ZnO Nanoparticle-Mediated Seed Priming Induces Biochemical and Antioxidant Changes in Chickpea to Alleviate Fusarium Wilt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Pathogen
2.1.1. Molecular Identification of Fungus
2.1.2. Phylogenetic Analysis of Isolated Fungus
2.2. Fugal Culture
2.3. Preparation of Mycological Zinc Oxide Nanoparticles (ZnO NPs)
2.4. Characterization of ZnO NPs
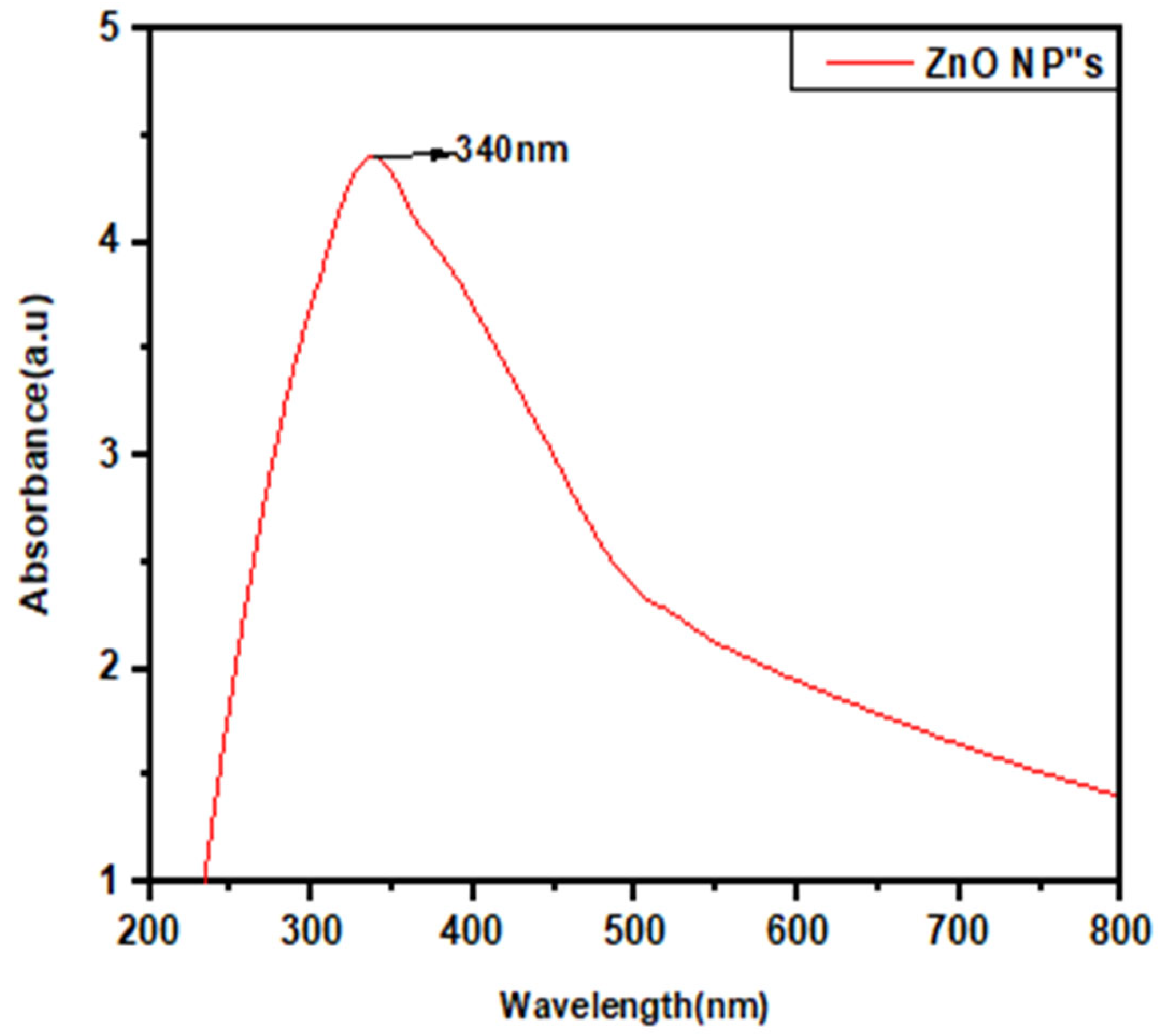
2.4.1. UV-Visible Spectroscopic Analysis
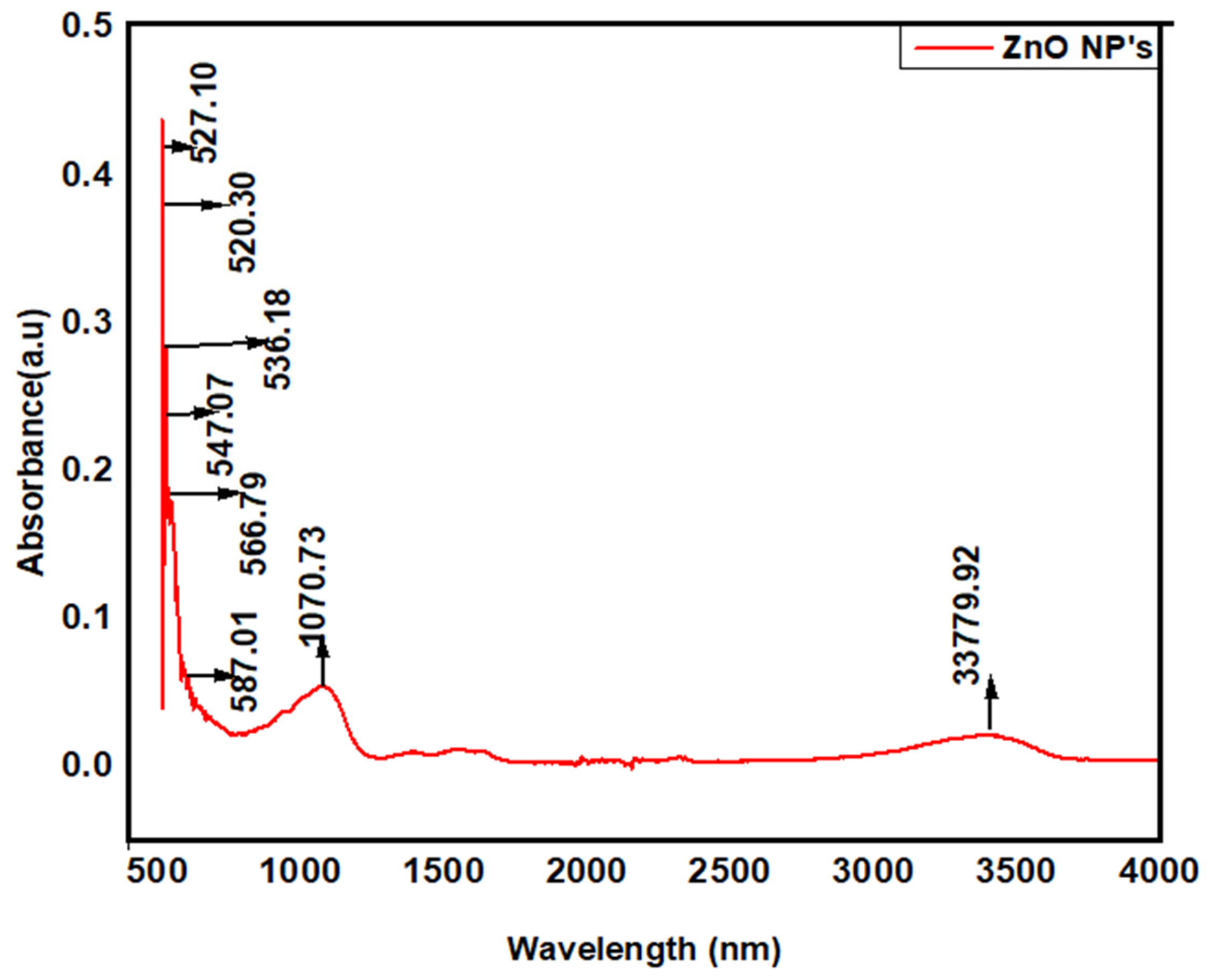
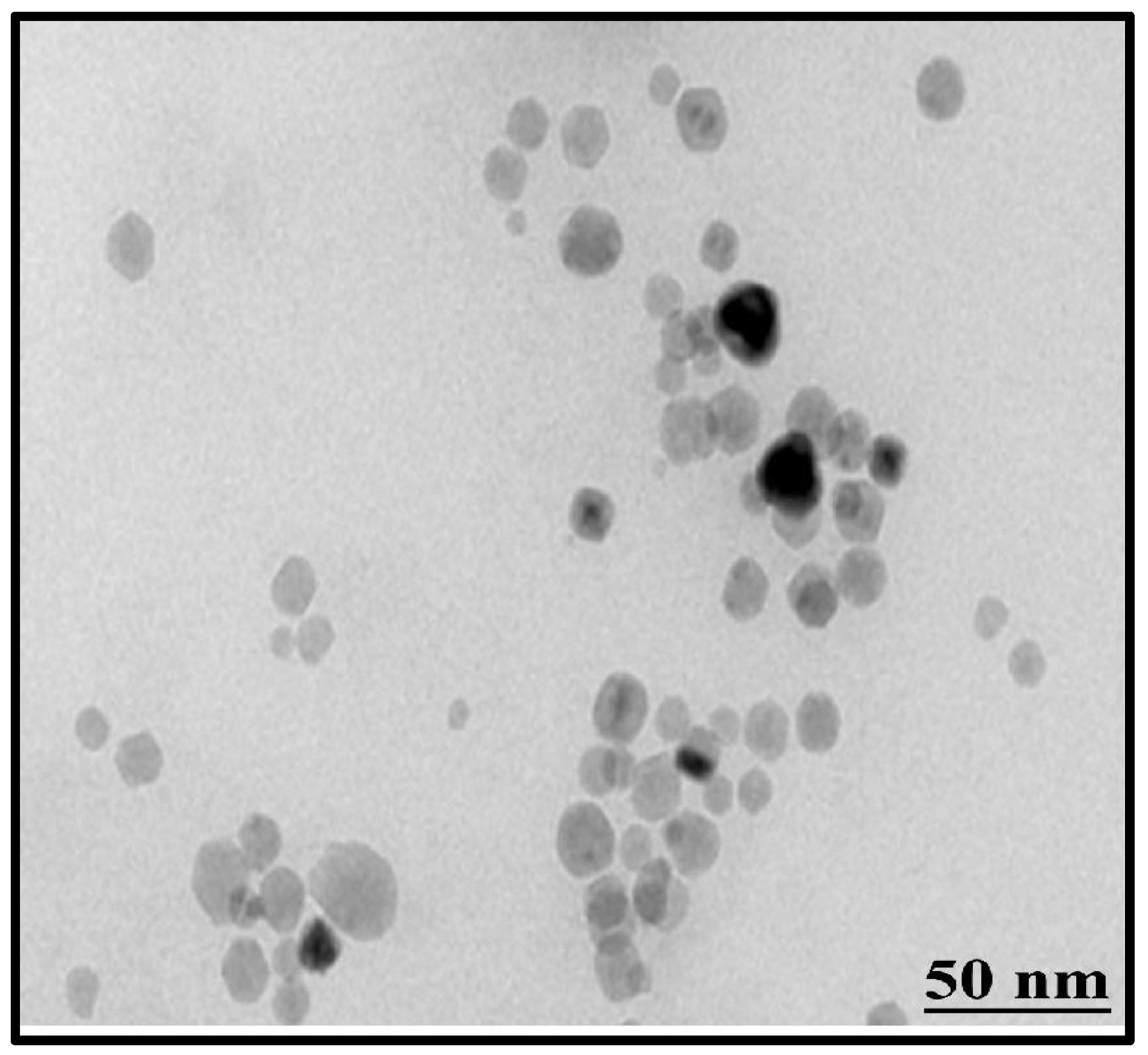
2.4.2. Fourier Transform Infrared (FTIR) Spectroscopy and Transmission Electron Microscopy (TEM)
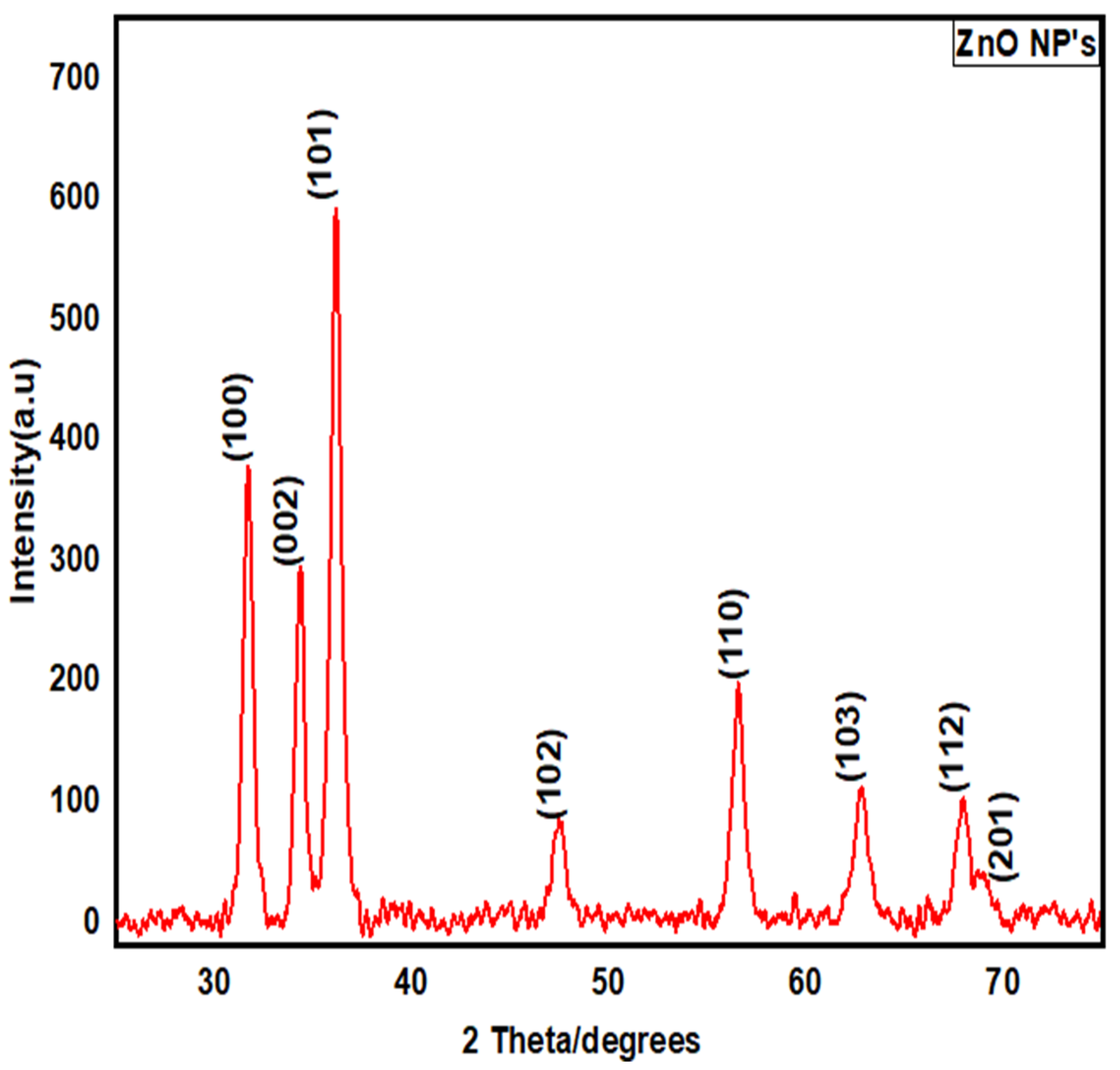
2.4.3. X-ray Diffraction (XRD)
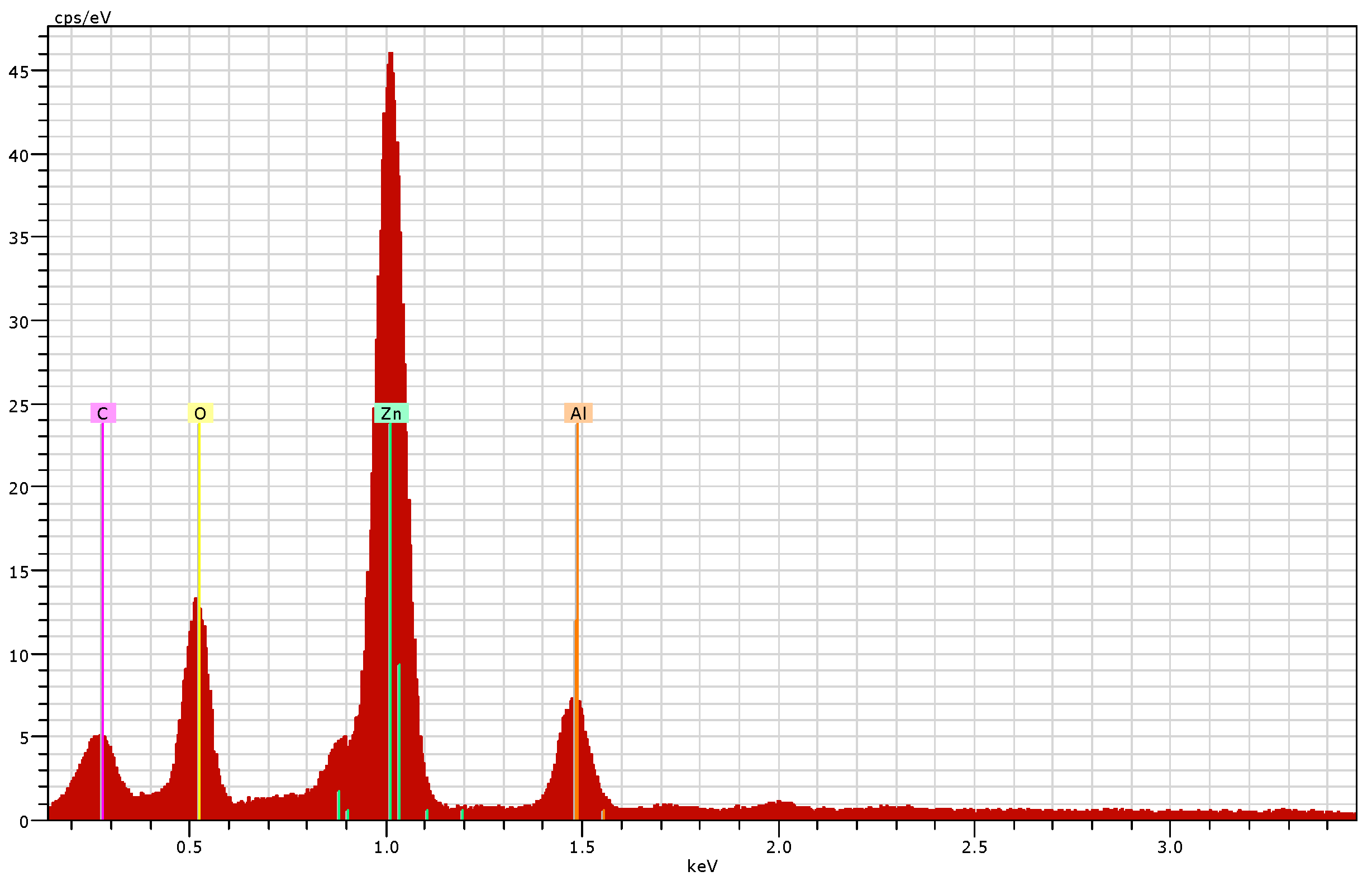
2.4.4. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDX) Analysis
2.5. Antifungal Assay
- T = average mycelial growth in treatments; C = average mycelial growth under control conditions.
2.6. Application of ZnO NPs, In Vivo
2.6.1. Preparation of Fungus Inoculum
2.6.2. Soil Preparation
2.6.3. Collection and Surface Sterilization of Seeds
2.6.4. Seed Priming and Sowing
2.6.5. Sowing and Germination of Seeds
2.7. Measurement of Disease Severity
2.8. Physiological Parameters
2.9. Biochemical Parameters
2.9.1. Estimation of Total Protein, Phenol, Sugar, and Flavonoid Contents
2.9.2. Enzymatic Activity (SOD, POD and CAT)
2.10. Statistical Analyses
3. Results
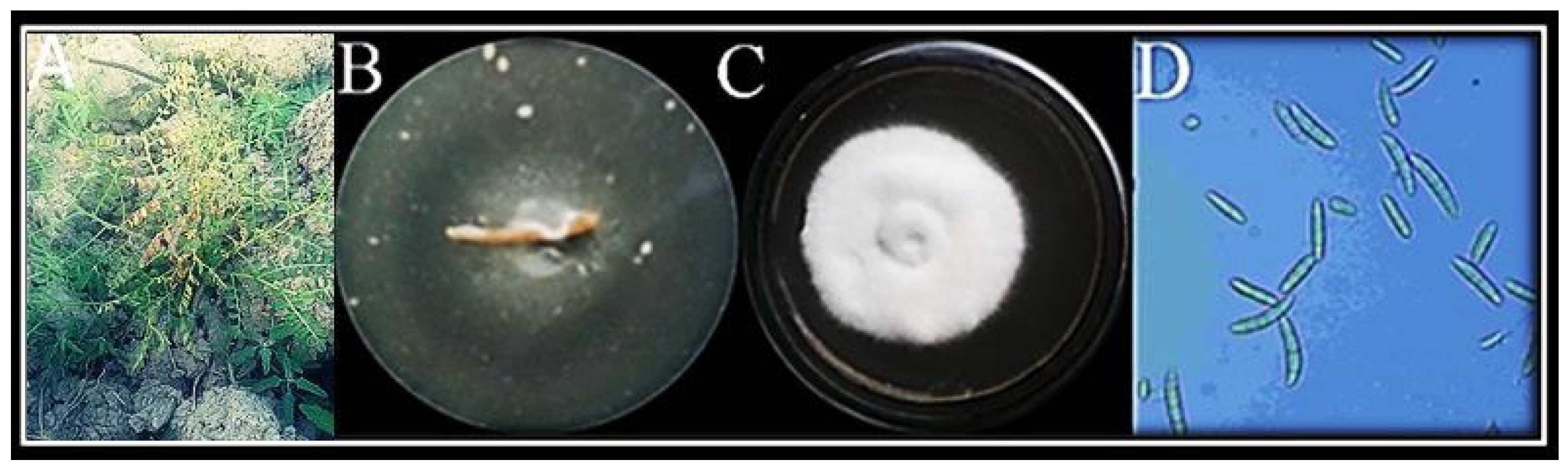
3.1. Isolation and Identification of Fungus
Molecular Identification of Fungus
3.2. Characterization of ZnO NPs
3.2.1. UV-Visible Spectroscopy
3.2.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.2.3. X-ray Diffraction (XRD) Structural Analysis
3.2.4. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDX) Analysis
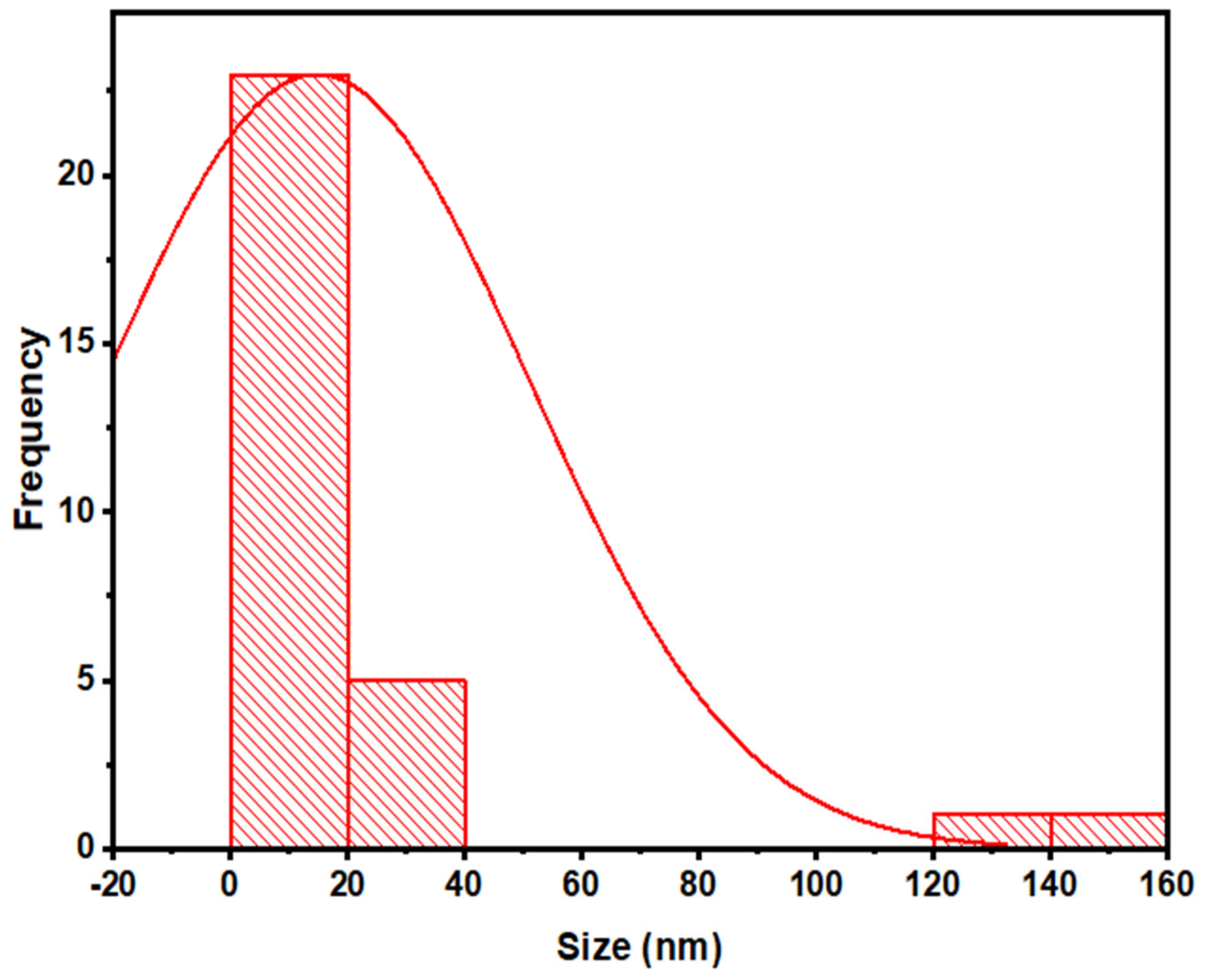
3.2.5. Transmission Electron Microscopy (TEM)
3.3. In Vitro Antagonism of F. oxysporum by T. harzianum Mediated ZnO NPs
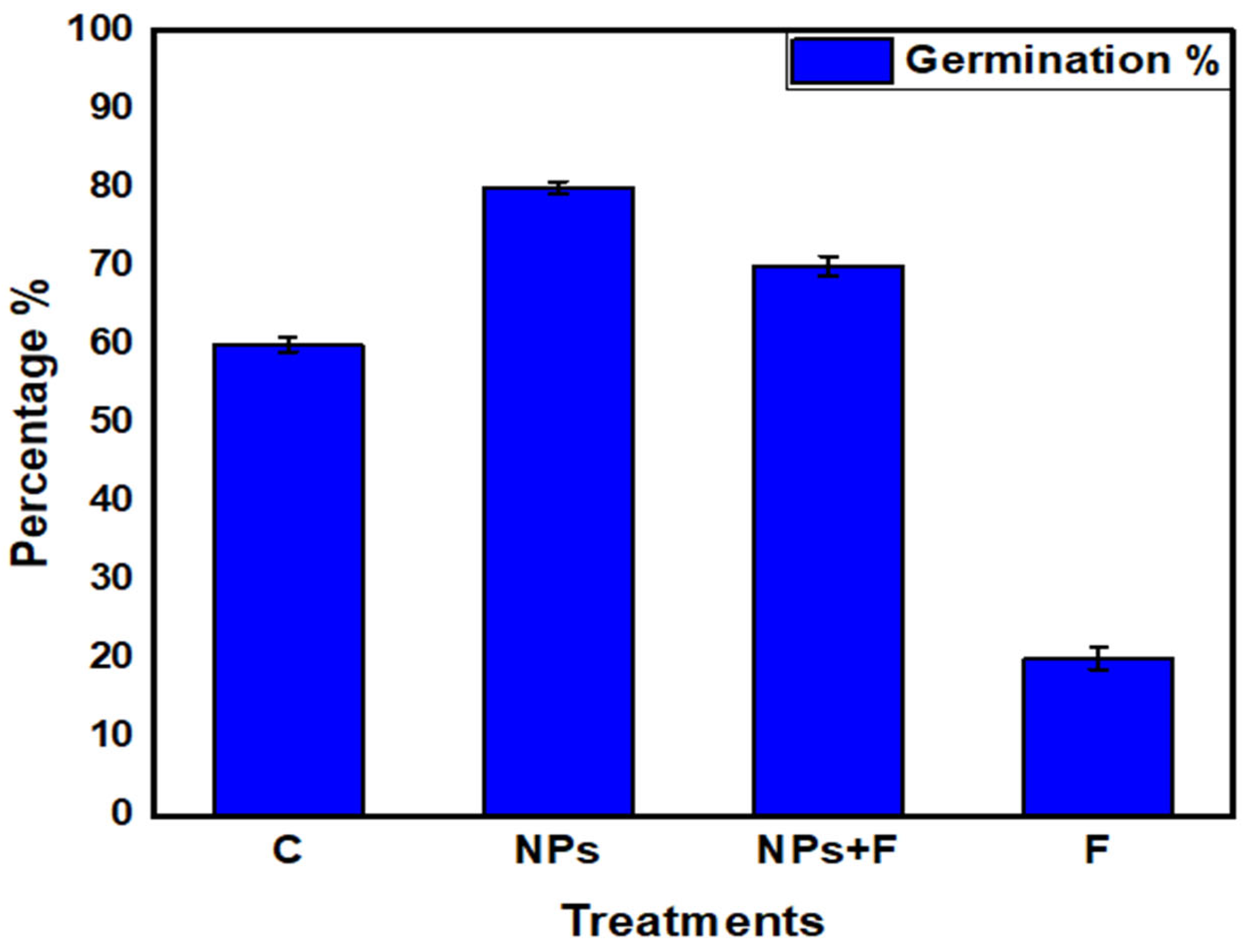
3.4. Germination Percentage
3.5. Disease Analyses
3.6. Disease Control Assay, In Vivo
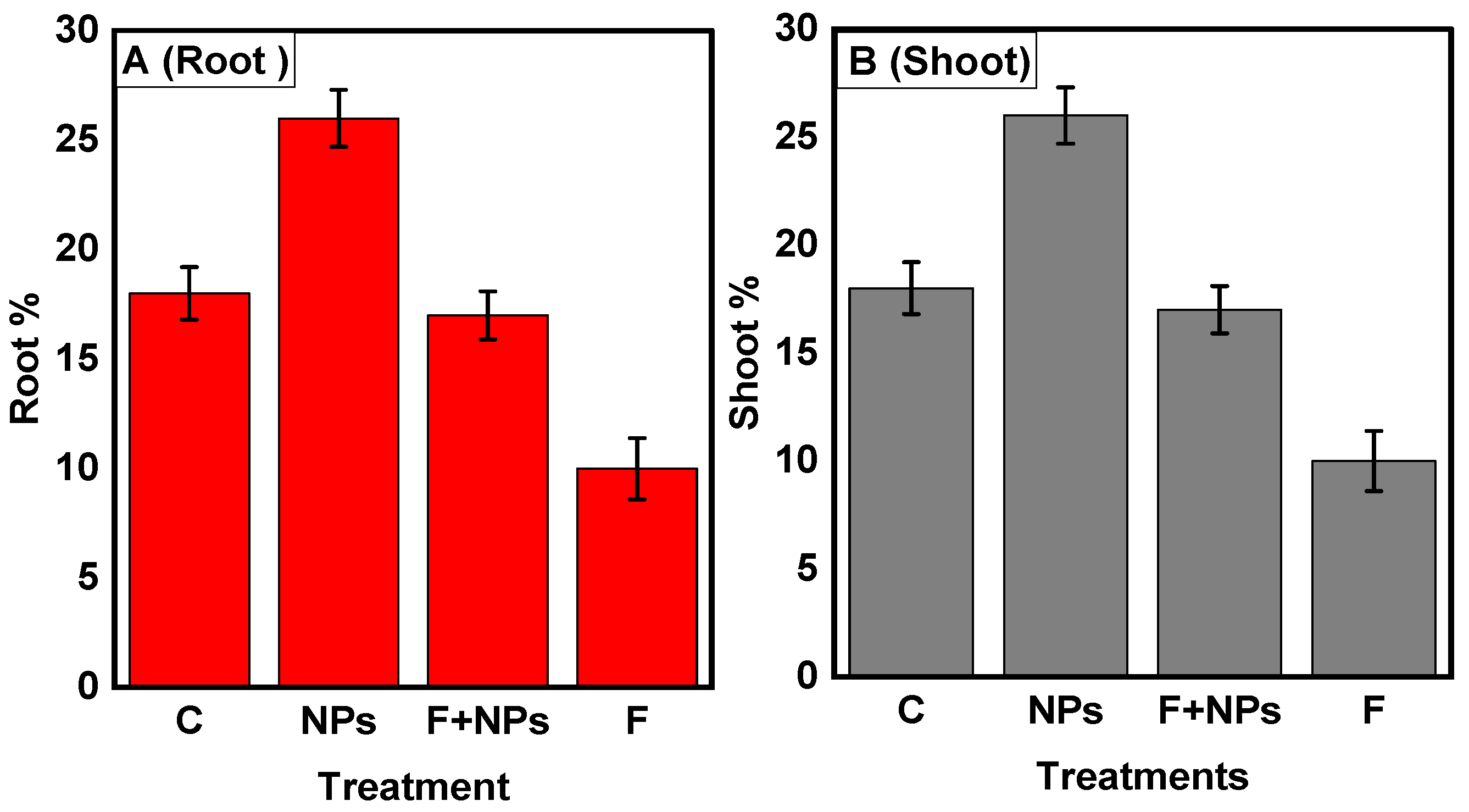
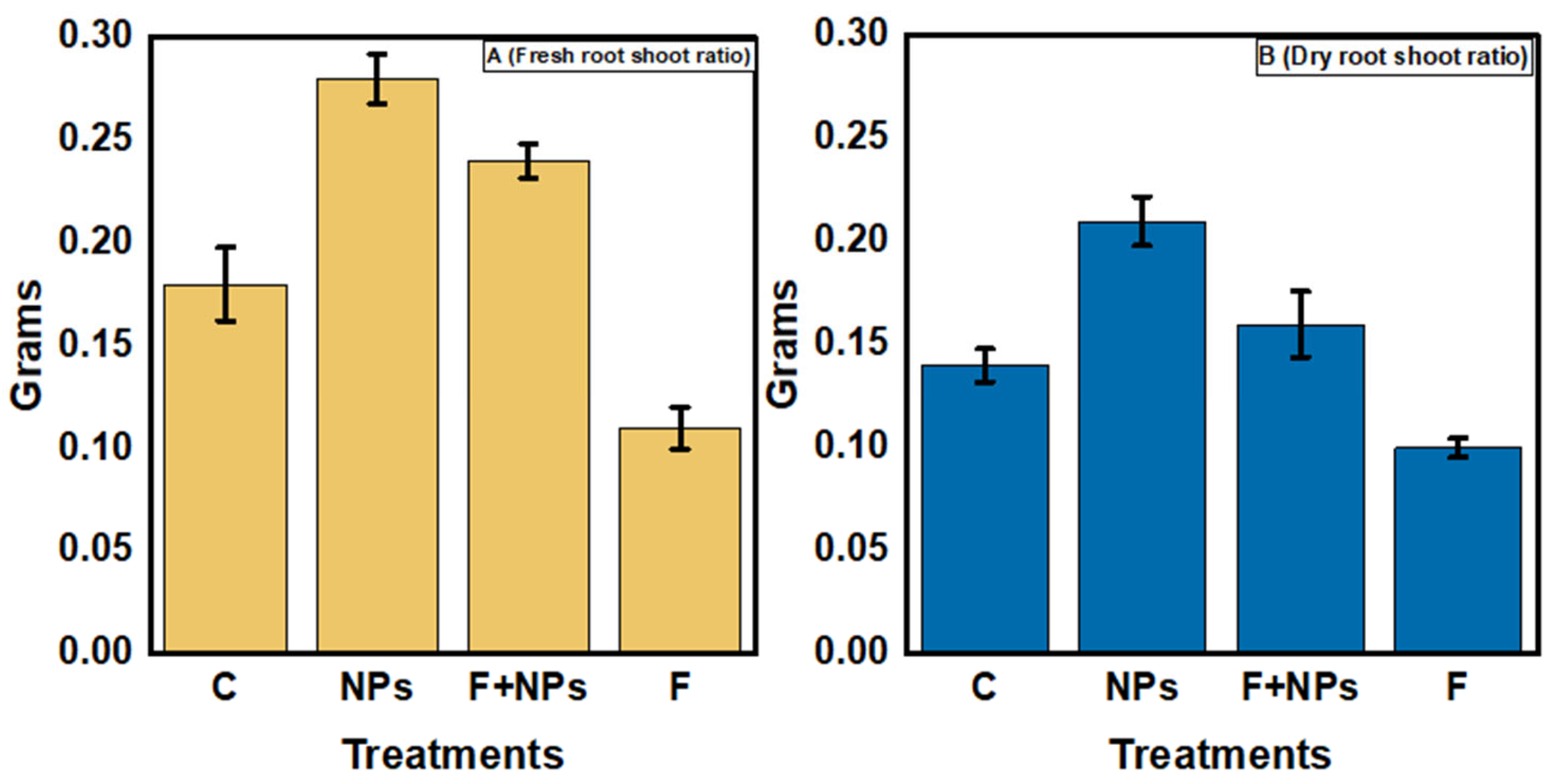
Physiological Parameters
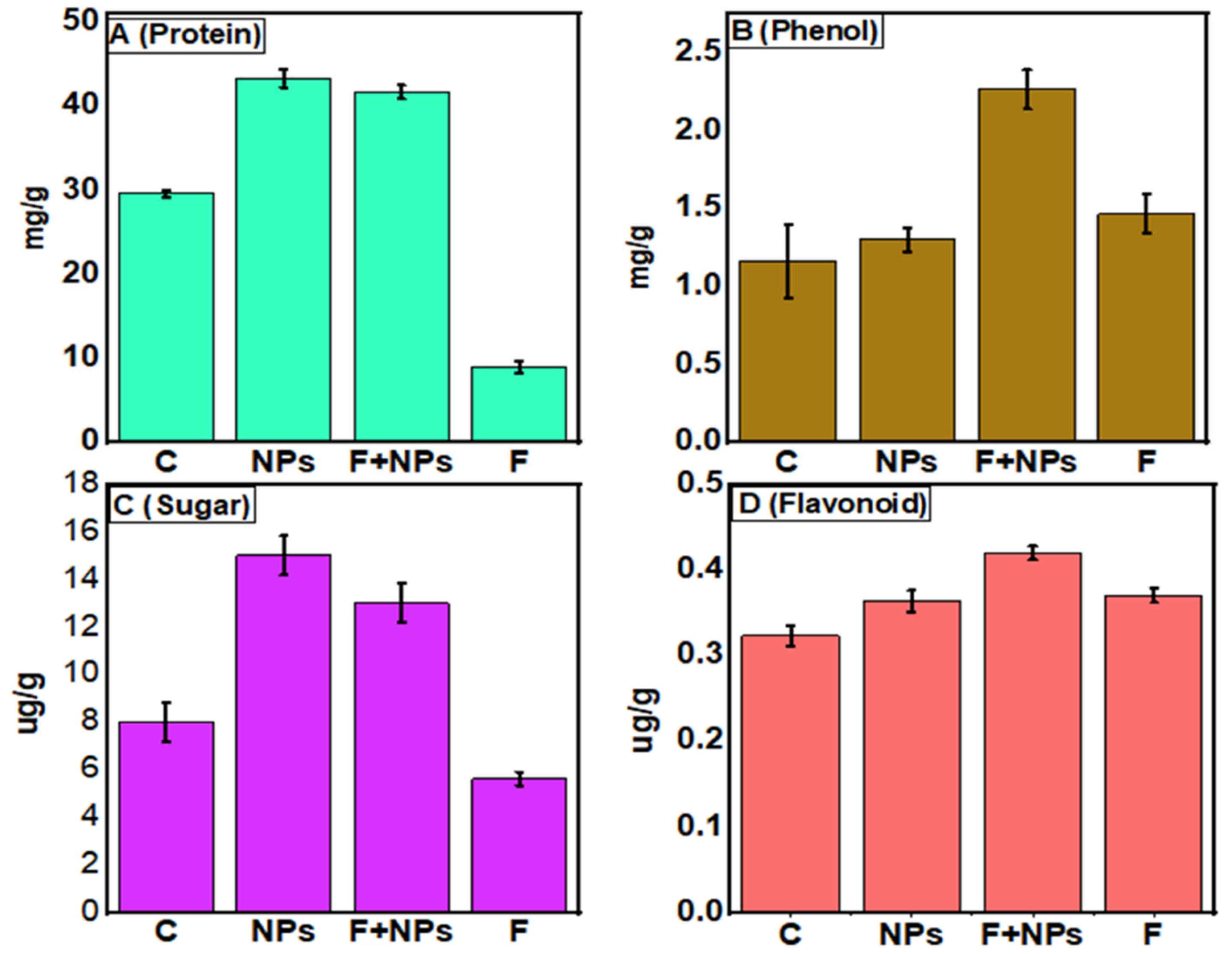
3.7. Biochemical Parameters
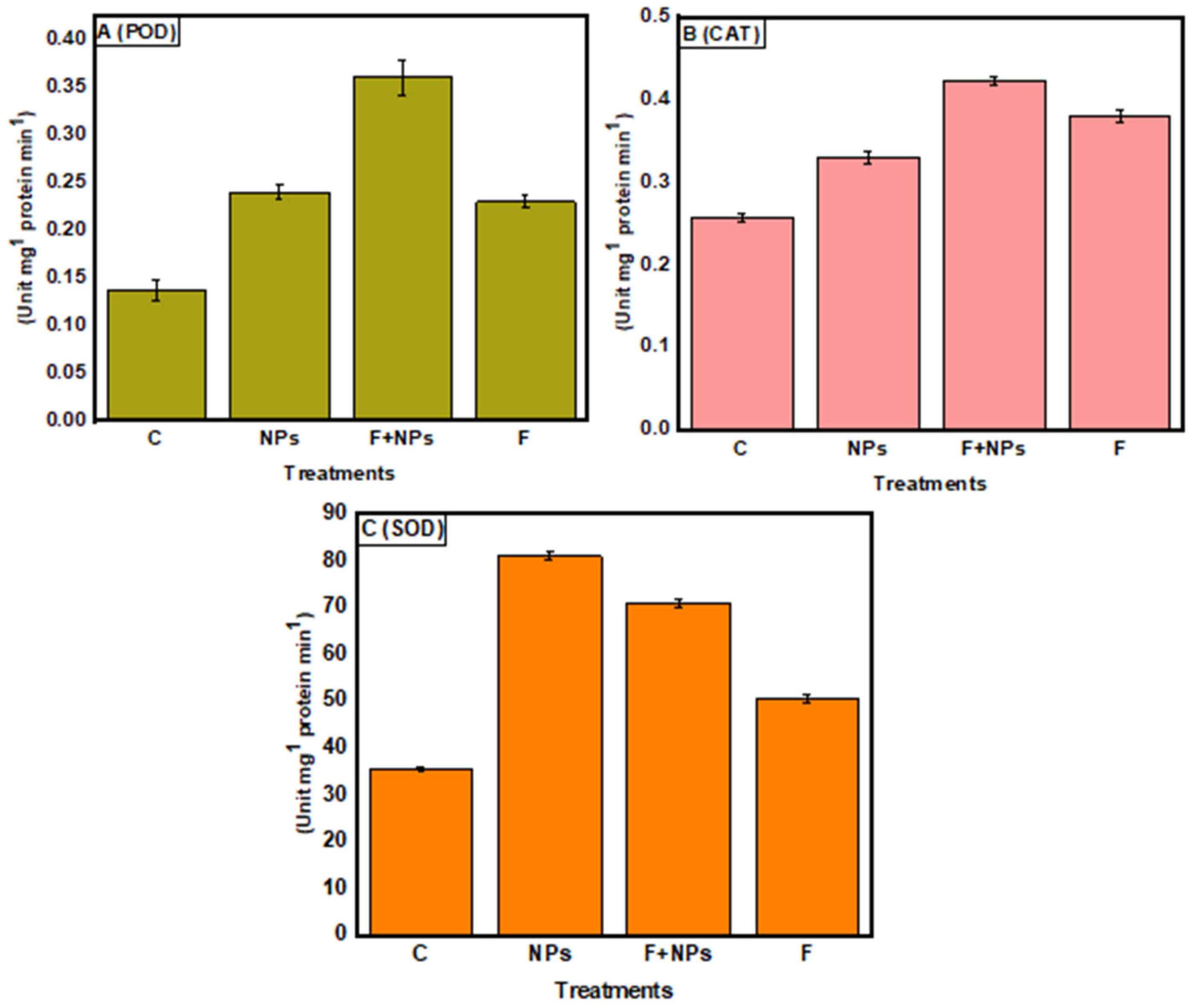
3.8. Enzymatic Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Merga, B.; Haji, J. Economic importance of chickpea: Production, value, and world trade. Cogent Food Agric. 2019, 5, 1615718. [Google Scholar] [CrossRef]
- Grasso, N.; Lynch, N.L.; Arendt, E.K.; O’Mahony, J.A. Chickpea protein ingredients: A review of composition, functionality, and applications. Compr. Rev. Food Sci. 2022, 21, 435–452. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, W.; Sankaran, S. High-throughput field phenotyping of Ascochyta blight disease severity in chickpea. J. Crop Prot. 2019, 125, 104885. [Google Scholar] [CrossRef]
- Maitlo, S.A.; Rajput, N.A.; Syed, R.N.; Khanzada, M.A.; Rajput, A.Q.; Lodhi, A.M. Microbial control of Fusarium wilt of chickpea caused by Fusarium oxysporum f. sp. ciceris. Pak. J. Bot. 2019, 51, 2261–2268. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Indhumathi, M. Chitosan thiamine nanoparticles intervene innate immunomodulation during Chickpea-Fusarium interaction. Int. J. Biol. Macromol. 2022, 198, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Fatima, I.; Hakim, S.; Imran, A.; Ahmad, N.; Imtiaz, M.; Ali, H.; Islam, E.-U.; Yousaf, S.; Mirza, M.S.; Mubeen, F. Exploring biocontrol and growth-promoting potential of multifaceted PGPR isolated from natural suppressive soil against the causal agent of chickpea wilt. Microbiol. Res. 2022, 260, 127015. [Google Scholar] [CrossRef]
- Sunkad, G.; Deepa, H.; Shruthi, T.H.; Singh, D. Chickpea wilt: Status, diagnostics and management. Indian Phytopathol. 2019, 72, 619–627. [Google Scholar] [CrossRef]
- Arie, T. Fusarium diseases of cultivated plants, control, diagnosis, and molecular and genetic studies. J. Pestic. Sci. 2019, 44, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Chittarath, K.; Nguyen, C.H.; Bailey, W.C.; Zheng, S.-J.; Mostert, D.; Viljoen, A.; Tazuba, A.F.; Ocimati, W.; Kearsley, E.; Chi, T.Y.; et al. Geographical Distribution and Genetic Diversity of the Banana Fusarium Wilt Fungus in Laos and Vietnam. J. Fungi 2022, 8, 46. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ahmad, R.; Ashraf, M. Plant-extract mediated green approach for the synthesis of ZnONPs: Characterization and evaluation of cytotoxic, antimicrobial and antioxidant potentials. J. Mol. Struct. 2019, 1189, 315–327. [Google Scholar] [CrossRef]
- Lipșa, F.-D.; Ursu, E.-L.; Ursu, C.; Ulea, E.; Cazacu, A. Evaluation of the Antifungal Activity of Gold–Chitosan and Carbon Nanoparticles on Fusarium oxysporum. Agronomy 2020, 10, 1143. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Haynes, T.E.; White, C.W.; MoberlyChan, W.J.; Roorda, S.; Aziz, M.J. Synthesis of Nearly Monodisperse Embedded Nanoparticles by Separating Nucleation and Growth in Ion Implantation. Nano Lett. 2005, 5, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Pantarotto, D.; Partidos, C.D.; Graff, R.; Hoebeke, J.; Briand, J.-P.; Prato, M.; Bianco, A. Synthesis, Structural Characterization, and Immunological Properties of Carbon Nanotubes Functionalized with Peptides. J. Am. Chem. Soc. 2003, 125, 6160–6164. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Ramanathan, R.; Bhargava, S.K. Fungus-mediated Biological Approaches Towards ‘Green’ Synthesis of Oxide Nanomaterials. Aust. J. Chem. 2011, 64, 279–293. [Google Scholar] [CrossRef]
- Rosado, I.; Rey, M.; Codón, A.C.; Govantes, J.; Moreno-Mateos, M.; Benítez, T. QID74 Cell wall protein of Trichoderma harzianum is involved in cell protection and adherence to hydrophobic surfaces. Fungal Genet. Biol. 2007, 44, 950–964. [Google Scholar] [CrossRef]
- Fraceto, L.F.; Maruyama, C.R.; Guilger, M.; Mishra, S.; Keswani, C.; Singh, H.B.; de Lima, R. Trichoderma harzianum-based novel formulations: Potential applications for management of Next-Gen agricultural challenges. J. Chem. Technol. Biotechnol. 2018, 93, 2056–2063. [Google Scholar] [CrossRef]
- Vahabi, K.; Mansoori, G.; Karimi, S. Biosynthesis of Silver Nanoparticles by Fungus Trichoderma reesei (A Route for Large-Scale Production of AgNPs). Insciences J. 2011, 1, 65–79. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Patra, S.; Biswas, M.K. Studies on cultural, morphological and pathogenic variability among the isolates of Fusarium oxysporum f. sp. ciceri causing wilt of chickpea. Int. J. Plant Anim. Environ. Sci. 2017, 7, 11–17. [Google Scholar] [CrossRef]
- De Carvalho Paulino, J.F.; de Almeida, C.P.; Barbosa, C.C.F.; de Moraes, G.; Gonçalves, C.; Bueno, C.J.; Harakava, R.; Carbonell, S.A.M.; Chiorato, A.F.; Benchimol-Reis, L.L. Molecular and pathogenicity characterization of Fusarium oxysporum species complex associated with Fusarium wilt of common bean in Brazil. Trop. Plant Pathol. 2022, 1–10. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombard, L.; Sandoval-Denis, M.; Lamprecht, S.C.; Crous, P. Epitypification of Fusarium oxysporum—Clearing the taxonomic chaos. Persoonia Mol. Phylogeny Evol. Fungi 2019, 43, 1–47. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.A.; Fouda, A.; Abdel-Rahman, M.A.; Hassan, S.E.-D.; El-Gamal, M.S.; Salem, S.S.; Shaheen, T.I. Fungal strain impacts the shape, bioactivity and multifunctional properties of green synthesized zinc oxide nanoparticles. Biocatal. Agric. Biotechnol. 2019, 19, 101103. [Google Scholar] [CrossRef]
- Kalsa, K.K.; Bekele, A. Influence of seed priming on seed germination and vigor traits of Vicia villosa ssp. dasycarpa (Ten.). Afr. J. Agric. Res. 2012, 7, 3202–3208. [Google Scholar] [CrossRef]
- Tyree, M.T.; Cochard, H. Vessel contents of leaves after excision: A test of the Scholander assumption. J. Exp. Bot. 2003, 54, 2133–2139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, B. Disease assessment and yield loss. In The Epidemiology of Plant Diseases; Springer: Berlin/Heidelberg, Germany, 2006; pp. 43–80. [Google Scholar] [CrossRef]
- Zheng, Z.; Shetty, K. Solid-State Bioconversion of Phenolics from Cranberry Pomace and Role of Lentinus edodes β-Glucosidase. J. Agric. Food Chem. 2000, 48, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J.-C.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef]
- Burman, U.; Saini, M.; Kumar, P. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- Kaur, P.; Bali, S.; Sharma, A.; Kohli, S.K.; Vig, A.P.; Bhardwaj, R.; Thukral, A.; Abd_Allah, E.; Wijaya, L.; Alyemeni, M.N.; et al. Cd induced generation of free radical species in Brassica juncea is regulated by supplementation of earthworms in the drilosphere. Sci. Total Environ. 2019, 655, 663–675. [Google Scholar] [CrossRef]
- Kalpana, V.; Kataru, B.A.S.; Sravani, N.; Vigneshwari, T.; Panneerselvam, A.; Rajeswari, V.D. Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus niger: Antimicrobial textiles and dye degradation studies. OpenNano 2018, 3, 48–55. [Google Scholar] [CrossRef]
- Housseiny, M.; Gomaa, E. Enhancement of antimicrobial and antitumor activities of zinc nanoparticles biosynthesized by Penicillium chrysogenum AUMC 10608 using gamma radiation. Egypt. J. Bot. 2019, 59, 319–337. [Google Scholar] [CrossRef]
- Jamdagni, P.; Khatri, P.; Rana, J. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud Univ. Sci. 2018, 30, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Pariona, N.; Paraguay-Delgado, F.; Basurto-Cereceda, S.; Morales-Mendoza, J.E.; Hermida-Montero, L.A.; Mtz-Enriquez, A.I. Shape-dependent antifungal activity of ZnO particles against phytopathogenic fungi. Appl. Nanosci. 2020, 10, 435–443. [Google Scholar] [CrossRef]
- Naznin, H.A.; Kiyohara, D.; Kimura, M.; Miyazawa, M.; Shimizu, M.; Hyakumachi, M. Systemic Resistance Induced by Volatile Organic Compounds Emitted by Plant Growth-Promoting Fungi in Arabidopsis thaliana. PLoS ONE 2014, 9, e86882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.; Hanjra, M.A. Sustainable land and water management policies and practices: A pathway to environmental sustainability in large irrigation systems. Land Degrad. Dev. 2008, 19, 469–487. [Google Scholar] [CrossRef]
- Alamri, S.; Nafady, N.A.; El-Sagheer, A.M.; El-Aal, M.A.; Mostafa, Y.S.; Hashem, M.; Hassan, E.A. Current Utility of Arbuscular Mycorrhizal Fungi and Hydroxyapatite Nanoparticles in Suppression of Tomato Root-Knot Nematode. Agronomy 2022, 12, 671. [Google Scholar] [CrossRef]
- Imran, M.; Abo-Elyousr, K.A.M.; El-Sharnouby, M.E.; Ali, E.F.; Sallam, N.M.A.; Bagy, H.M.M.K.; Abdel-Rahim, I.R. Biocontrol Potential of Trichoderma harzianum and Zinc Nanoparticles to Mitigate Gray Mold Disease of Tomato. Gesunde Pflanz. 2022, 1–13. [Google Scholar] [CrossRef]
- Akbar, M.; Haroon, U.; Ali, M.; Tahir, K.; Chaudhary, H.J.; Munis, M.F.H. Mycosynthesized Fe2O3 nanoparticles diminish brown rot of apple whilst maintaining composition and pertinent organoleptic properties. J. Appl. Microbiol. 2022, 132, 3735–3745. [Google Scholar] [CrossRef]
- Ali, M.; Haroon, U.; Khizar, M.; Chaudhary, H.J.; Munis, M.F.H. Scanning electron microscopy of bio-fabricated Fe2O3 nanoparticles and their application to control brown rot of citrus. Microsc. Res. Tech. 2021, 84, 101–110. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Jayaraj, M.; Manikandan, R.; Geetha, N.; Rene, E.R.; Sharma, N.; Sahi, S. Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: A physiochemical analysis. Plant Physiol. Biochem. 2017, 110, 59–69. [Google Scholar] [CrossRef]
- Jadhav, S.; Gaikwad, S.; Nimse, M.; Rajbhoj, A. Copper Oxide Nanoparticles: Synthesis, Characterization and Their Antibacterial Activity. J. Clust. Sci. 2011, 22, 121–129. [Google Scholar] [CrossRef]
- Bilesky-José, N.; Maruyama, C.; Germano-Costa, T.; Campos, E.; Carvalho, L.; Grillo, R.; Fraceto, L.F.; de Lima, R. Biogenic α-Fe2O3 Nanoparticles Enhance the Biological Activity of Trichoderma against the Plant Pathogen Sclerotinia sclerotiorum. ACS Sustain. Chem. Eng. 2021, 9, 1669–1683. [Google Scholar] [CrossRef]
- Santos, T.R.T.; Andrade, M.B.; Silva, M.F.; Bergamasco, R.; Hamoudi, S. Development of α- and γ-Fe2O3 decorated graphene oxides for glyphosate removal from water. Environ. Technol. 2019, 40, 1118–1137. [Google Scholar] [CrossRef] [PubMed]
- Vanathi, P.; Rajiv, P.; Narendhran, S.; Rajeshwari, S.; Rahman, P.K.; Venckatesh, R. Biosynthesis and characterization of phyto mediated zinc oxide nanoparticles: A green chemistry approach. Mater. Lett. 2014, 134, 13–15. [Google Scholar] [CrossRef]
- Yedurkar, S.; Maurya, C.; Mahanwar, P. Biosynthesis of Zinc Oxide Nanoparticles Using Ixora Coccinea Leaf Extract—A Green Approach. Open J. Synth. Theory Appl. 2016, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Nafady, N.A.; Alamri, S.A.; Hassan, E.A.; Hashem, M.; Mostafa, Y.S.; Abo-Elyousr, K.A. Application of ZnO-nanoparticles to manage Rhizopus soft rot of sweet potato and prolong shelf-life. Folia Hortic. 2019, 31, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Dimkpa, C.O.; McLean, J.E.; Britt, D.W.; Anderson, A.J. Antifungal activity of ZnO nanoparticles and their interactive effect with a biocontrol bacterium on growth antagonism of the plant pathogen Fusarium graminearum. BioMetals 2013, 26, 913–924. [Google Scholar] [CrossRef]
- Hajra, A.; Mondal, N.K. Effects of ZnO and TiO2 nanoparticles on germination, biochemical and morphoanatomical attributes of Cicer arietinum L. Energy Ecol. Environ. 2017, 2, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Rossi, L.; Fedenia, L.N.; Sharifan, H.; Ma, X.; Lombardini, L. Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant Physiol. Biochem. 2019, 135, 160–166. [Google Scholar] [CrossRef]
- Barea, J.-M.; Azcón, R.; Azcón-Aguilar, C. Mycorrhizal Fungi and Plant Growth Promoting Rhizobacteria. In Plant Surface Microbiology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 351–371. [Google Scholar] [CrossRef]
- Elsharkawy, M.M.; Omara, R.I.; Mostafa, Y.S.; Alamri, S.A.; Hashem, M.; Alrumman, S.A.; Ahmad, A.A. Mechanism of Wheat Leaf Rust Control Using Chitosan Nanoparticles and Salicylic Acid. J. Fungi 2022, 8, 304. [Google Scholar] [CrossRef]





| Concentration | ZnO NPs % Inhibition | Metalaxyl + Mancozeb Fungicide % Inhibition |
|---|---|---|
| 0.25 μg/mL | 78.0 ± 0.5 | 64.1 ± 0.5 |
| 0.5 μg/mL | 85.2 ± 0.5 | 66.3 ± 0.0 |
| 0.75 μg/mL | 75.1 ± 0.5 | 83.2 ± 0.5 |
| 1.0 μg/mL | 72.0 ± 0.5 | 85.0 ± 0.5 |
| Control | 95.0 ± 0.5 | 95.5.0 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhana; Munis, M.F.H.; Alamer, K.H.; Althobaiti, A.T.; Kamal, A.; Liaquat, F.; Haroon, U.; Ahmed, J.; Chaudhary, H.J.; Attia, H. ZnO Nanoparticle-Mediated Seed Priming Induces Biochemical and Antioxidant Changes in Chickpea to Alleviate Fusarium Wilt. J. Fungi 2022, 8, 753. https://doi.org/10.3390/jof8070753
Farhana, Munis MFH, Alamer KH, Althobaiti AT, Kamal A, Liaquat F, Haroon U, Ahmed J, Chaudhary HJ, Attia H. ZnO Nanoparticle-Mediated Seed Priming Induces Biochemical and Antioxidant Changes in Chickpea to Alleviate Fusarium Wilt. Journal of Fungi. 2022; 8(7):753. https://doi.org/10.3390/jof8070753
Chicago/Turabian StyleFarhana, Muhammad Farooq Hussain Munis, Khalid H. Alamer, Ashwaq T. Althobaiti, Asif Kamal, Fiza Liaquat, Urooj Haroon, Junaid Ahmed, Hassan Javed Chaudhary, and Houneida Attia. 2022. "ZnO Nanoparticle-Mediated Seed Priming Induces Biochemical and Antioxidant Changes in Chickpea to Alleviate Fusarium Wilt" Journal of Fungi 8, no. 7: 753. https://doi.org/10.3390/jof8070753
APA StyleFarhana, Munis, M. F. H., Alamer, K. H., Althobaiti, A. T., Kamal, A., Liaquat, F., Haroon, U., Ahmed, J., Chaudhary, H. J., & Attia, H. (2022). ZnO Nanoparticle-Mediated Seed Priming Induces Biochemical and Antioxidant Changes in Chickpea to Alleviate Fusarium Wilt. Journal of Fungi, 8(7), 753. https://doi.org/10.3390/jof8070753








