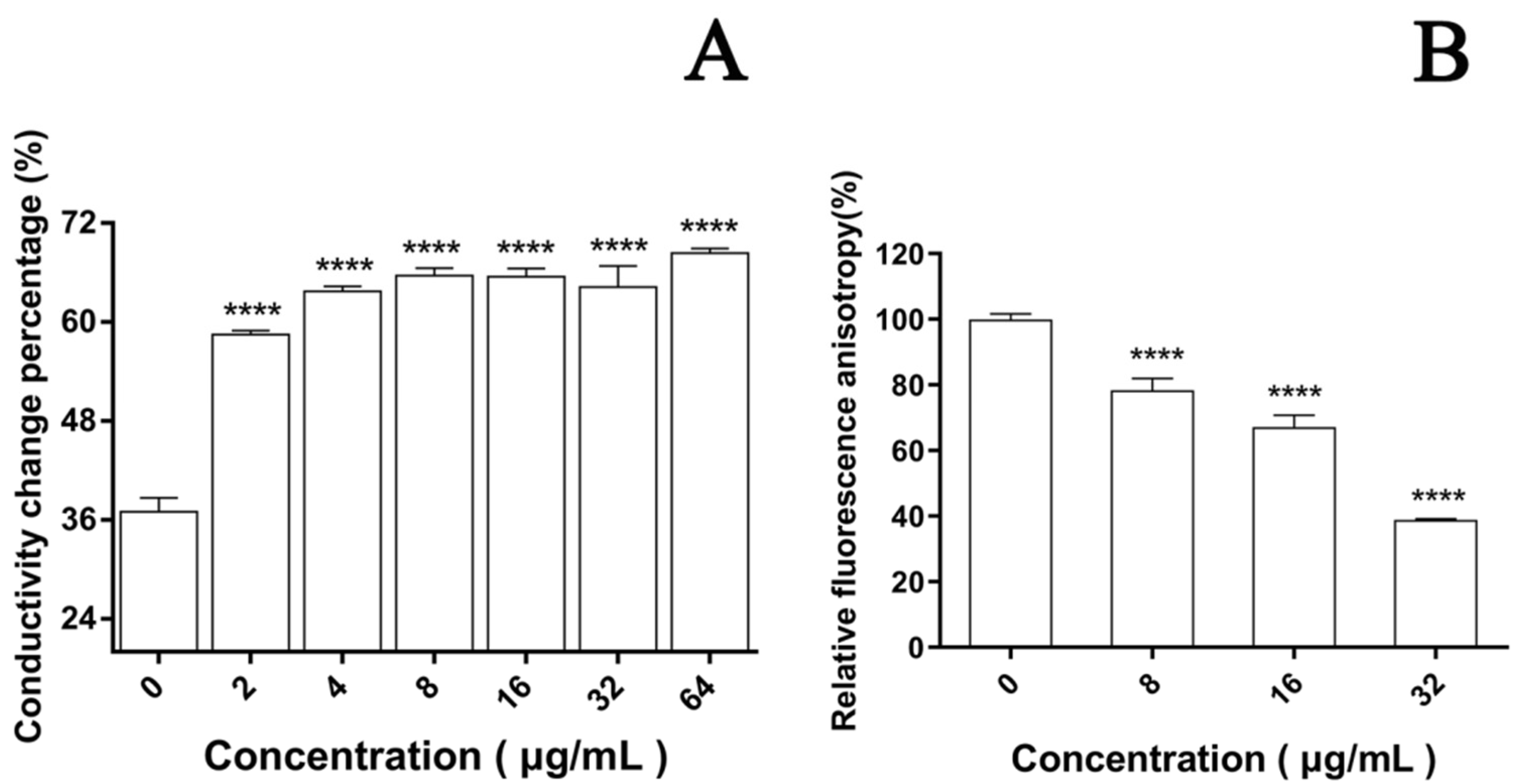
3.4. Effect of EA-Mediated PACT on Cell Membrane
3.4.1. Permeability of Cell Membrane
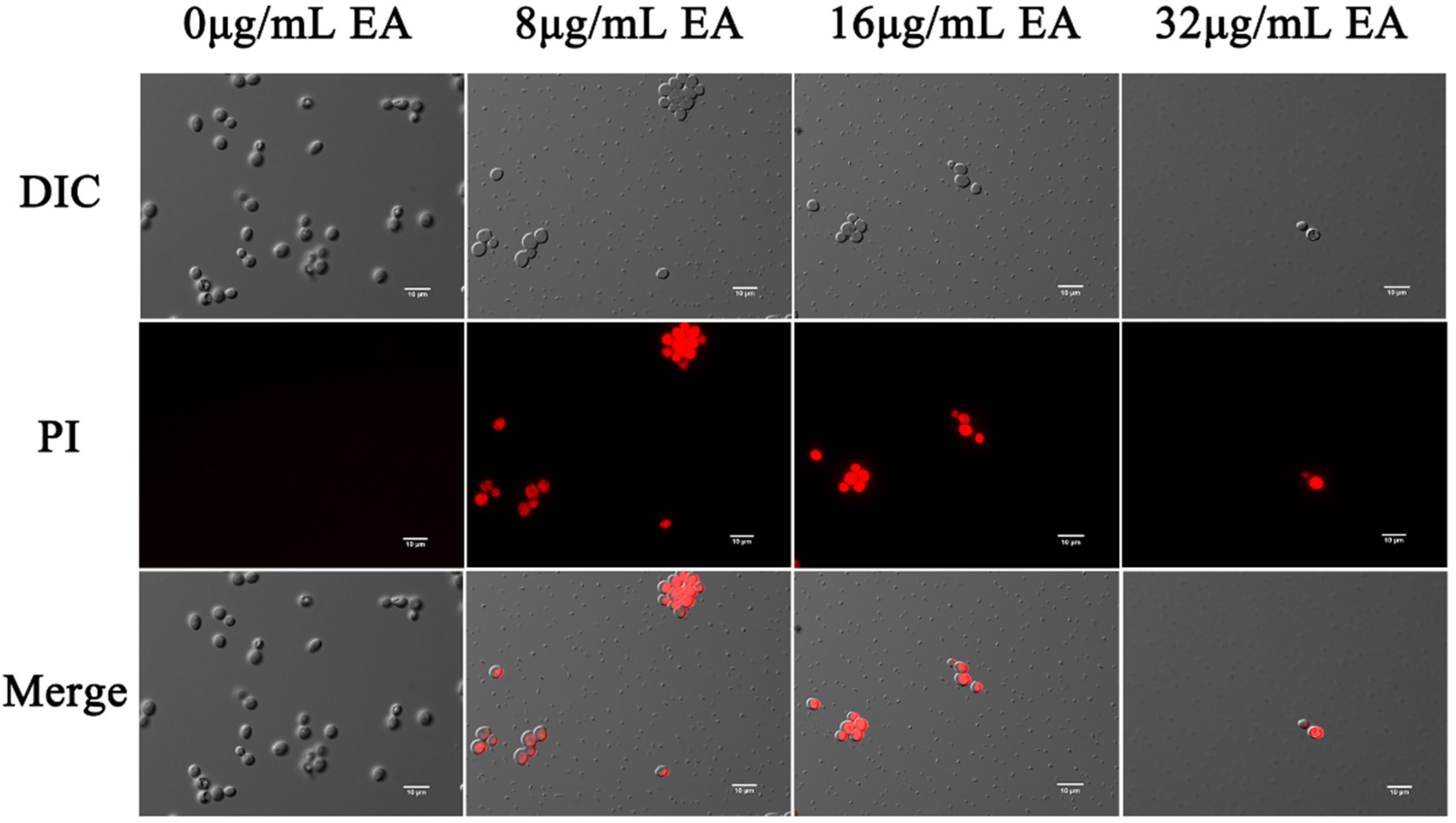
3.4.2. Integrity of Cell Membrane
3.5. EA-Mediated PACT Induces ROS Production
3.6. EA-Mediated PACT Decreases Mitochondrial Membrane Potential and Induces Nuclear Fragmentation
4. Discussion
4.1. EA-Mediated PACT Effect on C. albicans
4.2. EA-Mediated PACT Effect on C. albicans Biofilm
4.3. Mechanism of Action of EA-Mediated PACT
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dahiya, S.; Sharma, N.; Punia, A.; Choudhary, P.; Gulia, P.; Parmar, V.S.; Chhillar, A.K. Antimycotic Drugs and their Mechanisms of Resistance to Candida Species. Curr. Drug Targets 2021, 23, 116–125. [Google Scholar] [CrossRef]
- Ponde, N.O.; Lortal, L.; Ramage, G.; Naglik, J.R.; Richardson, J.P. Candida albicans biofilms and polymicrobial interactions. Crit. Rev. Microbiol. 2021, 47, 91–111. [Google Scholar] [CrossRef]
- Eix, E.F.; Nett, J.E. How Biofilm Growth Affects Candida-Host Interactions. Front. Microbiol. 2020, 11, 1437. [Google Scholar] [CrossRef] [PubMed]
- Souza, S.O.; Raposo, B.L.; Sarmento-Neto, J.F.; Rebouças, J.S.; Macêdo, D.P.C.; Figueiredo, R.C.B.Q.; Santos, B.S.; Freitas, A.Z.; Filho, P.E.C.; Ribeiro, M.S.; et al. Photoinactivation of Yeast and Biofilm Communities of Candida albicans Mediated by ZnTnHex-2-PyP4+ Porphyrin. J. Fungi 2022, 8, 556. [Google Scholar] [CrossRef]
- Moreira, L.M.; Dos Santos, F.V.; Lyon, J.P.; Maftoum-Costa, M.; Pacheco-Soares, C.; da Silva, N.S. Photodynamic Therapy: Porphyrins and Phthalocyanines as Photosensitizers. Aust. J. Chem. 2008, 61, 741–754. [Google Scholar] [CrossRef]
- Wiench, R.; Nowicka, J.; Pajączkowska, M.; Kuropka, P.; Skaba, D.; Kruczek-Kazibudzka, A.; Kuśka-Kiełbratowska, A.; Grzech-Leśniak, K. Influence of Incubation Time on Ortho-Toluidine Blue Mediated Antimicrobial Photodynamic Therapy Directed against Selected Candida Strains—An In Vitro Study. Int. J. Mol. Sci. 2021, 22, 10971. [Google Scholar] [CrossRef] [PubMed]
- Rosseti, I.B.; Chagas, L.R.; Costa, M.S. Photodynamic antimicrobial chemotherapy (PACT) inhibits biofilm formation by Candida albicans, increasing both ROS production and membrane permeability. Lasers Med. Sci. 2014, 29, 1059–1064. [Google Scholar] [CrossRef]
- Ma, W.; Liu, C.; Li, J.; Hao, M.; Ji, Y.; Zeng, X. The effects of aloe emodin-mediated antimicrobial photodynamic therapy on drug-sensitive and resistant Candida albicans. Photochem. Photobiol. Sci. 2020, 19, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Ziental, D.; Mlynarczyk, D.T.; Czarczynska-Goslinska, B.; Lewandowski, K.; Sobotta, L. Photosensitizers Mediated Photodynamic Inactivation against Fungi. Nanomaterials 2021, 11, 2883. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.A.; Romeiro, R.L.; Costa, A.C.B.P.; Machado, A.K.S.; Junqueira, J.C.; Jorge, A.O.C. Susceptibility of Candida albicans, Staphylococcus aureus, and Streptococcus mutans biofilms to photodynamic inactivation: An in vitro study. Lasers Med. Sci. 2009, 26, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.P.; Rosseti, I.B.; Carvalho, M.L.; da Silva, B.G.M.; Alberto-Silva, C.; Costa, M.S. Photodynamic Antimicrobial Chemotherapy (PACT), using Toluidine blue O inhibits the viability of biofilm produced by Candida albicans at different stages of development. Photodiagn. Photodyn. Ther. 2018, 21, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Chabrier-Roselloó, Y.; Foster, T.H.; Peórez-Nazario, N.; Mitra, S.; Haidaris, C.G. Sensitivity of Candida albicans Germ Tubes and Biofilms to Photofrin-Mediated Phototoxicity. Antimicrob. Agents Chemother. 2005, 49, 4288–4295. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Alves, C.T.; Raju, B.R.; Gonçalves, M.S.T.; Coutinho, P.J.; Henriques, M.; Belo, I. Application of benzo[a]phenoxazinium chlorides in antimicrobial photodynamic therapy of Candida albicans biofilms. J. Photochem. Photobiol. B Biol. 2014, 141, 93–99. [Google Scholar] [CrossRef]
- Song, S.; Sun, X.; Meng, L.; Wu, Q.; Wang, K.; Deng, Y. Antifungal activity of hypocrellin compounds and their synergistic effects with antimicrobial agents against Candida albicans. Microb. Biotechnol. 2020, 14, 430–443. [Google Scholar] [CrossRef]
- Khiralla, A.; Mohammed, A.O.; Yagi, S. Fungal perylenequinones. Mycol. Prog. 2022, 21, 38. [Google Scholar] [CrossRef]
- Chen, C.-T.; Nakanishi, K.; Natori, S. Biosynthesis of Elsinochrome A, the Perylenequinone from Elsinoe spp. I. Chem. Pharm. Bull. 1966, 14, 1434–1437. [Google Scholar] [CrossRef] [PubMed]
- Meille, S.V.; Malpezzi, L.; Allegra, G.; Nasini, G.; Weiss, U. Structure of elsinochrome A: A perylenequinone metabolite. Acta Crystallogr. C 1989, 45, 628–632. [Google Scholar] [CrossRef]
- Li, T.; Deng, H.; Zhao, J.; Gu, Y. Elsinochrome A photosensitizers: Alternative drugs for photodynamic therapy. J. Innov. Opt. Health Sci. 2015, 8, 1530001. [Google Scholar] [CrossRef]
- Ma, L.; Hong, T.; Li, C.; Zhang, Y.; Wang, Z.; Ji, W.-Z. Photodynamic inhibitory effects of three perylenequinones on human colorectal carcinoma cell line and primate embryonic stem cell line. World J. Gastroenterol. 2003, 9, 485–490. [Google Scholar] [CrossRef]
- Li, C.; He, Y.; Ou, L.; Tian, M.; Yao, Z.; Guo, M. Photophysical and photosensitive properties of Elsinochrome A. Chin. Sci. Bull. 2006, 51, 1050–1054. [Google Scholar] [CrossRef]
- Hirakawa, K.; Ishikawa, T. Phenothiazine dyes photosensitize protein damage through electron transfer and singlet oxygen generation. Dye. Pigment. 2017, 142, 183–188. [Google Scholar] [CrossRef]
- Chen, S.L.; Li, Q.; Yan, S.Z. Extraction Effects of Hypocrellin A and Elsinochrome A in Shiraia bambusicola Solid-State Fermentation with Different Polar Solvent Gradients. ZL201810359384.4, 10 August 2021. [Google Scholar]
- Donadu, M.; Peralta-Ruiz, Y.; Usai, D.; Maggio, F.; Molina-Hernandez, J.; Rizzo, D.; Bussu, F.; Rubino, S.; Zanetti, S.; Paparella, A.; et al. Colombian Essential Oil of Ruta graveolens against Nosocomial Antifungal Resistant Candida Strains. J. Fungi 2021, 7, 383. [Google Scholar] [CrossRef] [PubMed]
- Rukayadi, Y.; Hwang, J.K. In vitro activity of xanthorrhizol isolated from the rhizome of Javanese turmeric (Curcuma xanthorrhiza Roxb.) against Candida albicans biofilms. Phytother. Res. 2013, 27, 1061–1066. [Google Scholar] [CrossRef]
- Escobar, I.E.; Rossatto, F.C.P.; Kim, S.M.; Kang, M.H.; Kim, W.; Mylonakis, E. Repurposing Kinase Inhibitor Bay 11-7085 to Combat Staphylococcus aureus and Candida albicans Biofilms. Front. Pharmacol. 2021, 12, 675300. [Google Scholar] [CrossRef]
- Shichiri-Negoro, Y.; Tsutsumi-Arai, C.; Arai, Y.; Satomura, K.; Arakawa, S.; Wakabayashi, N. Ozone ultrafine bubble water inhibits the early formation of Candida albicans biofilms. PLoS ONE 2021, 16, e0261180. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Srivastava, V.; Ahmad, A. Dodonaea viscosa var angustifolia derived 5,6,8-trihydroxy-7,4’ dimethoxy flavone inhibits ergosterol synthesis and the production of hyphae and biofilm in Candida albicans. J. Ethnopharmacol. 2020, 259, 112965. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef]
- Zheng, S.; Chang, W.; Zhang, M.; Shi, H.; Lou, H. Chiloscyphenol A derived from Chinese liverworts exerts fungicidal action by eliciting both mitochondrial dysfunction and plasma membrane destruction. Sci. Rep. 2018, 8, 326. [Google Scholar] [CrossRef]
- Yun, J.; Lee, D.G. Cecropin A-induced apoptosis is regulated by ion balance and glutathione antioxidant system in Candida albicans. IUBMB Life 2016, 68, 652–662. [Google Scholar] [CrossRef]
- Lu, K.Q.; Zhang, M.X.; Shi, G.-X.; Yan, Y.-Y.; Shao, J.; Wu, D.-Q.; Wang, T.-M.; Wang, C.-Z. Inhibition of butanol extract from Paris polyphylla var. yunnanensis against Candida albicans biofilm formation. Chin. Tradit. Herb. Drugs 2016, 47, 440–446. [Google Scholar]
- Alonso, G.C.; Pavarina, A.C.; Sousa, T.V.; Klein, M.I. A quest to find good primers for gene expression analysis of Candida albicans from clinical samples. J. Microbiol. Methods 2018, 147, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Krom, B.P.; Sanglard, D.; Intapa, C.; Dawson, C.C.; Peters, B.M.; Shirtliff, M.E.; Jabra-Rizk, M.A. Farnesol-Induced Apoptosis in Candida albicans Is Mediated by Cdr1-p Extrusion and Depletion of Intracellular Glutathione. PLoS ONE 2011, 6, e28830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.X.; Lu, K.Q.; Xia, D.; Xia, X.; Shi, G.-X.; Shao, J.; Wu, D.-Q.; Wang, T.-M.; Wang, C.-Z. The inhibitory effects of Butyl alcohol extract of BaiTouWeng decoction on biofilm formation of Candida albicans. Chin. J. Mycol. 2015, 10, 321–327. [Google Scholar]
- Bajpai, V.K.; Sharma, A.; Baek, K.-H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Vincent, M.; England, L.; Trevors, J. Cytoplasmic membrane polarization in Gram-positive and Gram-negative bacteria grown in the absence and presence of tetracycline. Biochim. Biophys. Acta 2004, 1672, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Krysko, D.V.; Roels, F.; Leybaert, L.; D’Herde, K. Mitochondrial Transmembrane Potential Changes Support the Concept of Mitochondrial Heterogeneity During Apoptosis. J. Histochem. Cytochem. 2001, 49, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Isola, R.; Isola, M.; Conti, G.; Lantini, M.S.; Riva, A. Histatin-induced Alterations in Candida albicans: A Microscopic and Submicroscopic Comparison. Microsc. Res. Tech. 2007, 70, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Brunke, S.; Hube, B. Two unlike cousins: Candida albicans and C. glabrata infection strategies. Cell Microbiol. 2013, 15, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Garzón, A.C.; Wintaco-Martínez, L.M.; Velez, N.; Hernandez-Padilla, C.; De la Hoz, A.; Valderrama-Beltrán, S.L.; Moreno, C.A.A.; Le Pape, P.; Ramírez, J.D.; Parra-Giraldo, C.M. Persistence of clonal azole-resistant isolates of Candida albicans from a patient with chronic mucocutaneous candidiasis in Colombia. J. Glob. Infect. Dis. 2020, 12, 16–20. [Google Scholar] [CrossRef]
- El-Azizi, M.; Farag, N.; Khardori, N. Antifungal activity of amphotericin B and voriconazole against the biofilms and biofilm-dispersed cells of Candida albicans employing a newly developed in vitro pharmacokinetic model. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Yin, F.; Wang, S.; Zhao, A.; Li, Y.; Liu, Y. Helicobacter pylori Biofilm-Related Drug Resistance and New Developments in Its Anti-Biofilm Agents. Infect. Drug Res. 2022, 15, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, Y.-Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, Z.-Q.; Jin, H.; Sun, S.; Liu, T.-J.; Wang, X.; Fan, H.-J.; Hou, S.-K.; Ding, H. Photodynamic antimicrobial chemotherapy with the novel amino acid-porphyrin conjugate 4I: In vitro and in vivo studies. PLoS ONE 2017, 12, e0176529. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liu, W.; Huang, X.; Hao, L.; Li, Y.; Sun, S. Antifungal Activity and Potential Mechanism of N-Butylphthalide Alone and in Combination With Fluconazole Against Candida albicans. Front. Microbiol. 2019, 10, 1461. [Google Scholar] [CrossRef] [PubMed]
- Wall, G.; Montelongo-Jauregui, D.; Bonifacio, B.V.; Lopez-Ribot, J.L.; Uppuluri, P. Candida albicans biofilm growth and dispersal: Contributions to pathogenesis. Curr. Opin. Microbiol. 2019, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yount, N.Y.; Yeaman, M.R. Immunocontinuum: Perspectives in antimicrobial peptide mechanisms of action and resistance. Protein Pept. Lett. 2005, 12, 49–67. [Google Scholar] [CrossRef]
- Dananjaya, S.; Thao, N.T.; Wijerathna, H.; Lee, J.; Edussuriya, M.; Choi, D.; Kumar, R.S. In vitro and in vivo anticandidal efficacy of green synthesized gold nanoparticles using Spirulina maxima polysaccharide. Process Biochem. 2020, 92, 138–148. [Google Scholar] [CrossRef]
- Sun, L.; Liao, K.; Hang, C.; Wang, D. Honokiol induces reactive oxygen species-mediated apoptosis in Candida albicans through mitochondrial dysfunction. PLoS ONE 2017, 12, e0172228. [Google Scholar] [CrossRef] [PubMed]
- Atriwal, T.; Chawla, M.; Hussain, A.; Alajmi, M.F.; Abid, M. Reactive oxygen mediated apoptosis as a therapeutic approach against opportunistic Candida albicans. Adv. Protein Chem. Struct. Biol. 2021, 125, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.K.; Chand, H.; John, J.; Deshpande, M.V. Clerodane type diterpene as a novel antifungal agent from Polyalthia longifolia var. pendula. Eur. J. Med. Chem. 2015, 94, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Venditti, P.; Di Meo, S. The Role of Reactive Oxygen Species in the Life Cycle of the Mitochondrion. Int. J. Mol. Sci. 2020, 21, 2173. [Google Scholar] [CrossRef]
- Zhou, L.; Dong, C.; Wei, S.H.; Feng, Y.Y.; Zhou, J.H.; Liu, J.H. Water-soluble soft nano-colloid for drug delivery. Mater. Lett. 2009, 63, 1683–1685. [Google Scholar] [CrossRef]



| Irradiation | Time | Fluconazole | |||||
|---|---|---|---|---|---|---|---|
| 0 min | 5 min | 10 min | 20 min | 40 min | 60 min | ||
| SMIC80 (μg/Ml) | 256–512 | 256–512 | 128–256 | 64–128 | 16–32 | 16 | >1024 |
| MIC (μg/mL) | 128 | 16 | 8 | 4 | 2 | 1 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Yao, Y.; Zheng, H.; Yan, S.; Chen, S. Inhibitory Effects and Mechanism of Action of Elsinochrome A on Candida albicans and Its Biofilm. J. Fungi 2022, 8, 841. https://doi.org/10.3390/jof8080841
Pan L, Yao Y, Zheng H, Yan S, Chen S. Inhibitory Effects and Mechanism of Action of Elsinochrome A on Candida albicans and Its Biofilm. Journal of Fungi. 2022; 8(8):841. https://doi.org/10.3390/jof8080841
Chicago/Turabian StylePan, Lili, Yuanyuan Yao, Hailin Zheng, Shuzhen Yan, and Shuanglin Chen. 2022. "Inhibitory Effects and Mechanism of Action of Elsinochrome A on Candida albicans and Its Biofilm" Journal of Fungi 8, no. 8: 841. https://doi.org/10.3390/jof8080841





