Changes in Chemical Structure of Thermally Modified Spruce Wood Due to Decaying Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Spruce Wood
2.2. Thermal Treatment of Spruce Wood
2.3. Pure Fungal Culture Decomposition Test
2.4. Chemical Analyses of Thermally and Thermally–Fungally Attacked Spruce Wood
2.5. Statistical Evaluation
3. Results and Discussion
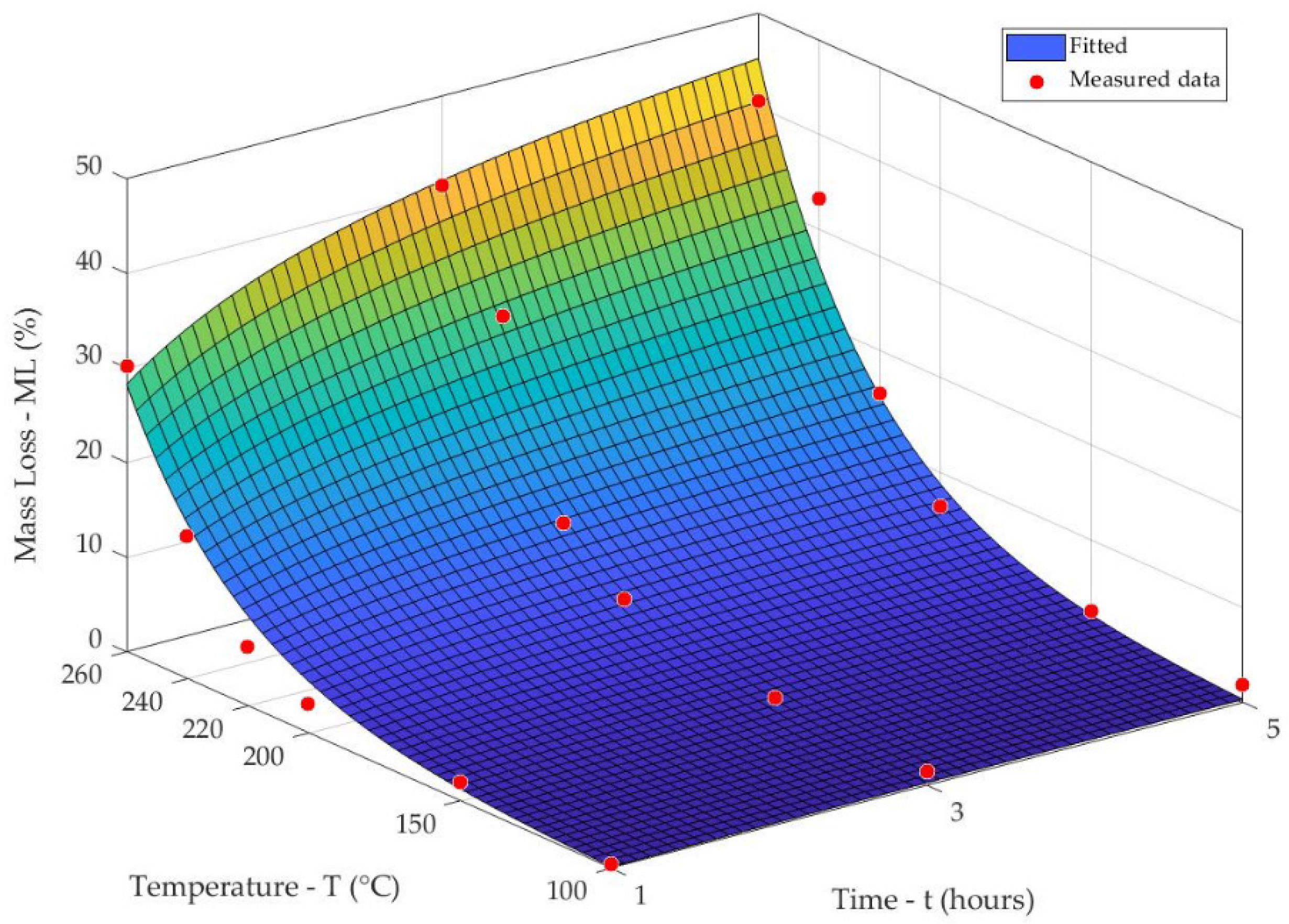
3.1. Mass Loss of Spruce Wood at Thermal Treatments
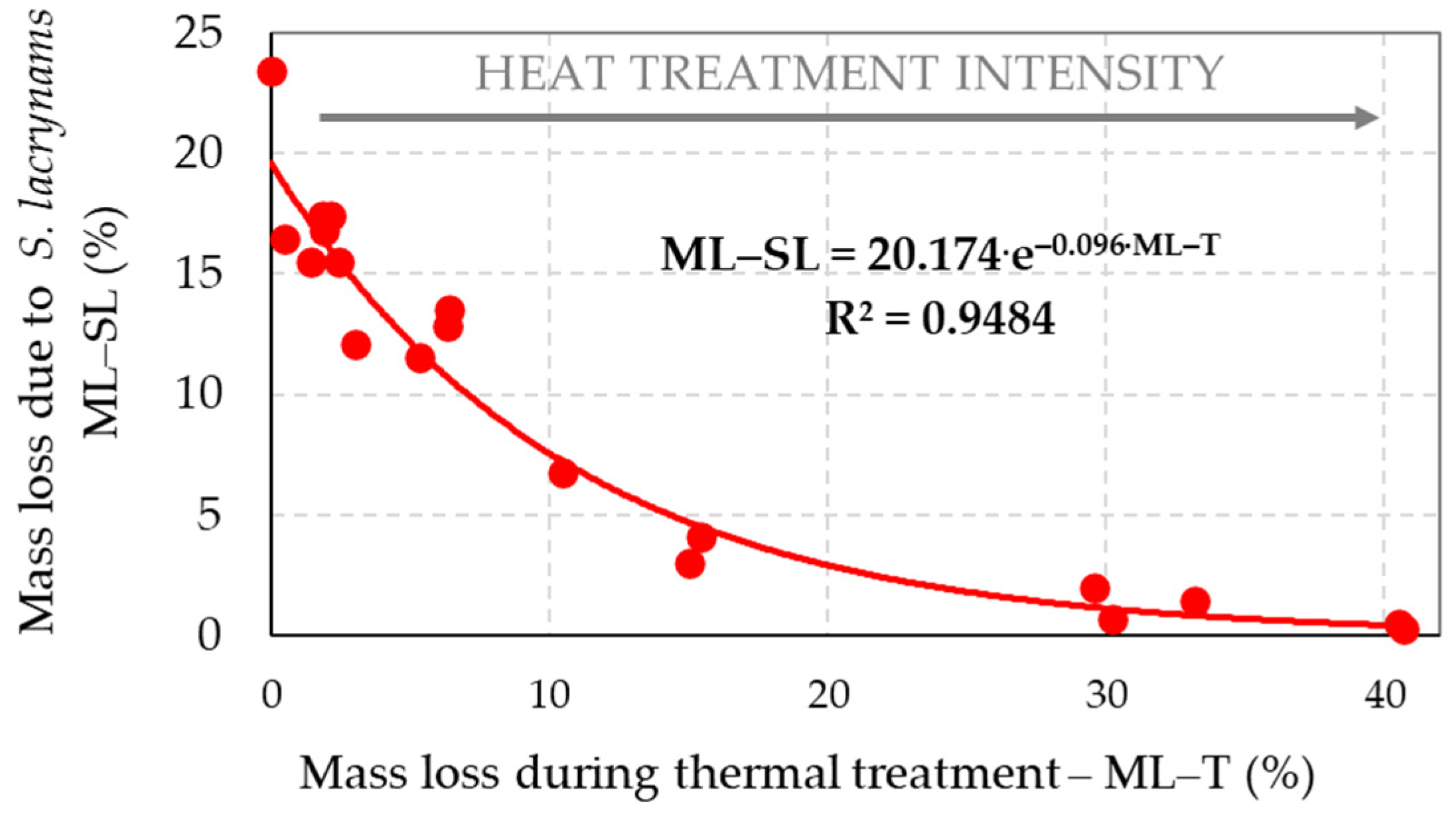
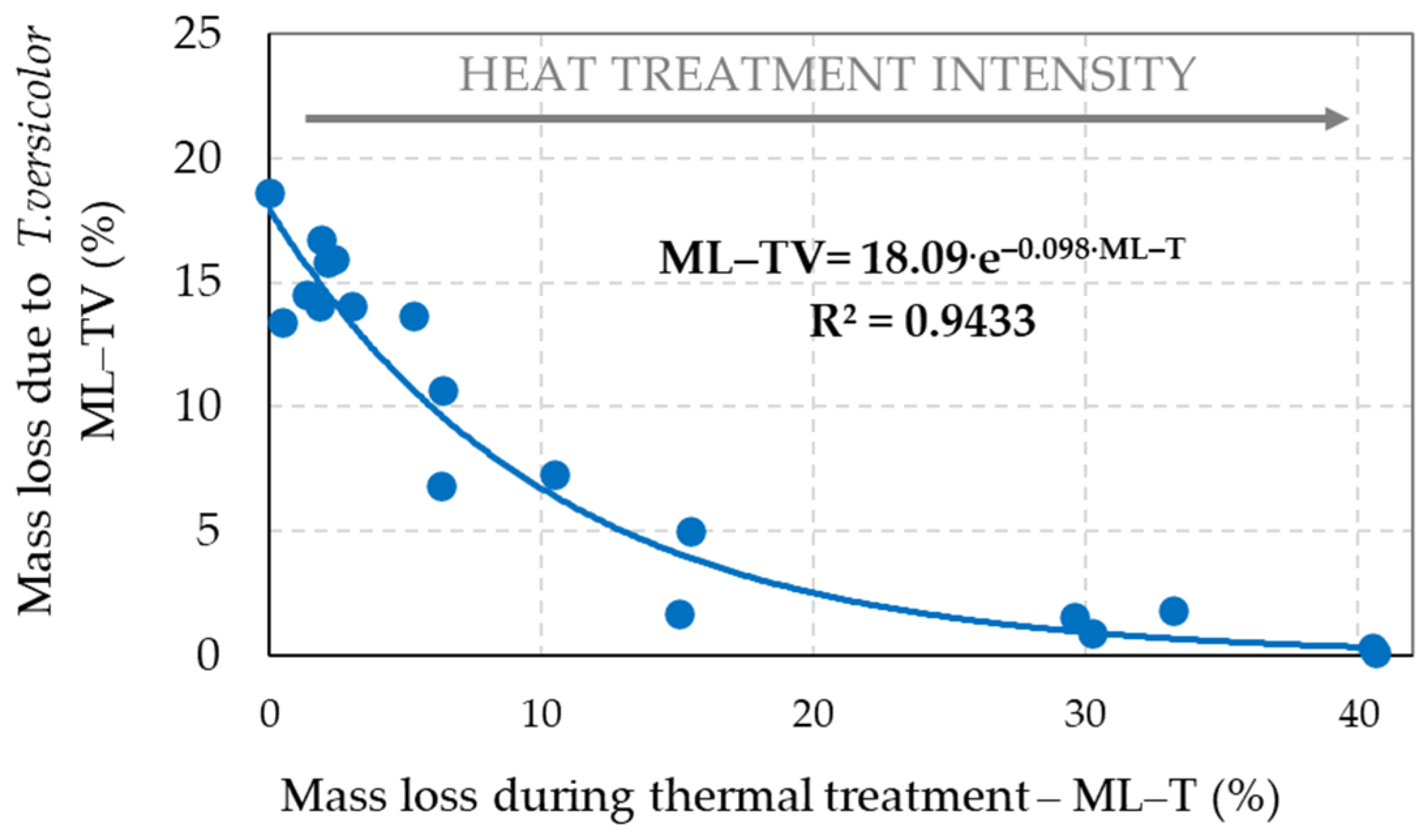
3.2. Mass Loss of Spruce Wood Caused by Decaying Fungi
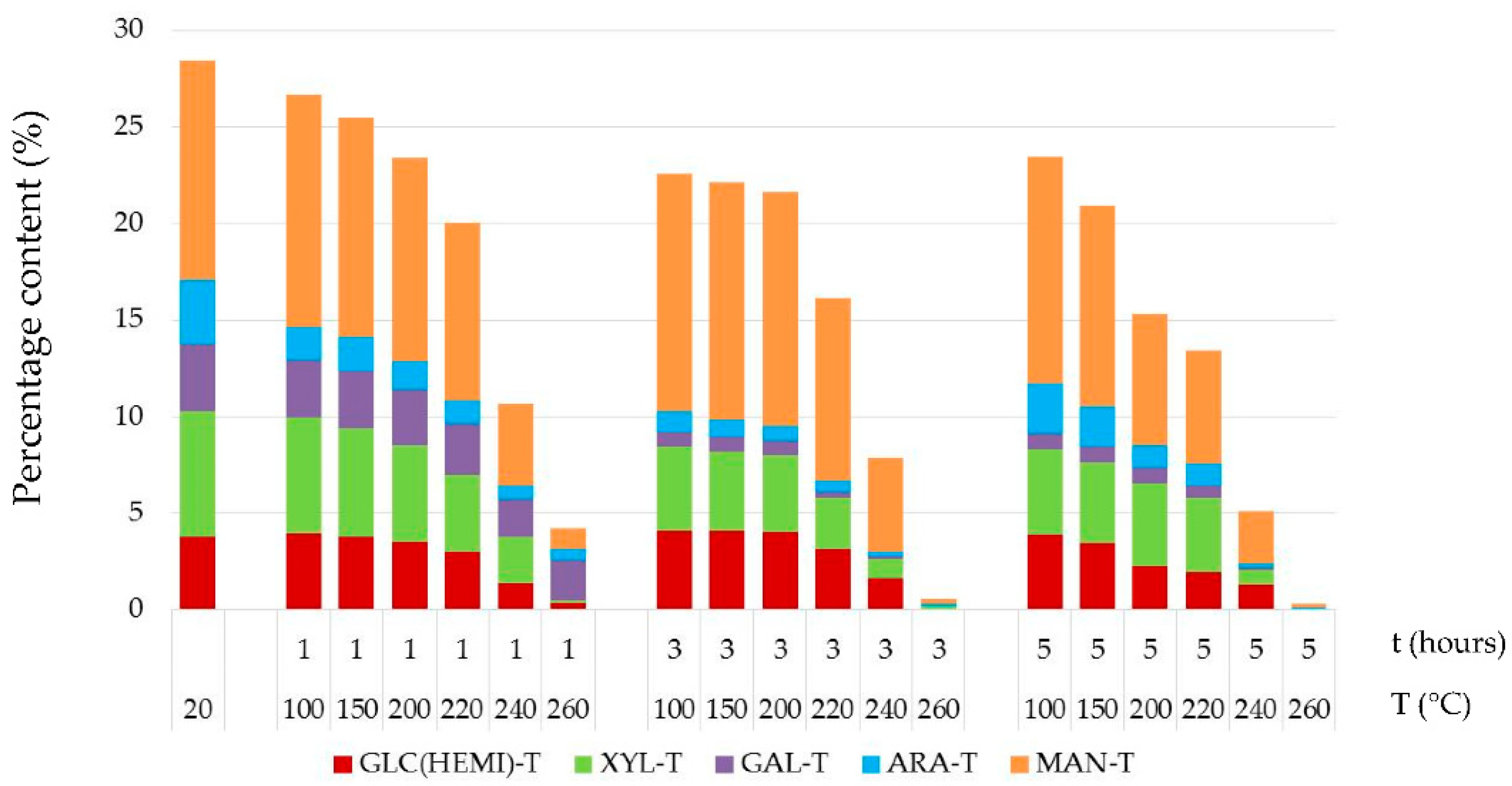
3.3. Chemical Structure of Thermally Treated Spruce Wood
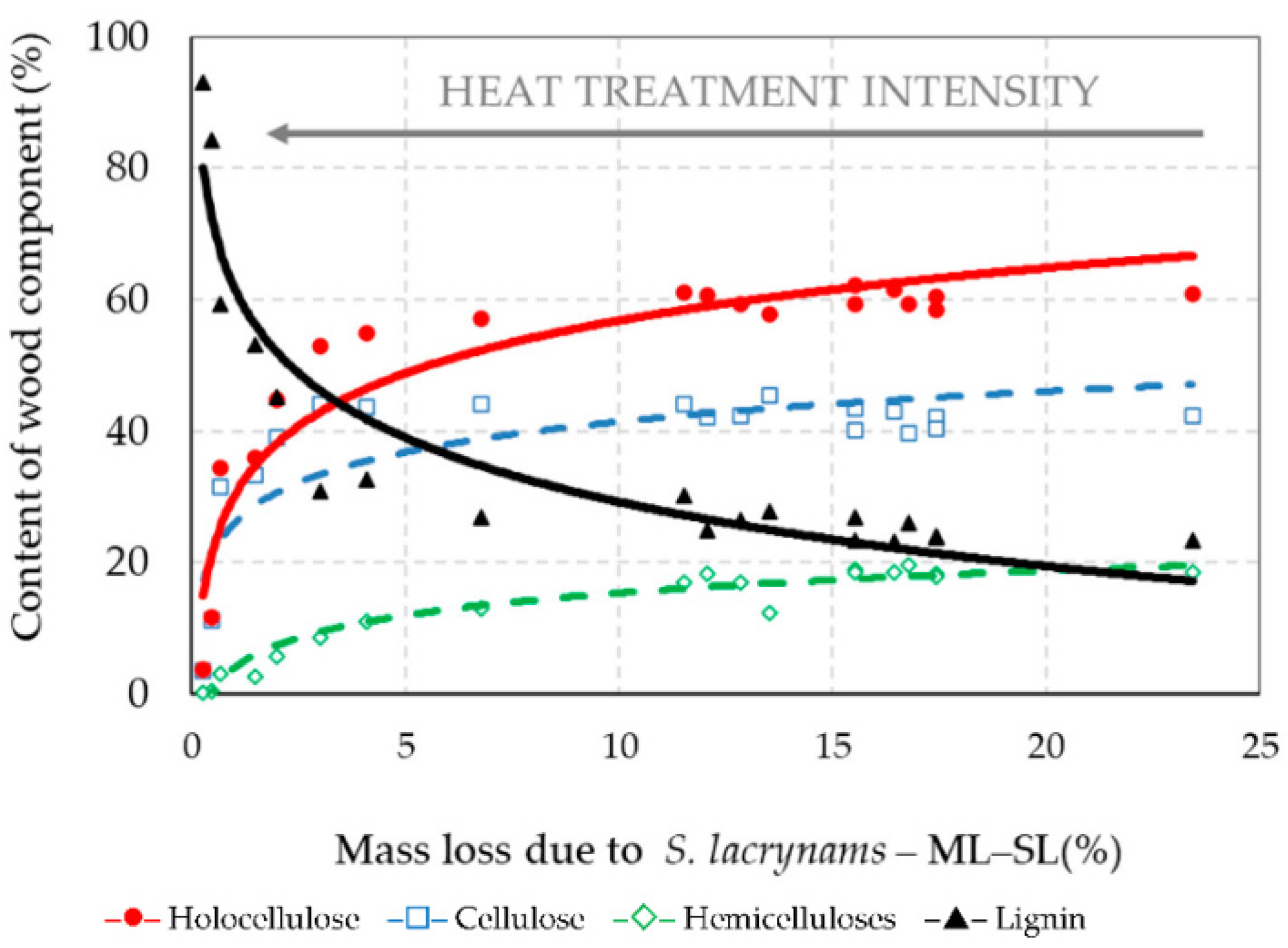
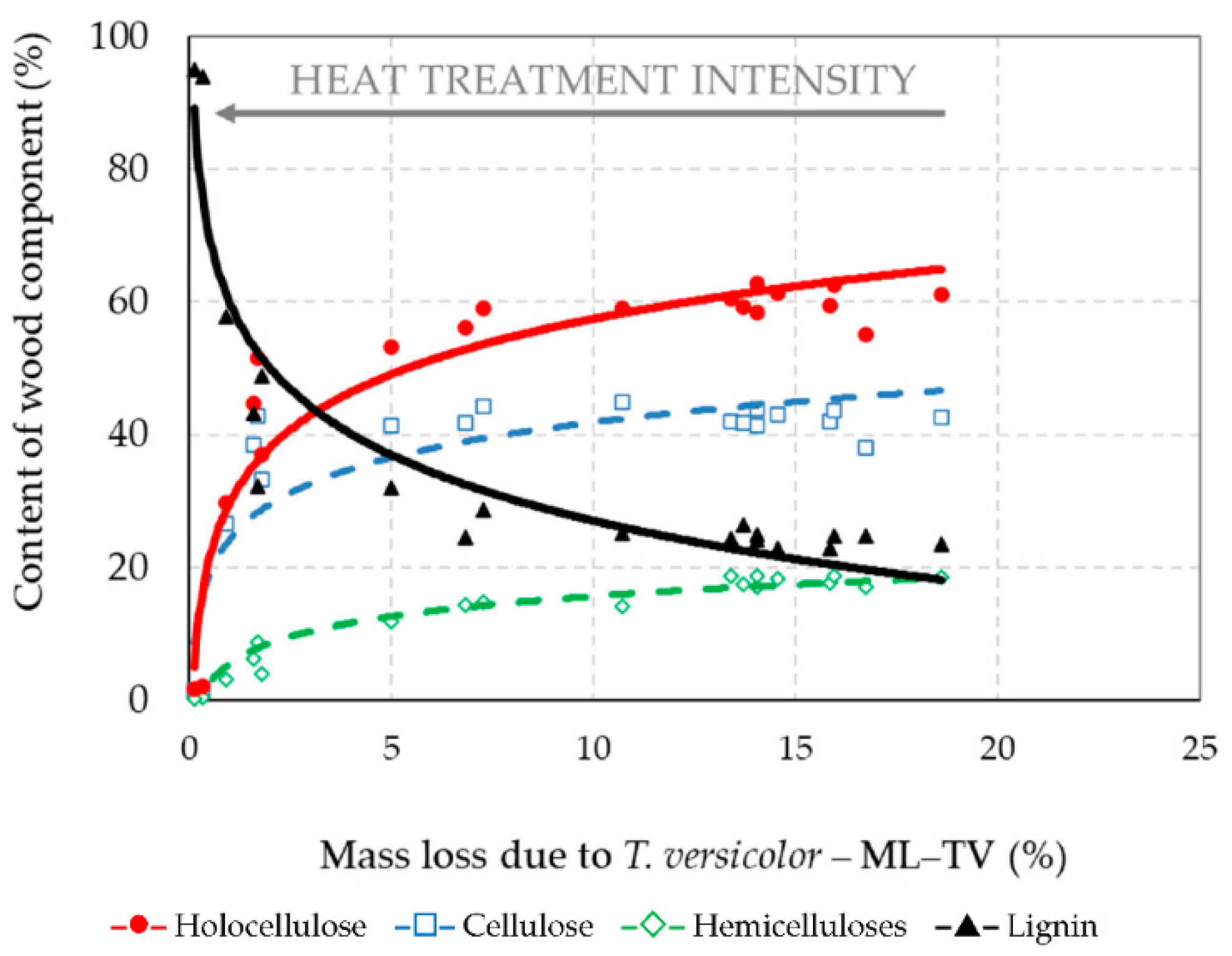
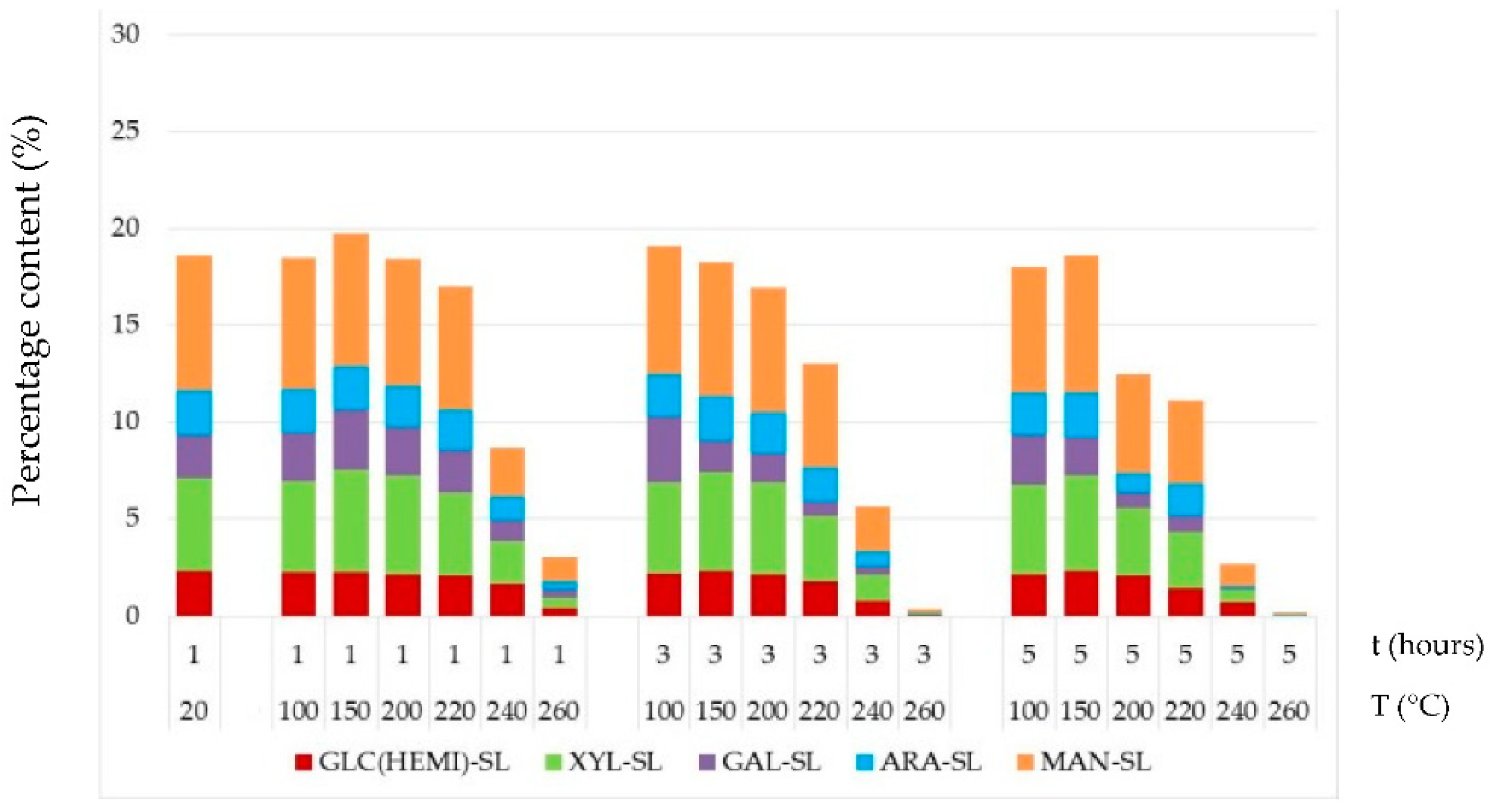
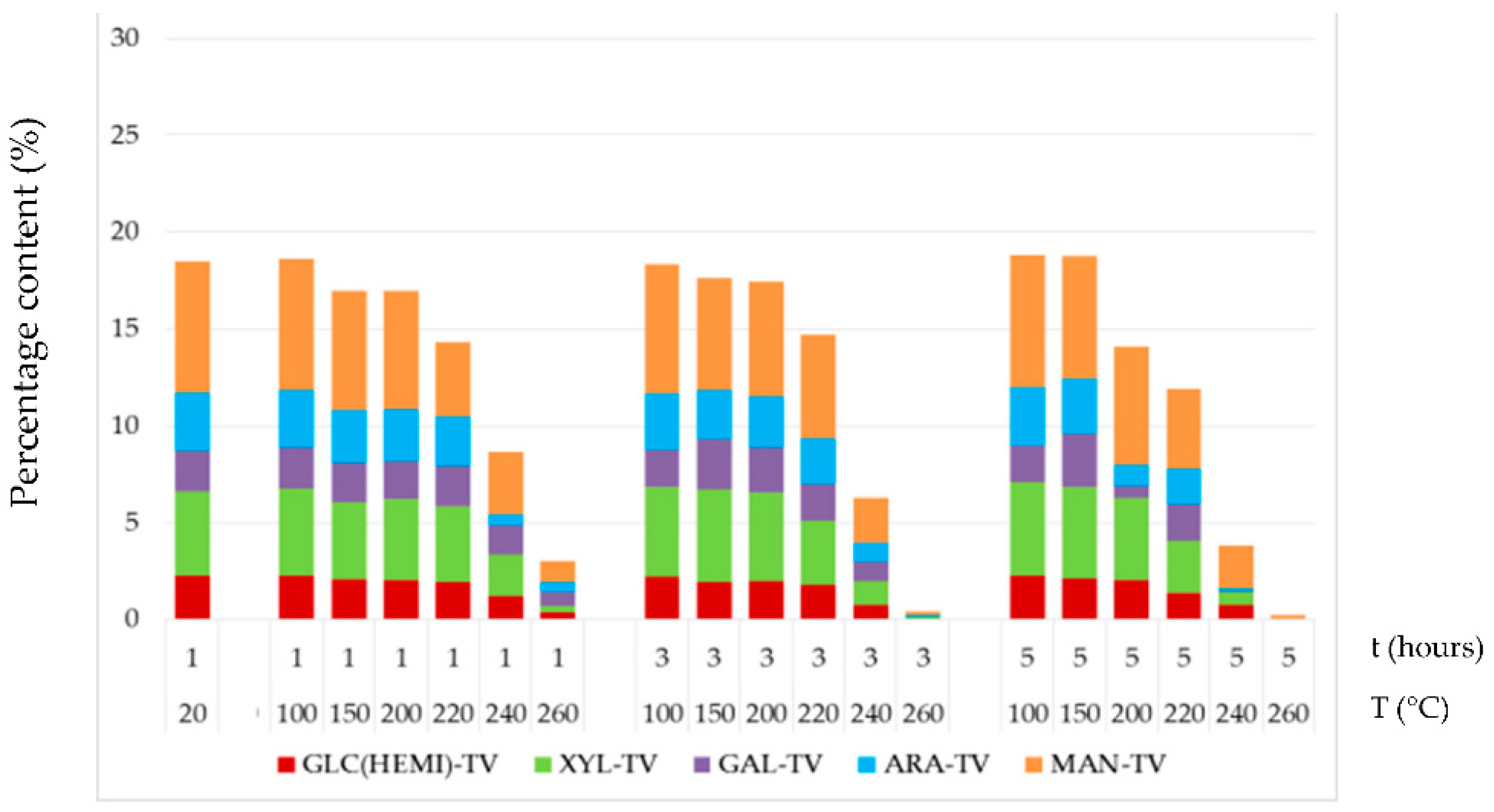
3.4. Chemical Structure of Thermally–Fungally Attacked Spruce Wood
4. Conclusions
- Different temperatures and times during the thermal treatments of spruce wood, from 100 °C to 260 °C/1–5 h, had varied effects on its chemical composition and decay resistance.
- Thermal treatments at temperatures equal to and above 200 °C caused high mass losses of spruce wood, from 3.04% to 40.67%, and great degradation of hemicelluloses, about 17.57–98.87%. Meanwhile, the holocellulose percent content reduced considerably and the percent content of lignin increased sharply.
- The decrease of holocellulose in spruce wood thermally modified at stronger thermal conditions leaded to the reduction of its bio-attacks by the brown-rot fungus S. lacrymans and the white-rot fungus T. versicolor, i.e., the decay resistance of such thermally modified woods can be significantly improved, more evidently against brown-rot fungi.
- Due to the fungal attacks by the brown-rot fungus S. lacrymans and the white-rot fungus T. versicolor the holocellulose, cellulose and hemicelluloses mass fractions were lower in those spruce wood samples in which primordial thermal degradation was highly intensive with achieving very high mass losses. On the contrary, the decay attacks were more intensive in those spruce samples that were not thermally treated, or their primordial thermal degradation was poorer with the minimal mass losses or even none.
- The mannose and glucose percent content in the thermally–fungally attacked spruce wood was mainly reduced, e.g., by 17% up to 98% in samples primordially treated at temperatures equal and above 200 °C.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandberg, D.; Haller, P.; Navi, P. Thermo-hydro and thermo-hydro-mechanical wood processing: An opportunity for future environmentally friendly wood products. Wood Mater. Sci. Eng. 2013, 8, 64–88. [Google Scholar] [CrossRef] [Green Version]
- Hakkou, M.; Pétrissans, M.; Zoulalian, A.; Gérardin, P. Investigation of wood wettability changes during heat treatment on the basis of chemical analysis. Polym. Degrad. Stab. 2005, 89, 70–84. [Google Scholar] [CrossRef]
- Čermák, P.; Hess, D.; Suchomelová, P. Mass loss kinetics of thermally modified wood species as a time–temperature function. Eur. J. Wood Prod. 2021, 79, 547–555. [Google Scholar] [CrossRef]
- González-Peña, M.M.; Curling, S.F.; Hale, M.D. On the effect of heat on the chemical composition and dimensions of thermally-modified wood. Polym. Degrad. Stab. 2009, 94, 2184–2193. [Google Scholar] [CrossRef]
- Yildiz, S.; Gezer, E.D.; Yildiz, U.C. Mechanical and chemical behaviour of spruce wood modified by heat. Build. Environ. 2006, 41, 1762–1766. [Google Scholar] [CrossRef]
- Reinprecht, L.; Repák, M. The impact of paraffin-thermal modification of beech wood on its biological, physical and mechanical properties. Forests 2019, 10, 1102. [Google Scholar] [CrossRef] [Green Version]
- Gaff, M.; Kačík, F.; Sandberg, D.; Babiak, M.; Turčani, M.; Niemz, P.; Hanzlík, P. The effect of chemical changes during thermal modification of European oak and Norway spruce on elasticity properties. Compos. Struct. 2019, 220, 529–538. [Google Scholar] [CrossRef]
- Kačíková, D.; Kačík, F.; Čabalová, I.; Ďurkovič, J. Effects of thermal treatment on chemical, mechanical and colour traits in Norway spruce wood. Bioresour. Technol. 2013, 144, 669–674. [Google Scholar] [CrossRef]
- Stamm, A.J.; Burr, H.K.; Kline, A.A. Staybwood. Heat stabilized wood. Ind. Eng. Chem. 1946, 38, 630–634. [Google Scholar] [CrossRef]
- Buro, A. Die Wirkung von Hitzebehandlung auf die Pilzresistenz von Kiefern- und Buchenholz. Holz Roh- Werkst. 1954, 12, 297–304. [Google Scholar] [CrossRef]
- Weiland, J.J.; Guyonnet, R. Study of chemical modifications and fungi degradation of thermally modified wood using DRIFT spectroscopy. Holz Roh- Werkst. 2003, 61, 216–220. [Google Scholar] [CrossRef]
- Mazela, B.; Zakrzewski, R.; Grześkowiak, W.; Cofta, G.; Bartkowiak, M. Resistance of thermally modified wood to basidiomycetes. Electron. J. Pol. Agric. Univ. Wood Technol. 2004, 7, 253–262. [Google Scholar]
- Hakkou, M.; Pètrissans, M.; Gèrardin, P.; Zoulalian, A. Investigations of the reasons for fungal durability of heat-treated beech wood. Polym. Degrad. Stab. 2006, 91, 393–397. [Google Scholar] [CrossRef]
- Welzbacher, C.R.; Brischke, C.; Rapp, A.O. Influence of treatment temperature and duration on selected biological, mechanical, physical and optical properties of thermally modified timber. Wood Mater. Sci. Eng. 2007, 2, 66–76. [Google Scholar] [CrossRef]
- Rapp, A.O.; Brischke, C.; Welzbacher, C.R.; Jazayeri, L. Increased resistance of thermally modified Norway spruce timber (TMT) against brown rot decay by Oligoporus placenta—Study on the mode of protective action. Wood Res. 2008, 53, 13–25. [Google Scholar]
- Reinprecht, L.; Vidholdová, Z. Mould resistance, water resistance and mechanical properties of OHT-thermowoods. In Proceedings of the Sustainability through New Technologies for Enhanced Wood Durability: Final Conference Proceedings, Bordeaux, France, 29–30 September 2008; Socio-Economic Perspectives of Treated Wood for the Common European Market. pp. 159–165. [Google Scholar]
- Chaouch, M.; Pétrissans, M.; Pétrissans, A.; Gérardin, P. Use of wood elemental composition to predict heat treatment intensity and decay resistance of different softwood and hardwood species. Polym. Degrad. Stab. 2010, 95, 2255–2259. [Google Scholar] [CrossRef]
- Thybring, E.E. The decay resistance of modified wood influenced by moisture exclusion and swelling reduction. Int. Biodeter. Biodegrad. 2013, 82, 87–95. [Google Scholar] [CrossRef]
- Sivrikaya, H.; Can, A.; de Troya, T.; Conde, M. Comparative biological resistance of differently thermal modified wood species against decay fungi, Reticulitermes grassei and Hylotrupes bajulus. Maderas-Cienc. Tecnol. 2015, 17, 559–570. [Google Scholar] [CrossRef] [Green Version]
- Candelier, K.; Thevenon, M.F.; Petrissans, A.; Dumarcay, S.; Gerardin, P.; Petrissans, M. Control of wood thermal treatment and its effects on decay resistance: A review. Ann. For. Sci. 2016, 73, 571–583. [Google Scholar] [CrossRef] [Green Version]
- Ringman, R.; Beck, G.; Pilgård, A. The importance of moisture for brown rot degradation of modified wood: A critical discussion. Forests 2019, 10, 522. [Google Scholar] [CrossRef] [Green Version]
- Severo, E.T.D.; Calonego, F.W.; Sansígolo, C.A.; Bond, B. Changes in the chemical composition and decay resistance of thermally-modified Hevea brasiliensis wood. PLoS ONE 2016, 11, e0151353. [Google Scholar] [CrossRef] [PubMed]
- Meihui, K.; Wang, W.; Jinzhen, C. Effects of thermal modification conditions on chemical composition and decay resistance of Pinus sylvestris. J. Beijing For. Univ. 2022, 44, 123–130. [Google Scholar] [CrossRef]
- Bagley, S.T.; Richter, D.L. Biodegradation by Brown Rot Fungi. In Industrial Applications; Osiewacz, H.D., Ed.; The Mycota; Springer: Berlin/Heidelberg, Germany, 2002; pp. 327–341. ISBN 978-3-662-10378-4. [Google Scholar]
- Forest Europe. State of Europe’s Forests 2020; Ministerial Conference on the Protection of Forests in Europe—Forest Europe Liaison Unit Bratislava: Zvolen, Slovakia, 2020; p. 394. Available online: https://foresteurope.org/wp-content/uploads/2016/08/SoEF_2020.pdf (accessed on 13 July 2022).
- Report on the Forestry Sector of the Slovak Republic 2020—Green Report; Ministry of Agriculture and Rural Development of the Slovak Republic: Bratislava, Slovakia, 2021; p. 69. Available online: https://www.mpsr.sk/en/index.php?navID=17&id=77 (accessed on 13 July 2022).
- EN 350; Durability of Wood and Wood-Based Products—Testing and Classification of the Durability to Biological Agents of Wood and Wood-Based Materials. European Committee for Standardization: Brussels, Belgium, 2016.
- Gabriel, J.; Švec, K. Occurrence of indoor wood decay basidiomycetes in Europe. Fungal Biol. Rev. 2017, 31, 212–217. [Google Scholar] [CrossRef]
- Gáper, J.; Gáperová, S.; Gašparcová, T.; Kvasnová, S.; Pristaš, P.; Náplavová, K. Indoor fungal destroyers of wooden materials Their identification in present review. Wood Res. 2018, 63, 203–214. [Google Scholar]
- Reinprecht, L.; Novotná, H.; Štefka, V. Density profiles of spruce wood changed by brown-rot and white-rot fungi. Wood Res. 2007, 52, 17–28. [Google Scholar]
- EN 113-2; Durability of Wood and Wood-Based Products—Test Method Against Wood Destroying Basidiomycetes—Part 2: Assessment of Inherent or Enhanced Durability. European Committee for Standardization: Brussels, Belgium, 2020.
- Karim, M.; Daryaei, M.G.; Torkaman, J.; Oladi, R.; Ghanbary, M.A.T.; Bari, E.; Yilgor, N. Natural decomposition of hornbeam wood decayed by the white rot fungus Trametes versicolor. An. Acad. Bras. Ciências 2017, 89, 2647–2655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witomski, P.; Olek, W.; Bonarski, J.T. Changes in strength of Scots pine wood (Pinus silvestris L.) decayed by brown rot (Coniophora puteana) and white rot (Trametes versicolor). Constr. Build. Mater. 2016, 102, 162–166. [Google Scholar] [CrossRef]
- Van Acker, J.; Stevens, M.; Carey, J.; Sierra-Alvarez, R.; Militz, H.; Le Bayon, I.; Kleist, G.; Peek, R.D. Biological durability of wood in relation to end-use. Holz Roh Werkst. 2003, 61, 35–45. [Google Scholar] [CrossRef]
- ASTM D1107-21; Standard Test Method for Ethanol-Toluene Solubility of Wood. ASTM International: West Conshohocken, PA, USA, 2021.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012; pp. 1–16.
- Allegretti, O.; Brunetti, M.; Cuccui, I.; Ferrari, S.; Nocetti, M.; Terziev, N. Thermo-vacuum modification of spruce (Picea abies Karst.) and fir (Abies alba Mill.) wood. BioResources 2012, 7, 3656–3669. [Google Scholar]
- Šušteršic, Ž.; Mohareb, A.; Chaouch, M.; Pétrissans, M.; Petrič, M.; Gérardin, P. Prediction of the decay resistance of heat treated wood on the basis of its elemental composition. Polym. Degrad. Stab. 2010, 95, 94–97. [Google Scholar] [CrossRef]
- Calonego, F.W.; Taylor, E.; Severo, D.; Furtado, E.L. Decay resistance of thermally-modified Eucalyptus grandis wood at 140 °C, 160 °C, 180 °C, 200 °C and 220 °C. Biores. Technol. 2010, 101, 9391–9394. [Google Scholar] [CrossRef] [PubMed]
- Ayata, U.; Akcay, C.; Esteves, B. Determination of decay resistance against Pleurotus ostreatus and Coniophora puteana fungus of heat-treated Scotch pine, oak and beech wood species. Maderas Cienc. Tecnol. 2017, 19, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Reinprecht, L.; Repák, M.; Iždinský, J.; Vidholdová, Z. Decay resistance of nano-zinc oxide, and PEG 6000, and thermally modified wood. Forests 2022, 13, 731. [Google Scholar] [CrossRef]
- Tripathi, S.; Pant, H.; Kashyap, A.K. Decay resistance against Basidiomycetes fungi of heat-treated Pinus roxburghii and Mangifera indica wood. J. Trop. For. Sci. 2014, 26, 203–207. [Google Scholar]
- Altgen, M.; Kyyrö, S.; Paajanen, O.; Rautkari, L. Resistance of thermally modified and pressurized hot water extracted Scots pine sapwood against decay by the brown-rot fungus Rhodonia placenta. Eur. J. Wood Wood Prod. 2020, 78, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Čabalová, I.; Kačík, F.; Kačíková, D.; Oravec, M. The influence of cross-section of spruce wood beams on saccharides changes at thermal loading. Acta Fac. Xylologiae 2014, 56, 81–86. [Google Scholar]
- Sikora, A.; Kačík, F.; Gaff, M.; Vondrová, V.; Bubeníková, T.; Kubovský, I. Impact of thermal modification on color and chemical changes of spruce and oak wood. J.Wood Sci. 2018, 64, 406–416. [Google Scholar] [CrossRef]
- Alén, R.; Kotilainen, R.; Zaman, A. Thermochemical behavior of Norway spruce (Picea abies) at 180–225 °C. Wood Sci. Technol. 2002, 36, 163–171. [Google Scholar] [CrossRef]
- Esteves, B.; Domingos, I.; Pereira, H. Pine wood modification by heat treatment in air. BioResources 2008, 3, 142–154. [Google Scholar] [CrossRef]
- Umar, I.; Zaidon, A.; Lee, S.H.; Halis, R. Oil-heat treatment of rubberwood for optimum changes in chemical constituents and decay resistance. J. Trop. For. Sci. 2016, 28, 88–96. [Google Scholar]
- Tümen, İ.; Aydemir, D.; Gündüz, G.; Üner, B.; Cetin, H. Changes in the chemical structure of thermally treated wood. Bioresources 2010, 5, 1936–1944. [Google Scholar] [CrossRef]
- Wang, D.; Fu, F.; Lin, L. Molecular-level characterization of changes in the mechanical properties of wood in response to thermal treatment. Cellulose 2022, 29, 3131–3142. [Google Scholar] [CrossRef]
- Jiang, J.; Peng, Y.; Ran, Y.; Cao, J. Pseudo lignin formed from hygrothermally treated holocellulose and its effect on fungal degradation. Ind. Crops Prod. 2022, 184, 115004. [Google Scholar] [CrossRef]
- Rowell, R.M.; Ibach, R.E.; McSweeny, J.; Nilsson, T. Understanding decay resistance, dimensional stability and strength changes in heat-treated and acetylated wood. Wood Mater Sci. Eng. 2009, 4, 14–22. [Google Scholar] [CrossRef]
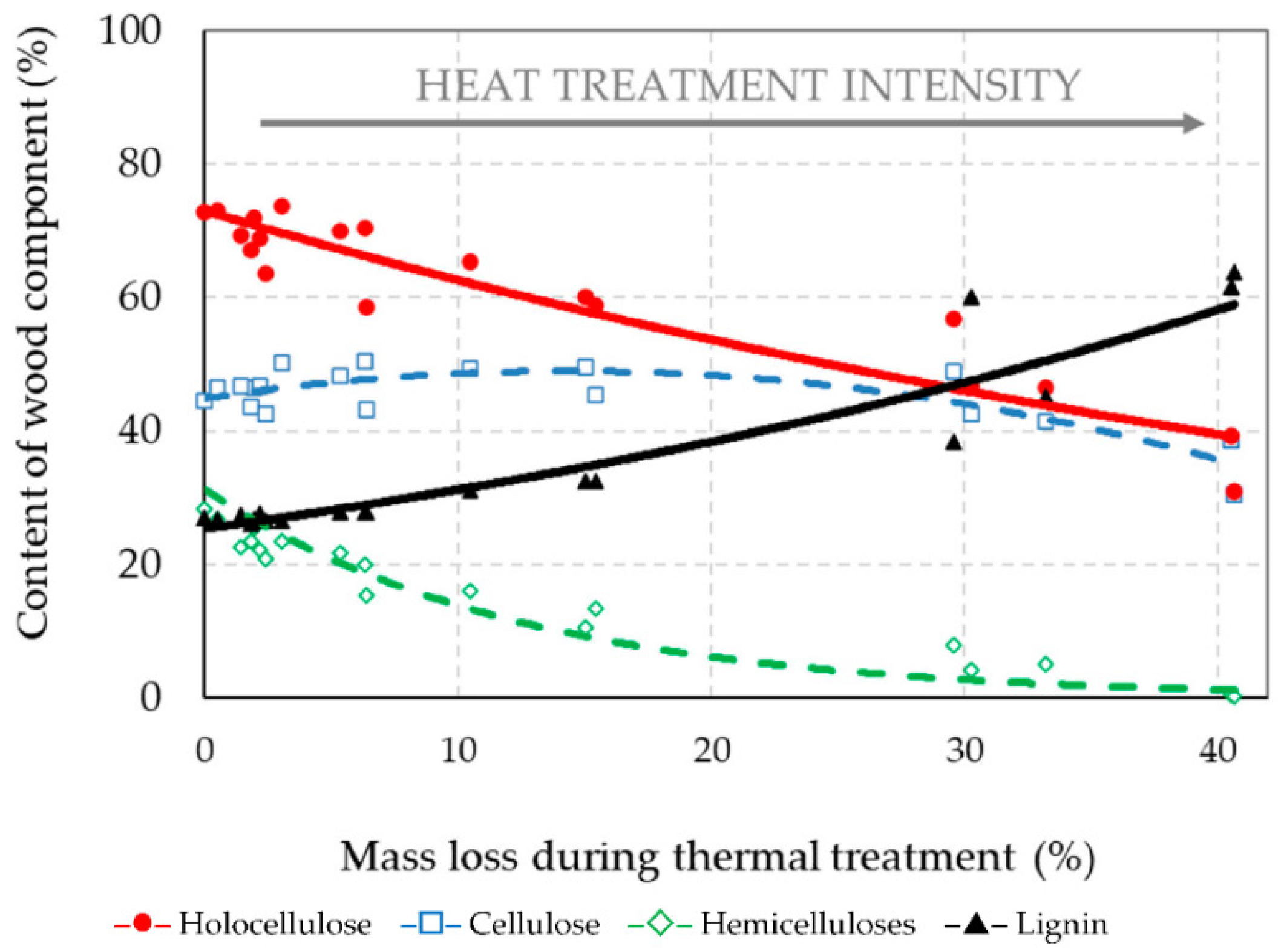
| Wood Component | Percentage Content of Wood Component = f (Mass Loss (ML–T)) | Coefficient of Determination (R2) |
|---|---|---|
| Holocellulose | HOLO–CEL = 72.99·e−0.015·(ML–T) | R2 = 0.868 |
| Cellulose | CEL = 44.89 + 0.574·(ML–T) − 0.020·(ML–T)2 | R2 = 0.678 |
| Hemicelluloses | HEMI–CEL = 31.26·e−0.081·(ML–T) | R2 = 0.941 |
| Lignin | LIGNIN = 25.34·e0.0208·(ML–T) | R2 = 0.900 |
| Wood Component | Percentage Content of Wood Component = f (Mass Loss (ML–SL)) | Coefficient of Determination (R2) |
|---|---|---|
| Holocellulose | HOLO–CEL = 30.24 + 11.56·ln(ML–SL) | R2 = 0.880 |
| Cellulose | CEL = 26.10 + 6.67·ln(ML–SL) | R2 = 0.672 |
| Hemicelluloses | HEMI–CEL = 4.14 + 4.89·ln(ML–SL) | R2 = 0.938 |
| Lignin | LIGNIN = 61.74 − 14.11·ln(ML–SL) | R2 = 0.885 |
| Wood Component | Percentage Content of Wood Component = f (Mass Loss (ML–TV)) | Coefficient of Determination (R2) |
|---|---|---|
| Holocellulose | HOLO–CEL = 29.68 + 12.07·ln(ML–TV) | R2 = 0.886 |
| Cellulose | CEL = 24.17 + 7.68·ln(ML–TV) | R2 = 0.743 |
| Hemicelluloses | HEMI–CEL = 5.50 + 4.99·ln(ML–TV) | R2 = 0.936 |
| Lignin | LIGNIN = 60.00 − 14.32·ln(ML–TV) | R2 = 0.878 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidholdová, Z.; Kačík, F.; Reinprecht, L.; Kučerová, V.; Luptáková, J. Changes in Chemical Structure of Thermally Modified Spruce Wood Due to Decaying Fungi. J. Fungi 2022, 8, 739. https://doi.org/10.3390/jof8070739
Vidholdová Z, Kačík F, Reinprecht L, Kučerová V, Luptáková J. Changes in Chemical Structure of Thermally Modified Spruce Wood Due to Decaying Fungi. Journal of Fungi. 2022; 8(7):739. https://doi.org/10.3390/jof8070739
Chicago/Turabian StyleVidholdová, Zuzana, František Kačík, Ladislav Reinprecht, Viera Kučerová, and Jana Luptáková. 2022. "Changes in Chemical Structure of Thermally Modified Spruce Wood Due to Decaying Fungi" Journal of Fungi 8, no. 7: 739. https://doi.org/10.3390/jof8070739
APA StyleVidholdová, Z., Kačík, F., Reinprecht, L., Kučerová, V., & Luptáková, J. (2022). Changes in Chemical Structure of Thermally Modified Spruce Wood Due to Decaying Fungi. Journal of Fungi, 8(7), 739. https://doi.org/10.3390/jof8070739








