Long-Term Follow-Up of Patients Diagnosed with COVID-19-Associated Pulmonary Aspergillosis (CAPA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Setting
2.2. Study Design
2.3. Definitions
- COVID-19 confirmed case: Positive result in the reverse transcription-polymerase chain reaction (RT-PCR) assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in nasopharyngeal swab.
- CAPA in immunosuppressed patients: was classified as possible/probable/proven invasive pulmonary aspergillosis according to the EORTC/MSG criteria [9].
- CAPA in non-immunocompromised patients: For these patients we used the 2020 European Confederation of Medical Mycology and International Society for Human and Animal Mycology (ECMM/ISHAM) consensus criteria for the diagnosis of CAPA [10]. According to these criteria, patients with positive SARS-CoV-2 RT-PCR and who develop respiratory insufficiency requiring intensive care could be classified as:
- ○
- Proven CAPA: Proven by histopathological and/or direct microscopic detection of fungal elements that are morphologically consistent with Aspergillus spp., showing invasive growth into tissues, or by PCR from material that was obtained by a sterile aspiration or biopsy from a pulmonary site, showing an infectious disease.
- ○
- Probable CAPA: Pulmonary infiltrate or nodules, preferably documented by chest CT, or cavitating infiltrate (not attributed to another cause), or both, combined with mycological evidence: microscopic detection of fungal elements in bronchoalveolar lavage (positive bronchoalveolar lavage culture); positive galactomannan in serum or bronchoalveolar lavage galactomannan; positive aspergillus PCR tests in plasma, serum, whole blood; or bronchoalveolar lavage fluid (modified from ECMM/ISHAM proposed criteria) [10].
- ○
- Possible CAPA: Pulmonary infiltrate or nodules, preferably documented by chest CT, or cavitating infiltrate (which is not attributed to another cause) in combination with mycological evidence (e.g., microscopy, culture, or galactomannan, alone or in combination) obtained via non-bronchoscopic lavage.
- Aspergillus colonization: Isolation of Aspergillus spp. in one or more respiratory samples for which the former criteria was not fulfilled.
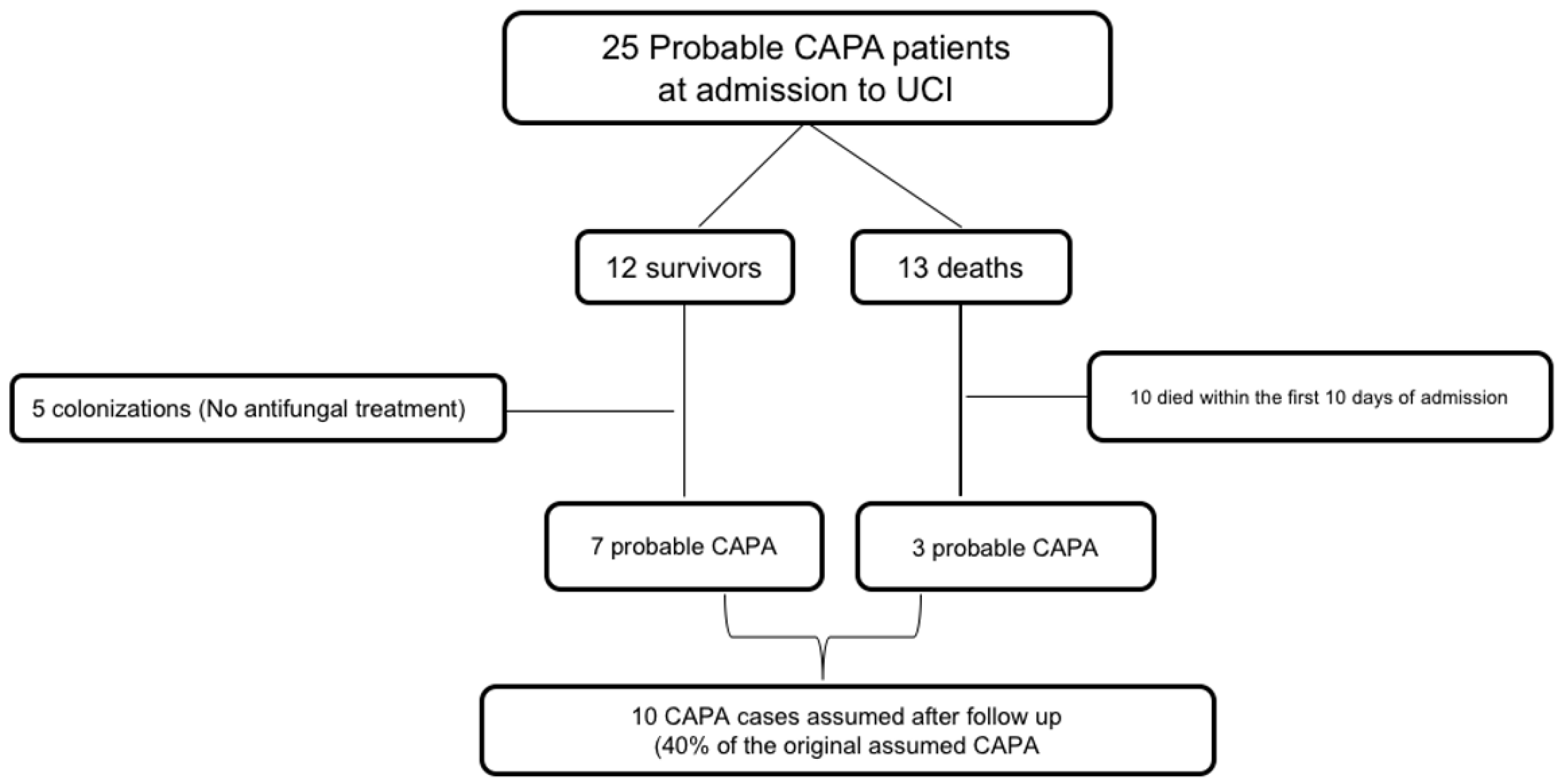
- CAPA confirmed cases after follow-up: Surviving patients from our cohort were followed up for at least 6 months after hospital discharge. Two clinicians (MRR and JMA) evaluated individually clinical and radiological evolution of these patients and classified them as true or false CAPA. We considered as CAPA those patients with compatible imaging evolution; cavitating infiltrate or nodules, infiltrates with air-crescent sign, or those with subacute evolution documented by lung CT. We considered as previously misidentified CAPA cases those presenting pulmonary infiltrates or nodules with an accelerated resolution, or those that could be attributed during the follow-up period to another cause.
- Dose and type of steroids used during study period:
- ○
- Metilprednisolone 250 mg/24 h for 3 days;
- ○
- Metilprednisolone 1 mg/Kg/day for 3–5 days;
- ○
- Metilprednisolone 40 mg/12 h for 3–5 days.
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schauwvlieghe, A.F.A.D.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van De Veerdonk, F.L.; Gommers, D.; et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- Liu, W.; Yu, W.; Chan, K.; Yang, C.; Wauters, J.; Verweij, P.E. Aspergillosis related to severe influenza: A worldwide phenomenon? Clin. Respir. J. 2019, 13, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Kasper, J.; van der Aa, S.; Andeweg, A.C.; Zaaraoui-Boutahar, F.; Goeijenbier, M.; Richard, M.; Herold, S.; Becker, C.; Scott, D.P.; et al. Influenza virus damages the alveolar barrier by disrupting epithelial cell tight junctions. Eur. Respir. J. 2016, 47, 954–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolilekas, L.; Loverdos, K.; Giannakaki, S.; Vlassi, L.; Levounets, A.; Zervas, E.; Gaga, M. Can steroids reverse the severe COVID-19 induced “cytokine storm”? J. Med. Virol. 2020, 92, 2866–2869. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, R.M.; Dingle, T.C.; Kula, B.E.; Vandermeer, B.; Sligl, W.I.; Schwartz, I.S. Defining COVID-19–associated pulmonary aspergillosis: Systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Salmanton-García, J.; Sprute, R.; Stemler, J.; Bartoletti, M.; Dupont, D.; Valerio, M.; Garcia-Vidal, C.; Falces-Romero, I.; Machado, M.; de la Villa, S.; et al. COVID-19-associated pulmonary aspergillosis, March–August 2020. Emerg. Infect. Dis. 2021, 27, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.H.; Neu, K.P. Incidence, diagnosis and outcomes of COVID-19-associated pulmonary aspergillosis (CAPA): A systematic review. J. Hosp. Infect. 2021, 113, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Brüggemann, R.J.M.; Azoulay, E.; Bassetti, M.; Blot, S.; Buil, J.B.; Calandra, T.; Chiller, T.; Clancy, C.J.; Cornely, O.A.; et al. Taskforce report on the diagnosis and clinical management of COVID-19 associated pulmonary aspergillosis. Intensive Care Med. 2021, 47, 819–834. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [Green Version]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Las-Florl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Bartoletti, M.; Pascale, R.; Cricca, M.; Rinaldi, M.; Maccaro, A.; Bussini, L.; Fornaro, G.; Tonetti, T.; Pizzilli, G.; Francalanci, E.; et al. Epidemiology of Invasive Pulmonary Aspergillosis Among Intubated Patients With COVID-19: A Prospective Study. Clin. Infect. Dis. 2021, 73, e3606–e3614. [Google Scholar] [CrossRef] [PubMed]
- Alanio, A.; Dellière, S.; Fodil, S.; Bretagne, S.; Mégarbane, B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir. Med. 2020, 8, e48–e49. [Google Scholar] [CrossRef]
- Machado, M.; Valerio, M.; Álvarez-Uría, A.; Olmedo, M.; Veintimilla, C.; Padilla, B.; De la Villa, S.; Guinea, J.; Escribano, P.; Ruiz-Serrano, M.J.; et al. Invasive pulmonary aspergillosis in the COVID-19 era: An expected new entity. Mycoses 2021, 64, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.A.K.; Ellis, J.; Gorton, R.; De, S.; Stone, N. Surveillance for COVID-19-associated pulmonary aspergillosis. Lancet Microbe 2020, 1, e152. [Google Scholar] [CrossRef]
- Verweij, P.E.; Gangneux, J.P.; Bassetti, M.; Brüggemann, R.J.M.; Cornely, O.A.; Koehler, P.; las-Florl, C.; van de Veerdonk, F.L.; Chakrabarti, A.; Hoenigl, M. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe 2020, 1, e53–e55. [Google Scholar] [CrossRef]
| Variable | n = 27 |
|---|---|
| Age, years [mean ± SD] | 65 ± 9.17 |
| Male gender [n (%)] | 21 (77.8) |
| Comorbidity | |
| Hypertension | 12 (44.4) |
| Diabetes Mellitus | 11 (40.7) |
| Diabetes [n (%)] | 12 (22.2) |
| COPD [n (%)] | 3 (11.1) |
| Chronic kidney disease [n (%)] | 4 (14.8) |
| Prior immunosuppression [n (%)] | 5 (18.5) |
| Mechanical ventilation [n (%)] | 25 (92.6) |
| COVID-19 Treatment | |
| Steroids [n (%)] | 26 (96.3) |
| Tocilizumab [n (%)] | 11 (40.7) |
| Antibiotic therapy [n (%)] | 25 (92.6) |
| Isolated Aspergillus species, n (%) | |
| Aspergillus fumigatus | 12 (44.4) |
| Aspergillus flavus | 3 (11.1) |
| Aspergillus niger | 3 (11.1) |
| Aspergillus terreus | 2 (7.4) |
| Aspergillus nidulans | 1 (3.7) |
| Aspergillus lentulus | 1 (3.7) |
| Aspergillus spp. | 2 (7.4) |
| More than one species in same sample | 3 (11.1) |
| Positive serum galactomannan assay a | 2 (11.1) |
| Compatible IFI images on thoracic CT scan b | 5 (41.7) |
| Antifungal therapy c | |
| Voriconazole | 13 (68.4) |
| Liposomal amphotericin B | 3 (15.7) |
| Isavuconazole | 8 (42.1) |
| Death | 14 (51.9) |
| Variable | |
|---|---|
| Age, years [mean ± SD] | 65 ± 10 |
| Male gender [n (%)] | 9 (75) |
| Follow-up, months [mean ± SD] | 15 ± 3.78 |
| COVID-19 Treatment [n (%)] | |
| Steroids | 12 (100) |
| Tocilizumab | 5 (41.7) |
| Antibiotic therapy | 12 (100) |
| IFI images on CT scan at CAPA diagnosis a | 2 (16.7) |
| Isolated Aspergillus species [n (%)] | |
| Aspergillus fumigatus | 3 (25) |
| Aspergillus flavus | 1 (8.3) |
| Aspergillus niger | 2 (16.7) |
| Aspergillus terreus | 2 (16.7) |
| Aspergillus nidulans | 1 (8.3) |
| Aspergillus spp. | 1 (8.3) |
| More than one species in same sample | 2 (16.7) |
| Positive galactomannan in respiratory sample | 0 (0) |
| Positive serum galactomannan assay b | 1 (8.3) |
| Antifungal therapy [n (%)] c | 7 (58.3) |
| Voriconazole | 5 (41.7) |
| Liposomal amphotericin B d | 1 (8.3) |
| Isavuconazole | 2 (16.7) |
| CAPA survivor cases assumed after follow-up [n (%)] e | 7 (58.3) |
| Control thoracic CT scan | 2 (28.6) |
| Radiological improvement after treatment | 2 (100) |
| Clinical improvement | 7 (100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Ruigómez, M.; Fernández-Ruiz, M.; Pérez-Ayala, A.; Aguado, J.M. Long-Term Follow-Up of Patients Diagnosed with COVID-19-Associated Pulmonary Aspergillosis (CAPA). J. Fungi 2022, 8, 840. https://doi.org/10.3390/jof8080840
Ruiz-Ruigómez M, Fernández-Ruiz M, Pérez-Ayala A, Aguado JM. Long-Term Follow-Up of Patients Diagnosed with COVID-19-Associated Pulmonary Aspergillosis (CAPA). Journal of Fungi. 2022; 8(8):840. https://doi.org/10.3390/jof8080840
Chicago/Turabian StyleRuiz-Ruigómez, María, Mario Fernández-Ruiz, Ana Pérez-Ayala, and José María Aguado. 2022. "Long-Term Follow-Up of Patients Diagnosed with COVID-19-Associated Pulmonary Aspergillosis (CAPA)" Journal of Fungi 8, no. 8: 840. https://doi.org/10.3390/jof8080840
APA StyleRuiz-Ruigómez, M., Fernández-Ruiz, M., Pérez-Ayala, A., & Aguado, J. M. (2022). Long-Term Follow-Up of Patients Diagnosed with COVID-19-Associated Pulmonary Aspergillosis (CAPA). Journal of Fungi, 8(8), 840. https://doi.org/10.3390/jof8080840






