Farnesol Boosts the Antifungal Effect of Fluconazole and Modulates Resistance in Candida auris through Regulation of the CDR1 and ERG11 Genes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of the C. auris Strains
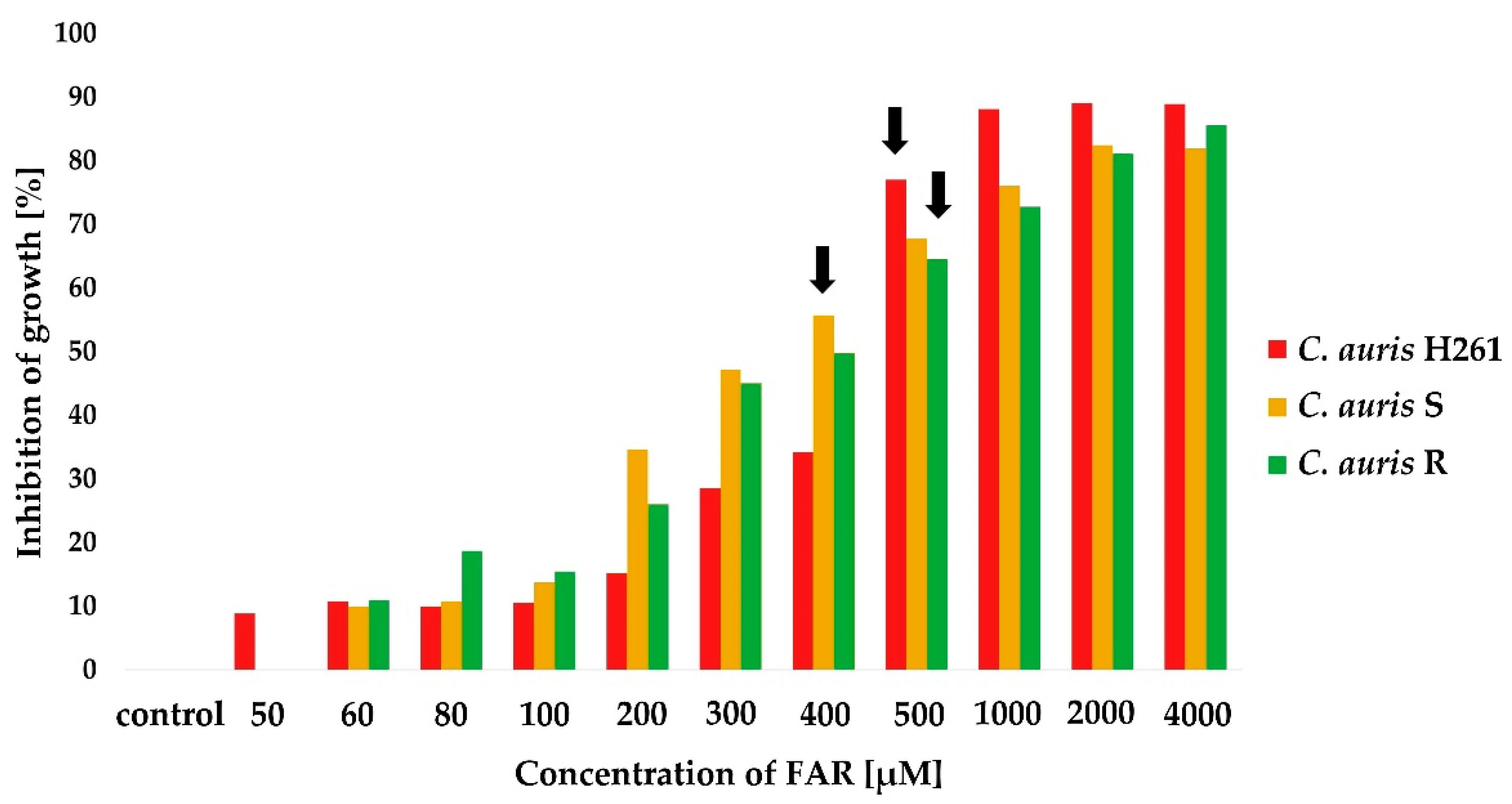
2.2. Susceptibility Testing of FAR Alone and in Combination with FLU
2.3. qPCR Analyses of Genes Related to Resistance in the Presence of FLU and Combination FLU/FAR
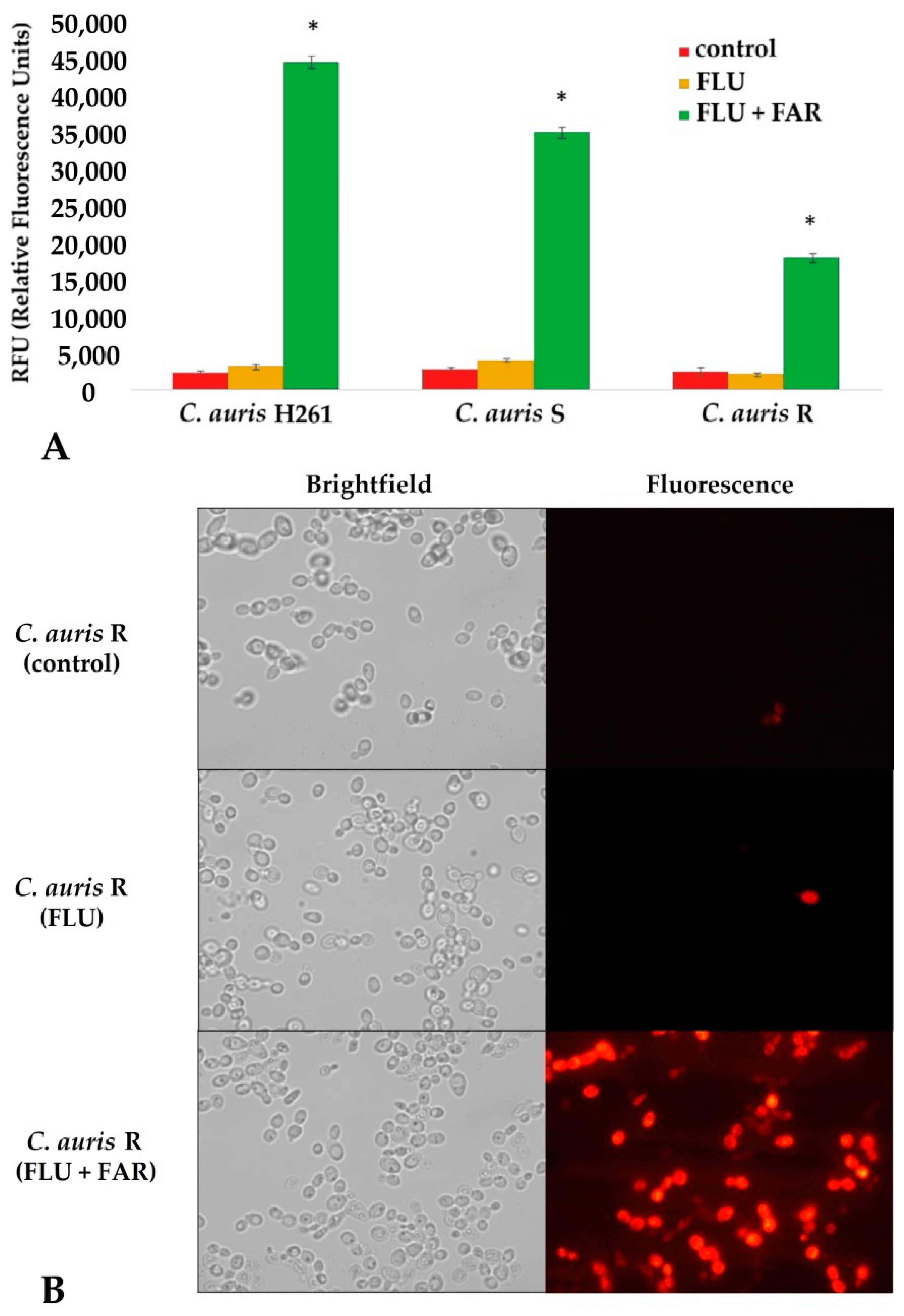
2.4. Rhodamine 6G Intracellular Accumulation Assay and Fluorescence Microscopy
2.5. Statistical Analysis
3. Results and Discussion
3.1. Identification of C. auris and Antifungal Susceptibility Profile of Tested Isolates
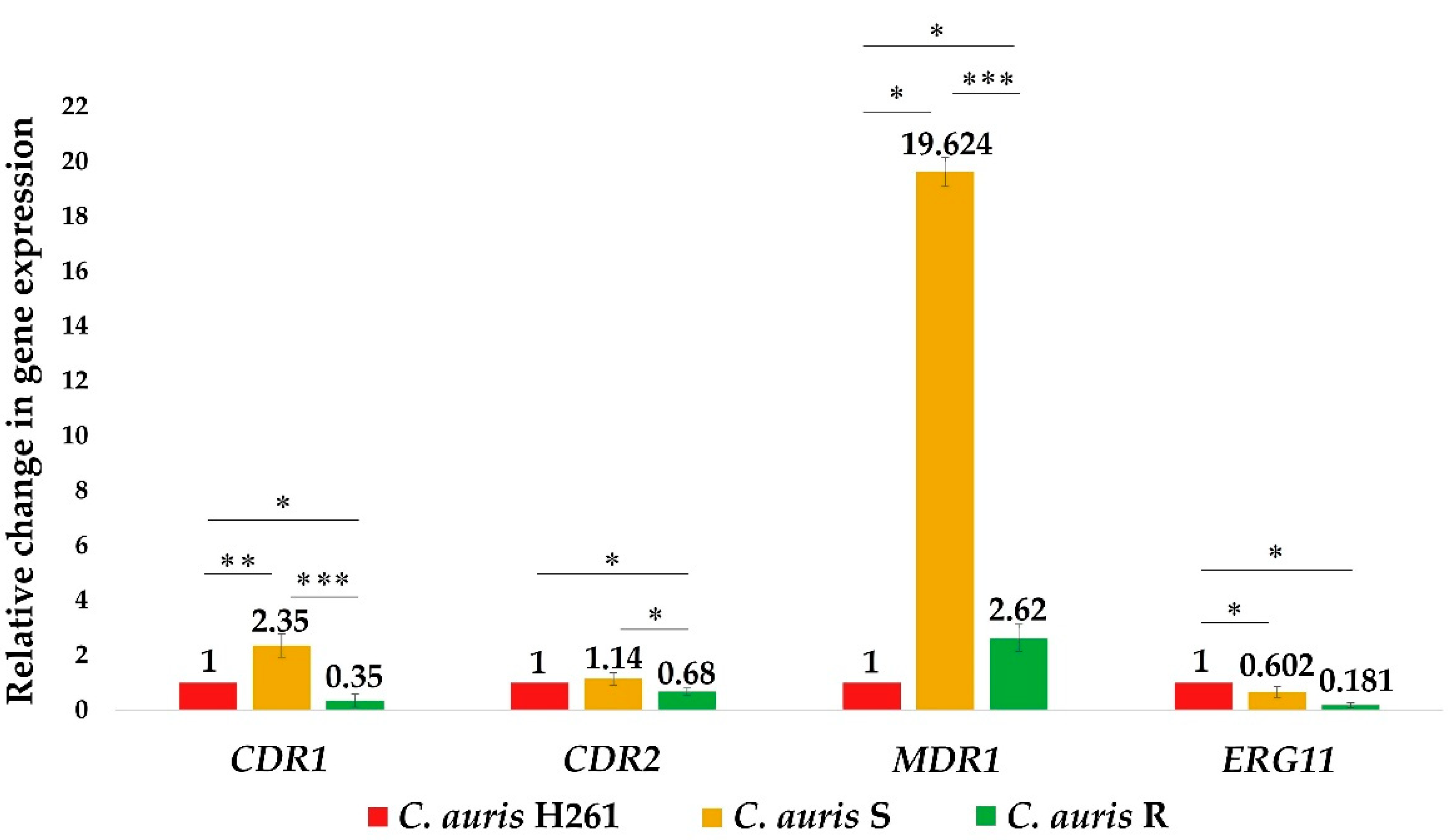
3.2. Relative Expression Levels of Genes Related to the Azole Resistance (CDR1, CDR2, MDR1, and ERG11) in Untreated C. auris Isolates and after FLU and FAR Exposure
3.3. The Synergy between FAR and FLU Modulates C. auris Resistance to FLU
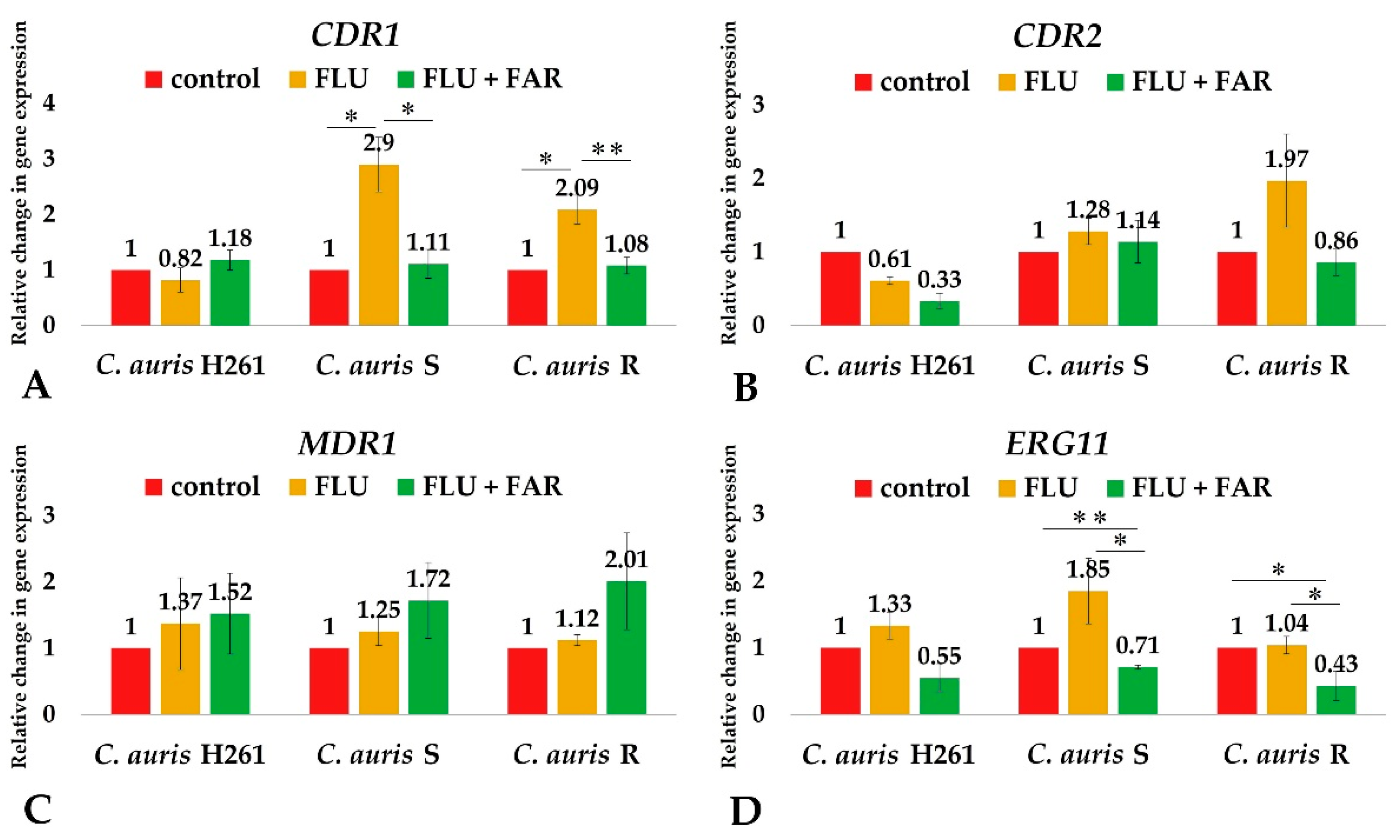
3.4. FAR Down-Regulates the Expression of Genes Involved in Resistance to FLU
3.5. FAR Inhibits the Cdr1 Pump Allowing for a High Intracellular Accumulation of Rhodamine 6G
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida Auris Sp. Nov., a Novel Ascomycetous Yeast Isolated from the External Ear Canal of an Inpatient in a Japanese Hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef]
- Lone, S.A.; Ahmad, A. Candida Auris-the Growing Menace to Global Health. Mycoses 2019, 62, 620–637. [Google Scholar] [CrossRef] [Green Version]
- Iguchi, S.; Itakura, Y.; Yoshida, A.; Kamada, K.; Mizushima, R.; Arai, Y.; Uzawa, Y.; Kikuchi, K. Candida Auris: A Pathogen Difficult to Identify, Treat, and Eradicate and Its Characteristics in Japanese Strains. J. Infect. Chemother. 2019, 25, 743–749. [Google Scholar] [CrossRef] [Green Version]
- Lockhart, S.R. Candida Auris and Multidrug Resistance: Defining the New Normal. Fungal Genet. Biol. 2019, 131, 103243. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Ahmad, A. Abrogation of Pathogenic Attributes in Drug Resistant Candida Auris Strains by Farnesol. PLoS ONE 2020, 15, e0233102. [Google Scholar] [CrossRef] [PubMed]
- Nagy, F.; Vitális, E.; Jakab, Á.; Borman, A.M.; Forgács, L.; Tóth, Z.; Majoros, L.; Kovács, R. In Vitro and in Vivo Effect of Exogenous Farnesol Exposure Against Candida Auris. Front. Microbiol. 2020, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Iyer, K.R.; Pardeshi, L.; Muñoz, J.F.; Robbins, N.; Cuomo, C.A.; Wong, K.H.; Cowen, L.E. Genetic Analysis of Candida Auris Implicates Hsp90 in Morphogenesis and Azole Tolerance and Cdr1 in Azole Resistance. mBio 2019, 10, e02529-18. [Google Scholar] [CrossRef] [Green Version]
- Romera, D.; Aguilera-Correa, J.J.; Gadea, I.; Viñuela-Sandoval, L.; García-Rodríguez, J.; Esteban, J. Candida Auris: A Comparison between Planktonic and Biofilm Susceptibility to Antifungal Drugs. J. Med. Microbiol. 2019, 68, 1353–1358. [Google Scholar] [CrossRef]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida Auris: Epidemiology, Biology, Antifungal Resistance, and Virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef]
- Sharma, M.; Prasad, R. The Quorum-Sensing Molecule Farnesol Is a Modulator of Drug Efflux Mediated by ABC Multidrug Transporters and Synergizes with Drugs in Candida Albicans. Antimicrob. Agents Chemother. 2011, 55, 4834–4843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasi, M.; Khandelwal, N.K.; Moorhouse, A.J.; Nair, R.; Vishwakarma, P.; Bravo Ruiz, G.; Ross, Z.K.; Lorenz, A.; Rudramurthy, S.M.; Chakrabarti, A.; et al. ABC Transporter Genes Show Upregulated Expression in Drug-Resistant Clinical Isolates of Candida Auris: A Genome-Wide Characterization of ATP-Binding Cassette (ABC) Transporter Genes. Front. Microbiol. 2019, 10, 1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bidaud, A.L.; Djenontin, E.; Botterel, F.; Chowdhary, A.; Dannaoui, E. Colistin Interacts Synergistically with Echinocandins against Candida Auris. Int. J. Antimicrob. Agents 2020, 55, 105901. [Google Scholar] [CrossRef] [PubMed]
- Iyer, K.R.; Camara, K.; Daniel-Ivad, M.; Trilles, R.; Pimentel-Elardo, S.M.; Fossen, J.L.; Marchillo, K.; Liu, Z.; Singh, S.; Muñoz, J.F.; et al. An Oxindole Efflux Inhibitor Potentiates Azoles and Impairs Virulence in the Fungal Pathogen Candida Auris. Nat. Commun. 2020, 11, 6429. [Google Scholar] [CrossRef] [PubMed]
- Černáková, L.; Dižová, S.; Gášková, D.; Jančíková, I.; Bujdáková, H. Impact of Farnesol as a Modulator of Efflux Pumps in a Fluconazole-Resistant Strain of Candida Albicans. Microb. Drug Resist. 2019, 25, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Kovács, R.; Bozó, A.; Gesztelyi, R.; Domán, M.; Kardos, G.; Nagy, F.; Tóth, Z.; Majoros, L. Effect of Caspofungin and Micafungin in Combination with Farnesol against Candida Parapsilosis Biofilms. Int. J. Antimicrob. Agents 2016, 47, 304–310. [Google Scholar] [CrossRef] [Green Version]
- Nagy, F.; Tóth, Z.; Daróczi, L.; Székely, A.; Borman, A.M.; Majoros, L.; Kovács, R. Farnesol Increases the Activity of Echinocandins against Candida Auris Biofilms. Med. Mycol. 2020, 58, 404–407. [Google Scholar] [CrossRef]
- Pekard-Amenitsch, S.; Schriebl, A.; Posawetz, W.; Willinger, B.; Kölli, B.; Buzina, W. Isolation of Candida Auris from Ear of Otherwise Healthy Patient, Austria, 2018. Emerg. Infect. Dis. 2018, 24, 1596–1597. [Google Scholar] [CrossRef] [Green Version]
- Orofino, F.; Truglio, G.I.; Fiorucci, D.; D’Agostino, I.; Borgini, M.; Poggialini, F.; Zamperini, C.; Dreassi, E.; Maccari, L.; Torelli, R.; et al. In Vitro Characterization, ADME Analysis, and Histological and Toxicological Evaluation of BM1, a Macrocyclic Amidinourea Active against Azole-Resistant Candida Strains. Int. J. Antimicrob. Agents 2020, 55, 105865. [Google Scholar] [CrossRef]
- EUCAST: Breakpoints for Antifungals. Version 10.0, Valid from 2020-02-04. Available online: https://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals/ (accessed on 4 May 2022).
- Odds, F.C. Synergy, Antagonism, and What the Chequerboard Puts between Them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Dawis, M.A.; Isenberg, H.D.; France, K.A.; Jenkins, S.G. In Vitro Activity of Gatifloxacin Alone and in Combination with Cefepime, Meropenem, Piperacillin and Gentamicin against Multidrug-Resistant Organisms. J. Antimicrob. Chemother. 2003, 51, 1203–1211. [Google Scholar] [CrossRef] [Green Version]
- Kean, R.; Brown, J.; Gulmez, D.; Ware, A.; Ramage, G. Candida Auris: A Decade of Understanding of an Enigmatic Pathogenic Yeast. J. Fungi 2020, 6, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.G.; Shin, J.H.; Uh, Y.; Kang, M.G.; Kim, S.H.; Park, K.H.; Jang, H.-C. First Three Reported Cases of Nosocomial Fungemia Caused by Candida Auris. J. Clin. Microbiol. 2011, 49, 3139–3142. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Tian, S.; Han, X.; Chu, Y.; Wang, Q.; Zhou, B.; Shang, H. Is the Superbug Fungus Really so Scary? A Systematic Review and Meta-Analysis of Global Epidemiology and Mortality of Candida Auris. BMC Infect. Dis. 2020, 20, 827. [Google Scholar] [CrossRef] [PubMed]
- Shahi, G.; Kumar, M.; Kumari, S.; Rudramurthy, S.M.; Chakrabarti, A.; Gaur, N.A.; Singh, A.; Prasad, R. A Detailed Lipidomic Study of Human Pathogenic Fungi Candida Auris. FEMS Yeast Res. 2020, 20, foaa045. [Google Scholar] [CrossRef] [PubMed]
- Rybak, J.M.; Doorley, L.A.; Nishimoto, A.T.; Barker, K.S.; Palmer, G.E.; Rogers, P.D. Abrogation of Triazole Resistance upon Deletion of CDR1 in a Clinical Isolate of Candida Auris. Antimicrob. Agents Chemother. 2019, 63, e00057-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ademe, M.; Girma, F. Candida Auris: From Multidrug Resistance to Pan-Resistant Strains. Infect. Drug Resist. 2020, 13, 1287–1294. [Google Scholar] [CrossRef]
- Ostrowsky, B.; Greenko, J.; Adams, E.; Quinn, M.; O’Brien, B.; Chaturvedi, V.; Berkow, E.; Vallabhaneni, S.; Forsberg, K.; Chaturvedi, S.; et al. Candida Auris Isolates Resistant to Three Classes of Antifungal Medications—New York, 2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 6–9. [Google Scholar] [CrossRef] [Green Version]
- Fayed, B.; Jayakumar, M.N.; Soliman, S.S.M. Caspofungin-Resistance in Candida Auris Is Cell Wall-Dependent Phenotype and Potential Prevention by Zinc Oxide Nanoparticles. Med. Mycol. 2021, 59, 1243–1256. [Google Scholar] [CrossRef]
- Sharma, D.; Paul, R.A.; Rudramurthy, S.M.; Kashyap, N.; Bhattacharya, S.; Soman, R.; Shankarnarayan, S.A.; Chavan, D.; Singh, S.; Das, P.; et al. Impact of FKS1 Genotype on Echinocandin In Vitro Susceptibility in Candida Auris and In Vivo Response in a Murine Model of Infection. Antimicrob. Agents Chemother. 2022, 66, e0165221. [Google Scholar] [CrossRef]
- Kathuria, S.; Singh, P.K.; Sharma, C.; Prakash, A.; Masih, A.; Kumar, A.; Meis, J.F.; Chowdhary, A. Multidrug-Resistant Candida Auris Misidentified as Candida Haemulonii: Characterization by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry and DNA Sequencing and Its Antifungal Susceptibility Profile Variability by Vitek 2, CLSI Broth Microdilution, and Etest Method. J. Clin. Microbiol. 2015, 53, 1823–1830. [Google Scholar] [CrossRef] [Green Version]
- Escandón, P.; Chow, N.A.; Caceres, D.H.; Gade, L.; Berkow, E.L.; Armstrong, P.; Rivera, S.; Misas, E.; Duarte, C.; Moulton-Meissner, H.; et al. Molecular Epidemiology of Candida Auris in Colombia Reveals a Highly Related, Countrywide Colonization With Regional Patterns in Amphotericin B Resistance. Clin. Infect. Dis. 2019, 68, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida Albicans and Emerging Non-Albicans Candida Species. Front. Microbiol. 2016, 7, 2173. [Google Scholar] [CrossRef] [Green Version]
- Wirsching, S.; Michel, S.; Köhler, G.; Morschhäuser, J. Activation of the Multiple Drug Resistance Gene MDR1 in Fluconazole-Resistant, Clinical Candida Albicans Strains Is Caused by Mutations in a Trans-Regulatory Factor. J. Bacteriol. 2000, 182, 400–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourakbari, B.; Teymuri, M.; Mahmoudi, S.; Valian, S.K.; Movahedi, Z.; Eshaghi, H.; Mamishi, S. Expression of Major Efflux Pumps in Fluconazole-Resistant Candida Albicans. Infect. Disord. Drug Targets 2017, 17, 178–184. [Google Scholar] [CrossRef]
- Kean, R.; Ramage, G. Combined Antifungal Resistance and Biofilm Tolerance: The Global Threat of Candida Auris. mSphere 2019, 4, e00458-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamith-Miranda, D.; Heyman, H.M.; Cleare, L.G.; Couvillion, S.P.; Clair, G.C.; Bredeweg, E.L.; Gacser, A.; Nimrichter, L.; Nakayasu, E.S.; Nosanchuk, J.D. Multi-Omics Signature of Candida Auris, an Emerging and Multidrug-Resistant Pathogen. mSystems 2019, 4, e00257-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Healey, K.R.; Kordalewska, M.; Jiménez Ortigosa, C.; Singh, A.; Berrío, I.; Chowdhary, A.; Perlin, D.S. Limited ERG11 Mutations Identified in Isolates of Candida Auris Directly Contribute to Reduced Azole Susceptibility. Antimicrob. Agents Chemother. 2018, 62, e01427-18. [Google Scholar] [CrossRef] [Green Version]
- AlJindan, R.; AlEraky, D.M.; Mahmoud, N.; Abdalhamid, B.; Almustafa, M.; AbdulAzeez, S.; Borgio, J.F. Drug Resistance-Associated Mutations in ERG11 of Multidrug-Resistant Candida Auris in a Tertiary Care Hospital of Eastern Saudi Arabia. J. Fungi 2020, 7, 18. [Google Scholar] [CrossRef]
- Katragkou, A.; McCarthy, M.; Alexander, E.L.; Antachopoulos, C.; Meletiadis, J.; Jabra-Rizk, M.A.; Petraitis, V.; Roilides, E.; Walsh, T.J. In Vitro Interactions between Farnesol and Fluconazole, Amphotericin B or Micafungin against Candida Albicans Biofilms. J. Antimicrob. Chemother. 2015, 70, 470–478. [Google Scholar] [CrossRef]
- Bozó, A.; Domán, M.; Majoros, L.; Kardos, G.; Varga, I.; Kovács, R. The in Vitro and in Vivo Efficacy of Fluconazole in Combination with Farnesol against Candida Albicans Isolates Using a Murine Vulvovaginitis Model. J. Microbiol. 2016, 54, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, D.R.; Arias, L.S.; Fernandes, R.A.; Deszo da Silva, L.F.; de Castilho, M.O.V.F.; da Rosa, T.O.; Vieira, A.P.M.; Straioto, F.G.; Barbosa, D.B.; Delbem, A.C.B. Antifungal Activity of Tyrosol and Farnesol Used in Combination against Candida Species in the Planktonic State or Forming Biofilms. J. Appl. Microbiol. 2017, 123, 392–400. [Google Scholar] [CrossRef]
- Zhu, J.; Krom, B.P.; Sanglard, D.; Intapa, C.; Dawson, C.C.; Peters, B.M.; Shirtliff, M.E.; Jabra-Rizk, M.A. Farnesol-Induced Apoptosis in Candida Albicans Is Mediated by Cdr1-p Extrusion and Depletion of Intracellular Glutathione. PLoS ONE 2011, 6, e28830. [Google Scholar] [CrossRef] [Green Version]
- Hiller, D.; Sanglard, D.; Morschhäuser, J. Overexpression of the MDR1 Gene Is Sufficient to Confer Increased Resistance to Toxic Compounds in Candida Albicans. Antimicrob. Agents Chemother. 2006, 50, 1365–1371. [Google Scholar] [CrossRef] [Green Version]
- Kong, E.F.; Tsui, C.; Kucharíková, S.; Van Dijck, P.; Jabra-Rizk, M.A. Modulation of Staphylococcus Aureus Response to Antimicrobials by the Candida Albicans Quorum Sensing Molecule Farnesol. Antimicrob. Agents Chemother. 2017, 61, e01573-17. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Wei, X.; Ma, M.; Chen, X.; Xu, S. Possible Inhibitory Molecular Mechanism of Farnesol on the Development of Fluconazole Resistance in Candida Albicans Biofilm. Antimicrob. Agents Chemother. 2012, 56, 770–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.-J.; Liu, Z.-H. Systematic Analysis of Protein Expression in Candida Albicans Exposed to Farnesol. Chin. Med. J. Engl. 2019, 132, 2348–2353. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Holowka, T.; Orner, E.P.; Fries, B.C. Gene Duplication Associated with Increased Fluconazole Tolerance in Candida Auris Cells of Advanced Generational Age. Sci. Rep. 2019, 9, 5052. [Google Scholar] [CrossRef] [Green Version]
- Maesaki, S.; Marichal, P.; Vanden Bossche, H.; Sanglard, D.; Kohno, S. Rhodamine 6G Efflux for the Detection of CDR1-Overexpressing Azole-Resistant Candida Albicans Strains. J. Antimicrob. Chemother. 1999, 44, 27–31. [Google Scholar] [CrossRef]
- Ruiz-Gaitán, A.C.; Fernández-Pereira, J.; Valentin, E.; Tormo-Mas, M.A.; Eraso, E.; Pemán, J.; de Groot, P.W.J. Molecular identification of Candida auris by PCR amplification of species-specific GPI protein-encoding genes. Int. J. Med. Microbiol. 2018, 308, 812–818. [Google Scholar] [CrossRef]
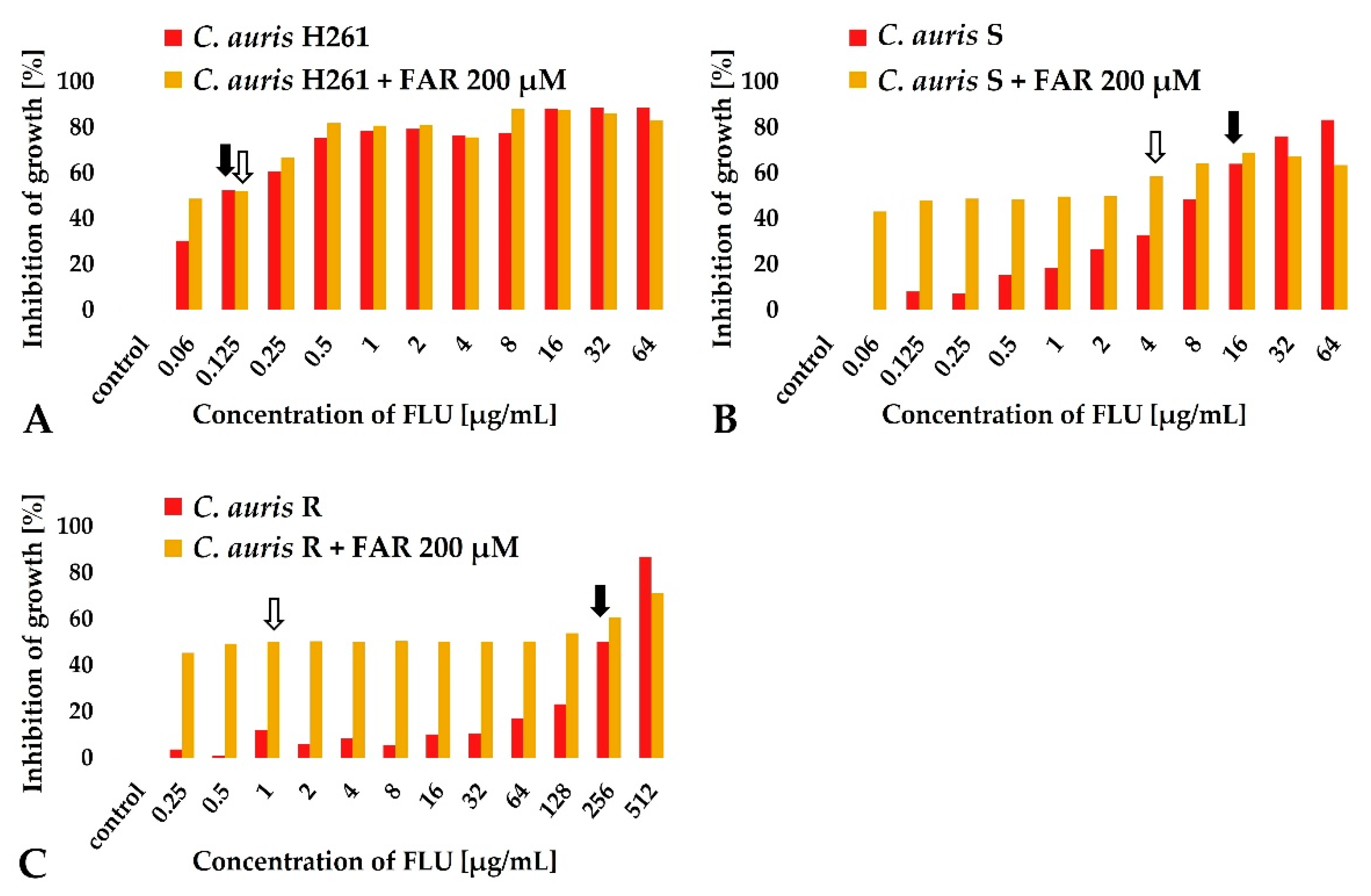
| Strain | MIC50 FLU (Alone) (μg/mL) | Interpretation | MIC50 FAR (Alone) (μM) | MIC50 FLU with 200 μM FAR (μg/mL) | FIC Index | Interpretation |
|---|---|---|---|---|---|---|
| C. auris H261 | 0.125 | Susceptible | 500 | 0.125 | 1.4 | Indifferent |
| C. auris S | 16 | Susceptible * | 400 | 4 | 0.75 | Partial synergy |
| C. auris R | 256 | Resistant | 500 | 1 | 0.4 | Synergy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dekkerová, J.; Černáková, L.; Kendra, S.; Borghi, E.; Ottaviano, E.; Willinger, B.; Bujdáková, H. Farnesol Boosts the Antifungal Effect of Fluconazole and Modulates Resistance in Candida auris through Regulation of the CDR1 and ERG11 Genes. J. Fungi 2022, 8, 783. https://doi.org/10.3390/jof8080783
Dekkerová J, Černáková L, Kendra S, Borghi E, Ottaviano E, Willinger B, Bujdáková H. Farnesol Boosts the Antifungal Effect of Fluconazole and Modulates Resistance in Candida auris through Regulation of the CDR1 and ERG11 Genes. Journal of Fungi. 2022; 8(8):783. https://doi.org/10.3390/jof8080783
Chicago/Turabian StyleDekkerová, Jaroslava, Lucia Černáková, Samuel Kendra, Elisa Borghi, Emerenziana Ottaviano, Birgit Willinger, and Helena Bujdáková. 2022. "Farnesol Boosts the Antifungal Effect of Fluconazole and Modulates Resistance in Candida auris through Regulation of the CDR1 and ERG11 Genes" Journal of Fungi 8, no. 8: 783. https://doi.org/10.3390/jof8080783
APA StyleDekkerová, J., Černáková, L., Kendra, S., Borghi, E., Ottaviano, E., Willinger, B., & Bujdáková, H. (2022). Farnesol Boosts the Antifungal Effect of Fluconazole and Modulates Resistance in Candida auris through Regulation of the CDR1 and ERG11 Genes. Journal of Fungi, 8(8), 783. https://doi.org/10.3390/jof8080783








