Update on the Epidemiology, Diagnosis, and Treatment of Coccidioidomycosis
Abstract
1. Introduction
2. Epidemiology
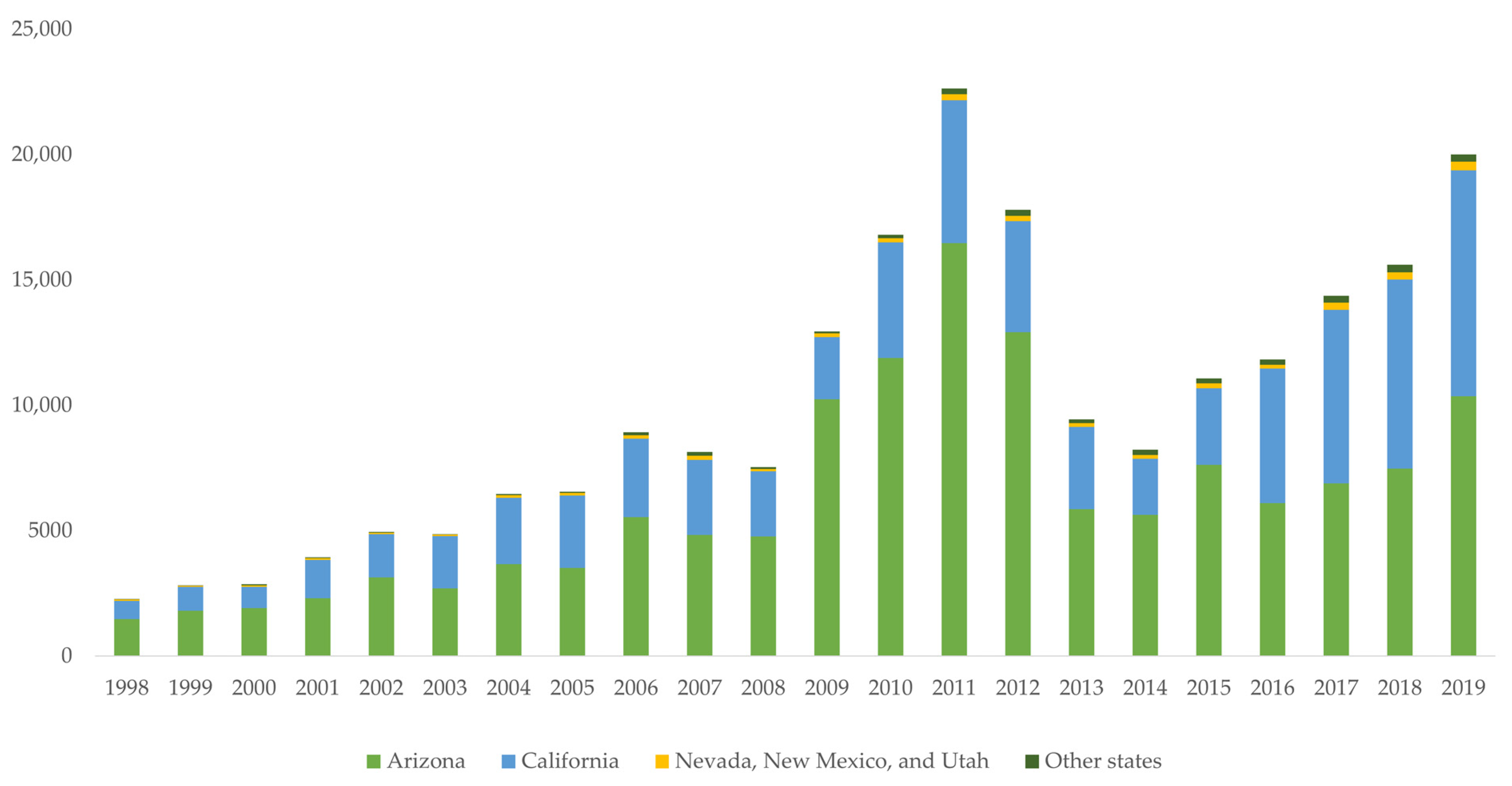
2.1. Increased Number of Reported Cases
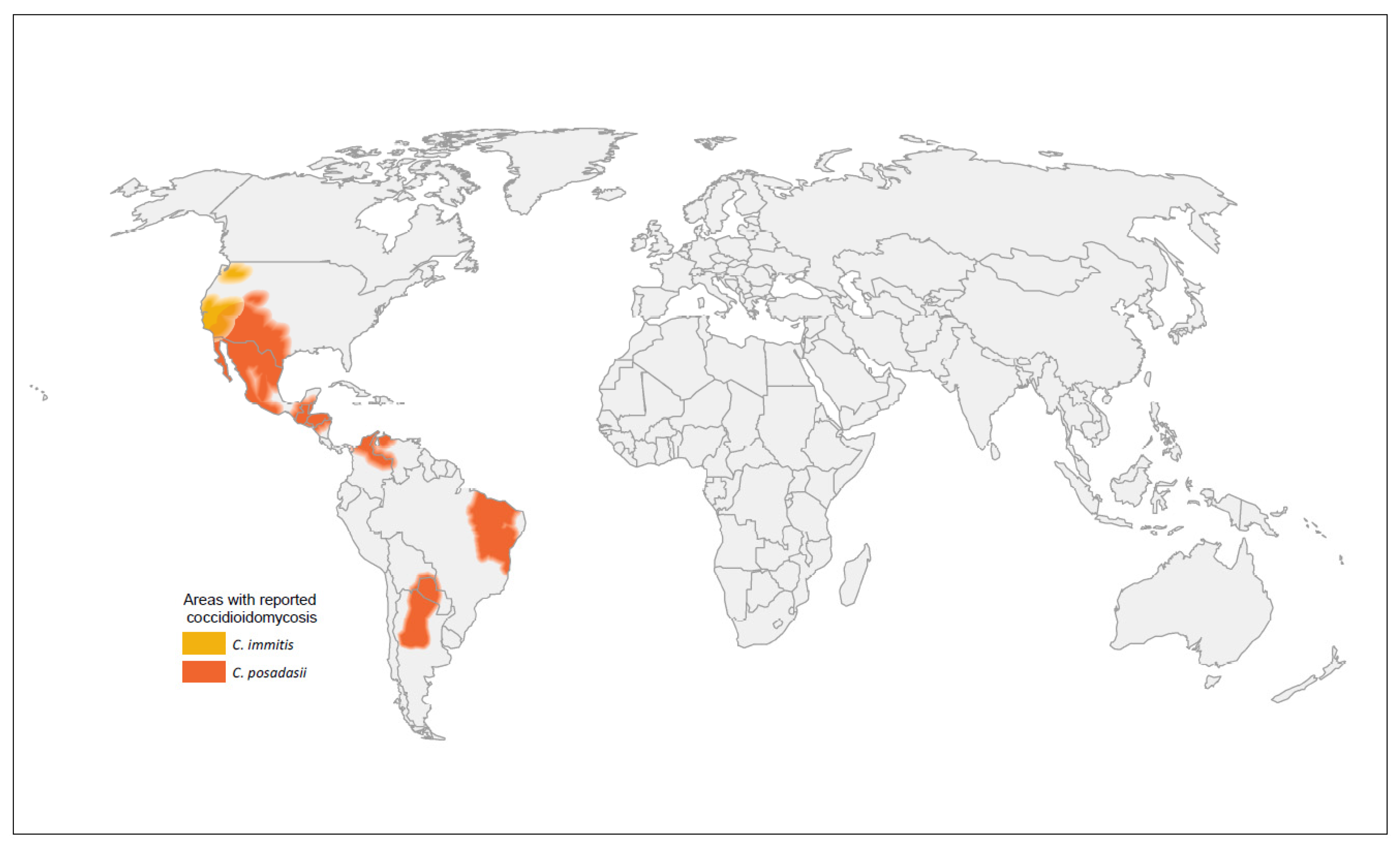
2.2. Geographic Expansion of Coccidioides Species
2.3. Risk Factors
2.4. Coccidioidomycosis and COVID-19
3. Diagnosis
3.1. Diagnostic Challenges
3.2. Serology
3.3. Antigen Detection
3.4. Microscopy and Culture
3.5. Additional Laboratory Diagnostic Methods
| Test | Sensitivity | Specificity ‡ | Considerations |
|---|---|---|---|
| Serology | |||
| Antibody | Antibody production may lag behind symptom onset. Sensitivity is often lower in immunosuppressed patients. | ||
| EIA IgG or IgM [103,104,105] | 59–88% | 68–96% | Rapid performance time within hours. Often used as a screening test, later confirmed by ID or CF. IgM only may lead to more false positives than IgG only. |
| EIA IgG [103,104,105] | 47–87% | 89–97% | |
| EIA IgM [103,104,105] | 22–61% | 70–99% | |
| ID § [103,118] | 60–91% | 99–100% | Results may take several days to receive. Some specialized training is required. Methods are not standardized across laboratories. |
| CF § [103,108,109,118] | 65–98% | 80–98% | Titers may offer prognostic value of disease progression. Measurement of IgG only. Highly specialized training is required. Methods are not standardized across laboratories. |
| LFA § [117,118] | 31–99% | 92–98% | Rapid 1-h performance time. |
| Antigen | |||
| Urine and serum [113] | 57% | 99% | May detect Coccidioides in the early stages of the disease [112]. May be preferred to antibody tests for immunocompromised patients. Substantial cross-reactivity with other dimorphic fungi. |
| Urine [111,113] | 37–71% | 99% | |
| Serum [119] | 73% | 100% | |
| Microscopy and culture | |||
| Culture [114] | 23–93% | High | Considered the gold standard of coccidioidomycosis diagnosis. Biosafety level 3 lab needed for safe isolation of Coccidioides. Culture growth may take up to a week. Sensitivity is heavily dependent on specimen quality. |
| Histopathology [114] | 23–84% | High | |
| Cytology [114] | 15–75% | High | |
| Additional laboratory methods | |||
| PCR [115,116] | 56–75% | 99–100% | Rapid 4-h performance time. Site of specimen collection may influence results. |
| (1→3) β-d-glucan [117] | 44% | 91% | Lower sensitivity among patients with acute pulmonary coccidioidomycosis. Values correlate poorly with CF titers. Test cannot detect specific pathogens. |
4. Treatment
4.1. Azoles
4.2. Polyenes
4.3. Treatment Duration and Follow-Up
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Smith, C.E.; Whiting, E.G.; Baker, E.E.; Rosenberger, H.G.; Beard, R.R.; Saito, M.T. The Use of Coccidioidin1, 2. Am. Rev. Tuberc. 1948, 57, 330–360. [Google Scholar] [CrossRef] [PubMed]
- Donovan, F.M.; Wightman, P.; Zong, Y.; Gabe, L.; Majeed, A.; Ynosencio, T.; Bedrick, E.J.; Galgiani, J.N. Delays in Coccidioidomycosis Diagnosis and Associated Healthcare Utilization, Tucson, Arizona, USA. Emerg. Infect. Dis. 2019, 25, 1745–1747. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.E.; Beard, R.R. Varieties of Coccidioidal Infection in Relation to the Epidemiology and Control of the Diseases. Am. J. Public Health Nations Health 1946, 36, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Galgiani, J.N.; Ampel, N.M.; Blair, J.E.; Catanzaro, A.; Geertsma, F.; Hoover, S.E.; Johnson, R.H.; Kusne, S.; Lisse, J.; MacDonald, J.D.; et al. 2016 Infectious Diseases Society of America (IDSA) Clinical Practice Guideline for the Treatment of Coccidioidomycosis. Clin. Infect. Dis. 2016, 63, e112–e146. [Google Scholar] [CrossRef]
- Benedict, K.; Jackson, B.R.; Chiller, T.; Beer, K.D. Estimation of direct healthcare costs of fungal diseases in the United States. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 68, 1791–1797. [Google Scholar] [CrossRef]
- Benedict, K.; Whitham, H.K.; Jackson, B.R. Economic Burden of Fungal Diseases in the United States. Open Forum Infect. Dis. 2022, 9, ofac097. [Google Scholar] [CrossRef]
- Wilson, L.; Ting, J.; Lin, H.; MacLean, M.; Peterson, M.W.; Stockamp, N.; Libke, R.; Brown, P. The Rise of Valley Fever: Prevalence and Cost Burden of Coccidioidomycosis Infection in California. Int. J. Environ. Res. Public Health 2019, 16, 1113. [Google Scholar] [CrossRef]
- Grizzle, A.J.; Wilson, L.; Nix, D.E.; Galgiani, J.N. Clinical and Economic Burden of Valley Fever in Arizona: An Incidence-Based Cost-of-Illness Analysis. Open Forum Infect. Dis. 2020, 8, ofaa623. [Google Scholar] [CrossRef]
- Edwards, P.Q.; Palmer, C.E. Prevalence of Sensitivity to Coccidioidin, With Special Reference to Specific and Nonspecific Reactions to Coccidioidin and to Histoplasmin. Dis. Chest. 1957, 31, 35–60. [Google Scholar] [CrossRef]
- Marsden-Haug, N.; Goldoft, M.; Ralston, C.; Limaye, A.P.; Chua, J.; Hill, H.; Jecha, L.; Thompson, G.R.; Chiller, T. Coccidioidomycosis Acquired in Washington State. Clin. Infect. Dis. 2013, 56, 847–850. [Google Scholar] [CrossRef]
- Benedict, K.; Thompson, G.R.; Deresinski, S.; Chiller, T. Mycotic Infections Acquired outside Areas of Known Endemicity, United States. Emerg. Infect. Dis. 2015, 21, 1935–1941. [Google Scholar] [CrossRef]
- Laniado-Laborín, R.; Arathoon, E.G.; Canteros, C.; Muñiz-Salazar, R.; Rendon, A. Coccidioidomycosis in Latin America. Med. Mycol. 2019, 57 (Suppl. 1), S46–S55. [Google Scholar] [CrossRef]
- Ashraf, N.; Kubat, R.C.; Poplin, V.; Adenis, A.A.; Denning, D.W.; Wright, L.; McCotter, O.; Schwartz, I.S.; Jackson, B.R.; Chiller, T.; et al. Re-drawing the Maps for Endemic Mycoses. Mycopathologia 2020, 185, 843–865. [Google Scholar] [CrossRef]
- Benedict, K.; Ireland, M.; Weinberg, M.P.; Gruninger, R.J.; Weigand, J.; Chen, L.; Perez-Lockett, K.; Bledsoe, C.; Denny, L.; Cibulskas, K.; et al. Enhanced Surveillance for Coccidioidomycosis, 14 US States, 2016. Emerg. Infect. Dis. 2018, 24, 1444–1452. [Google Scholar] [CrossRef]
- Benedict, K.; Li, Y.; Molinari, N.A.M.; Jackson, B.R. Health Care Providers’ Testing Practices for Coccidioidomycosis and Histoplasmosis in Patients With Community-Acquired Pneumonia—United States, 2020. Open Forum Infect. Dis. 2021, 8, ofab020. [Google Scholar] [CrossRef]
- Reportable Fungal Diseases by State Fungal Diseases CDC. Published 19 November 2021. Available online: https://www.cdc.gov/fungal/fungal-disease-reporting-table.html (accessed on 1 April 2022).
- Valley Fever Statistics Coccidioidomycosis Types of Fungal Diseases Fungal CDC. Published 15 October 2021. Available online: https://www.cdc.gov/fungal/diseases/coccidioidomycosis/statistics.html (accessed on 26 March 2022).
- Epidemiologic Summary of Valley Fever (Coccidioidomycosis) in California, 2019; California Department of Public Health: Sacramento, CA, USA, 2019. Available online: https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/CocciEpiSummary2019.pdf (accessed on 12 April 2022).
- Valley Fever 2019 Annual Report; Arizona Department of Health Services. Available online: https://www.azdhs.gov/documents/preparedness/epidemiology-disease-control/valley-fever/reports/valley-fever-2019.pdf (accessed on 12 April 2022).
- Coccidioidomycosis in California Provisional Monthly Report, January–February 2022; California Department of Public Health. Available online: https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/CocciinCAProvisionalMonthlyReport.pdf (accessed on 12 April 2022).
- Year-to-Date Communicable Disease Summary; Arizona Department of Health Services: 2022. Available online: https://www.azdhs.gov/documents/preparedness/epidemiology-disease-control/disease-data-statistics-reports/data-statistics-archive/2021/2021-ytd-communicable-disease-summary.pdf (accessed on 12 April 2022).
- Gorris, M.E.; Cat, L.A.; Zender, C.S.; Treseder, K.K.; Randerson, J.T. Coccidioidomycosis Dynamics in Relation to Climate in the Southwestern United States. GeoHealth 2018, 2, 6–24. [Google Scholar] [CrossRef]
- Head, J.R.; Sondermeyer-Cooksey, G.; Heaney, A.K.; Yu, A.T.; Jones, I.; Bhattachan, A.; Campo, S.; Wagner, R.; Mgbara, W.; Phillips, S.; et al. Influence of Meteorological Factors and Drought on Coccidioidomycosis Incidence in California, 2000–2020. Epidemiology 2022. [Google Scholar] [CrossRef]
- Gorris, M.E.; Treseder, K.K.; Zender, C.S.; Randerson, J.T. Expansion of Coccidioidomycosis Endemic Regions in the United States in Response to Climate Change. GeoHealth 2019, 3, 308–327. [Google Scholar] [CrossRef]
- Baptista-Rosas, R.C.; Hinojosa, A.; Riquelme, M. Ecological Niche Modeling of Coccidioides spp. in Western North American Deserts. Ann. N. Y. Acad. Sci. 2007, 1111, 35–46. [Google Scholar] [CrossRef]
- Kolivras, K.N.; Comrie, A.C. Modeling valley fever (coccidioidomycosis) incidence on the basis of climate conditions. Int. J. Biometeorol. 2003, 47, 87–101. [Google Scholar] [CrossRef]
- Tamerius, J.D.; Comrie, A.C. Coccidioidomycosis Incidence in Arizona Predicted by Seasonal Precipitation. PLoS ONE 2011, 6, e21009. [Google Scholar] [CrossRef]
- Talamantes, J.; Behseta, S.; Zender, C.S. Fluctuations in Climate and Incidence of Coccidioidomycosis in Kern County, California. Ann. N. Y. Acad. Sci. 2007, 1111, 73–82. [Google Scholar] [CrossRef]
- Zender, C.S.; Talamantes, J. Climate controls on valley fever incidence in Kern County, California. Int. J. Biometeorol. 2006, 50, 174–182. [Google Scholar] [CrossRef]
- Comrie, A.C. Climate Factors Influencing Coccidioidomycosis Seasonality and Outbreaks. Environ. Health Perspect. 2005, 113, 688–692. [Google Scholar] [CrossRef]
- Park, B.J.; Sigel, K.; Vaz, V.; Komatsu, K.; McRill, C.; Phelan, M.; Colman, T.; Comrie, A.C.; Warnock, D.W.; Galgiani, J.N.; et al. An Epidemic of Coccidioidomycosis in Arizona Associated with Climatic Changes, 1998–2001. J. Infect. Dis. 2005, 191, 1981–1987. [Google Scholar] [CrossRef]
- US EPA, O. Particulate Matter (PM10) Trends. Published 19 July 2016. Available online: https://www.epa.gov/air-trends/particulate-matter-pm10-trends (accessed on 1 April 2022).
- Kollath, D.R.; Mihaljevic, J.R.; Barker, B.M. PM10 and Other Climatic Variables Are Important Predictors of Seasonal Variability of Coccidioidomycosis in Arizona. Microbiol. Spectr. 2022, 10, e0148321. [Google Scholar] [CrossRef]
- U.S. Census Bureau QuickFacts. Available online: https://www.census.gov/quickfacts/fact/table/maricopacountyarizona,CA,AZ,US/AGE775220 (accessed on 26 March 2022).
- Colson, A.J.; Vredenburgh, L.; Guevara, R.E.; Rangel, N.P.; Kloock, C.T.; Lauer, A. Large-Scale Land Development, Fugitive Dust, and Increased Coccidioidomycosis Incidence in the Antelope Valley of California, 1999–2014. Mycopathologia 2017, 182, 439–458. [Google Scholar] [CrossRef]
- Guevara, R.E.; Motala, T.; Terashita, D. The Changing Epidemiology of Coccidioidomycosis in Los Angeles (LA) County, California, 1973–2011. PLoS ONE 2015, 10, e0136753. [Google Scholar] [CrossRef]
- Leake, J.A.D.; Mosley, D.G.; England, B.; Graham, J.V.; Plikaytis, B.D.; Ampel, N.M.; Perkins, B.A.; Hajjeh, R.A. Risk Factors for Acute Symptomatic Coccidioidomycosis among Elderly Persons in Arizona, 1996–1997. J. Infect. Dis. 2000, 181, 1435–1440. [Google Scholar] [CrossRef]
- Center for International Blood and Marrow Transplant Research Transplant Activity Report Covering 2009–2013. Available online: https://bloodstemcell.hrsa.gov/data/donation-and-transplantation-statistics/transplant-activity-report (accessed on 26 March 2022).
- Organ Procurement and Transplantation Network: View Data Reports. Available online: https://optn.transplant.hrsa.gov/data/view-data-reports/ (accessed on 26 March 2022).
- Casadevall, A. Fungal Diseases in the 21st Century: The Near and Far Horizons. Pathog. Immun. 2018, 3, 183–196. [Google Scholar] [CrossRef]
- Brown, J.; Benedict, K.; Park, B.J.; Thompson, G.R. Coccidioidomycosis: Epidemiology. Clin. Epidemiol. 2013, 5, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Odio, C.D.; Marciano, B.E.; Galgiani, J.N.; Holland, S.M. Risk Factors for Disseminated Coccidioidomycosis, United States. Emerg. Infect. Dis. 2017, 23, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.A.; Anderson, S.M.; Imholte, S.B.; Erhart, L.M.; Chen, S.; Park, B.J.; Christ, C.; Komatsu, K.K.; Chiller, T.; Sunenshine, R.H. Enhanced Surveillance of Coccidioidomycosis, Arizona, USA, 2007–2008. Emerg. Infect. Dis. 2010, 16, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.; Molinari, N.A.M.; Jackson, B.R. Public Awareness of Invasive Fungal Diseases—United States, 2019. Morb. Mortal. Wkly. Rep. 2020, 69, 1343–1346. [Google Scholar] [CrossRef]
- Hurd-Kundeti, G.; Sondermeyer Cooksey, G.L.; Jain, S.; Vugia, D.J. Valley Fever (Coccidioidomycosis) Awareness—California, 2016–2017. Morb. Mortal. Wkly. Rep. 2020, 69, 1512–1516. [Google Scholar] [CrossRef]
- Werner, S.B.; Pappagianis, D. Coccidioidomycosis in Northern California—An Outbreak among Archeology Students near Red Bluff. Calif. Med. 1973, 119, 16–20. [Google Scholar]
- Petersen, L.R.; Marshall, S.L.; Barton, C.; Hajjeh, R.A.; Lindsley, M.D.; Warnock, D.W.; Panackal, A.A.; Shaffer, J.B.; Haddad, M.B.; Fisher, F.S.; et al. Coccidioidomycosis among Workers at an Archeological Site, Northeastern Utah. Emerg. Infect. Dis. 2004, 10, 637–642. [Google Scholar] [CrossRef]
- Werner, S.B.; Pappagianis, D.; Heindl, I.; Mickel, A. An Epidemic of Coccidioidomycosis among Archeology Students in Northern California. N. Engl. J. Med. 1972, 286, 507–512. [Google Scholar] [CrossRef]
- Factors Influencing Distribution of Coccidioides Immitis in Soil, Washington State, 2016-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/34730378/ (accessed on 23 March 2022).
- Kobziar, L.N.; Thompson, G.R. Wildfire smoke, a potential infectious agent. Science 2020, 370, 1408–1410. [Google Scholar] [CrossRef]
- Laws, R.L.; Jain, S.; Cooksey, G.S.; Mohle-Boetani, J.; McNary, J.; Wilken, J.; Harrison, R.; Leistikow, B.; Vugia, D.J.; Windham, G.C.; et al. Coccidioidomycosis outbreak among inmate wildland firefighters: California, 2017. Am. J. Ind. Med. 2021, 64, 266–273. [Google Scholar] [CrossRef]
- Tong, D.Q.; Wang, J.X.L.; Gill, T.E.; Lei, H.; Wang, B. Intensified dust storm activity and Valley fever infection in the southwestern United States. Geophys. Res. Lett. 2017, 44, 4304–4312. [Google Scholar] [CrossRef]
- Comrie, A.C. No Consistent Link Between Dust Storms and Valley Fever (Coccidioidomycosis). GeoHealth 2021, 5, e2021GH000504. [Google Scholar] [CrossRef]
- Gade, L.; McCotter, O.Z.; Bowers, J.R.; Waddell, V.; Brady, S.; Carvajal, J.A.; Sunenshine, R.; Komatsu, K.K.; Engelthaler, D.M.; Chiller, T.; et al. The detection of Coccidioides from ambient air in Phoenix, Arizona: Evidence of uneven distribution and seasonality. Med. Mycol. 2020, 58, 552–559. [Google Scholar] [CrossRef]
- Emmons, C.W. Coccidioidomycosis in Wild Rodents. A Method of Determining the Extent of Endemic Areas. Public Health Rep. (1896–1970) 1943, 58, 1–5. [Google Scholar] [CrossRef]
- Health ASD of Proceedings of Symposium on Coccidioidomycosis: Held at Phoenix, Ariz.-Feb. 11–13, 1957; U.S. Department of Health, Education, and Welfare Public Health Service, Bureau of State Services, Communicable Disease Center: Washington, DC, USA, 1957.
- Kollath, D.R.; Miller, K.J.; Barker, B.M. The mysterious desert dwellers: Coccidioides immitis and Coccidioides posadasii, causative fungal agents of coccidioidomycosis. Virulence 2019, 10, 222–233. [Google Scholar] [CrossRef]
- Soil Ecology of Coccidioides Immitis at Amerindian Middens in California Applied Microbiology. Available online: https://journals.asm.org/doi/abs/10.1128/am.27.2.379-388.1974 (accessed on 23 March 2022).
- Cordeiro, R.A.; Brilhante, R.S.N.; Rocha, M.F.G.; Fechine, M.A.B.; Camara, L.M.C.; Camargo, Z.P.; Sidrim, J.J.C. Phenotypic characterization and ecological features of Coccidioides spp. from Northeast Brazil. Med. Mycol. 2006, 44, 631–639. [Google Scholar] [CrossRef]
- Sharpton, T.J.; Stajich, J.E.; Rounsley, S.D.; Gardner, M.J.; Wortman, J.R.; Jordar, V.S.; Maiti, R.; Kodira, C.D.; Neafsey, D.E.; Zeng, Q.; et al. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 2009, 19, 1722–1731. [Google Scholar] [CrossRef]
- Ampel, N.M.; Dols, C.L.; Galgiani, J.N. Coccidioidomycosis during human immunodeficiency virus infection: Results of a prospective study in a coccidioidal endemic area. Am. J. Med. 1993, 94, 235–240. [Google Scholar] [CrossRef]
- Woods, C.W.; McRill, C.; Plikaytis, B.D.; Rosenstein, N.E.; Mosley, D.; Boyd, D.; England, B.; Perkins, B.A.; Ampel, N.M.; Hajjeh, R.A. Coccidioidomycosis in Human Immunodeficiency Virus–Infected Persons in Arizona, 1994–1997: Incidence, Risk Factors, and Prevention. J. Infect. Dis. 2000, 181, 1428–1434. [Google Scholar] [CrossRef]
- Masannat, F.Y.; Ampel, N.M. Coccidioidomycosis in Patients with HIV-1 Infection in the Era of Potent Antiretroviral Therapy. Clin. Infect. Dis. 2010, 50, 1–7. [Google Scholar] [CrossRef]
- Blair, J.E.; Logan, J.L. Coccidioidomycosis in Solid Organ Transplantation. Clin. Infect. Dis. 2001, 33, 1536–1544. [Google Scholar] [CrossRef]
- Bergstrom, L.; Yocum, D.E.; Ampel, N.M.; Villanueva, I.; Lisse, J.; Gluck, O.; Tesser, J.; Posever, J.P.; Miller, M.; Araujo, J.; et al. Increased risk of coccidioidomycosis in patients treated with tumor necrosis factor α antagonists. Arthritis Rheum. 2004, 50, 1959–1966. [Google Scholar] [CrossRef]
- Blair, J.E.; Douglas, D.D.; Mulligan, D.C. Early results of targeted prophylaxis for coccidioidomycosis in patients undergoing orthotopic liver transplantation within an endemic area. Transpl. Infect. Dis. 2003, 5, 3–8. [Google Scholar] [CrossRef]
- Kahn, A.; Carey, E.J.; Blair, J.E. Universal fungal prophylaxis and risk of coccidioidomycosis in liver transplant recipients living in an endemic area. Liver Transpl. 2015, 21, 353–361. [Google Scholar] [CrossRef]
- Keckich, D.W.; Blair, J.E.; Vikram, H.R.; Seville, M.T.; Kusne, S. Reactivation of Coccidioidomycosis Despite Antifungal Prophylaxis in Solid Organ Transplant Recipients. Transplantation 2011, 92, 88–93. [Google Scholar] [CrossRef]
- Truong, C.N.; Nailor, M.D.; Walia, R.; Cherrier, L.; Nasar, A.; Goodlet, K.J. Universal lifelong fungal prophylaxis and risk of coccidioidomycosis in lung transplant recipients living in an endemic area. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 74, ciab752. [Google Scholar] [CrossRef]
- Al-Obaidi, M.M.; Nematollahi, S.; Nix, D.E.; Zangeneh, T.T. Remarks on the universal lifelong coccidioidomycosis prophylaxis in lung transplant recipients. Clin. Infect. Dis. 2021, 74, ciab878. [Google Scholar] [CrossRef]
- Bercovitch, R.S.; Catanzaro, A.; Schwartz, B.S.; Pappagianis, D.; Watts, D.H.; Ampel, N.M. Coccidioidomycosis During Pregnancy: A Review and Recommendations for Management. Clin. Infect. Dis. 2011, 53, 363–368. [Google Scholar] [CrossRef]
- Crum, N.F.; Ballon-Landa, G. Coccidioidomycosis in Pregnancy: Case Report and Review of the Literature. Am. J. Med. 2006, 119, e11–e993. [Google Scholar] [CrossRef]
- Crum, N.F.; Lederman, E.R.; Stafford, C.M.; Parrish, J.S.; Wallace, M.R. Coccidioidomycosis: A Descriptive Survey of a Reemerging Disease. Clinical Characteristics and Current Controversies. Medicine 2004, 83, 149–175. [Google Scholar] [CrossRef]
- Sondermeyer Cooksey, G.L.; Nguyen, A.; Vugia, D.; Jain, S. Regional Analysis of Coccidioidomycosis Incidence—California, 2000–2018. Morb. Mortal. Wkly. Rep. 2020, 69, 1817–1821. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.C.; Anderson, S.; Wannemuehler, K.; Engelthaler, D.M.; Erhart, L.; Sunenshine, R.H.; Burwell, L.A.; Park, B.J. Testing for Coccidioidomycosis among Patients with Community-Acquired Pneumonia. Emerg. Infect. Dis. 2008, 14, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Valley Fever (Coccidioidomycosis), Jobs at Risk NIOSH CDC. Published 11 March 2022. Available online: https://www.cdc.gov/niosh/topics/valleyfever/risk.html (accessed on 26 March 2022).
- de Perio, M.A.; Niemeier, R.T.; Burr, G.A. Coccidioides Exposure and Coccidioidomycosis among Prison Employees, California, United States. Emerg. Infect. Dis. 2015, 21, 1031–1033. [Google Scholar] [CrossRef] [PubMed]
- Wilken, J.A.; Marquez, P.; Terashita, D.; McNary, J.; Windham, G.; Materna, B. Coccidioidomycosis Among Cast and Crew Members at an Outdoor Television Filming Event—California, 2012. MMWR Morb. Mortal Wkly Rep. 2014, 63, 20. [Google Scholar]
- Durry, E.; Pappagianis, D.; Werner, S.B.; Hutwagner, L.; Sun, R.K.; Maurer, M.; McNeil, M.M.; Pinner, R.W. Coccidioidomycosis in Tulare County, California, 1991: Reemergence of an endemic disease. J. Med. Vet. Mycol. 1997, 35, 321–326. [Google Scholar] [CrossRef][Green Version]
- Sondermeyer, G.; Lee, L.; Gilliss, D.; Tabnak, F.; Vugia, D. Coccidioidomycosis-associated Hospitalizations, California, USA, 2000–2011. Emerg. Infect. Dis. 2013, 19, 1590–1597. [Google Scholar] [CrossRef]
- Einstein, H.E.; Johnson, R.H. Coccidioidomycosis: New Aspects of Epidemiology and Therapy. Clin. Infect. Dis. 1993, 16, 349–356. [Google Scholar] [CrossRef]
- McHardy, I.; Reagan, K.L.; Sebastian, J.F.; Barker, B.; Bays, D.J.; Dandekar, S.; Cohen, S.H.; Jennings, K.E.; Sykes, J.; Thompson, G.R. Sex Differences in Susceptibility to Coccidioidomycosis. Open Forum Infect. Dis. 2022, 9, ofab543. [Google Scholar] [CrossRef]
- Shah, A.S.; Heidari, A.; Civelli, V.F.; Sharma, R.; Clark, C.S.; Munoz, A.D.; Ragland, A.S.; Johnson, R.H. The Coincidence of 2 Epidemics, Coccidioidomycosis and SARS-CoV-2: A Case Report. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620930540. [Google Scholar] [CrossRef]
- Chang, C.C.; Senining, R.; Kim, J.; Goyal, R. An Acute Pulmonary Coccidioidomycosis Coinfection in a Patient Presenting With Multifocal Pneumonia With COVID-19. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620972244. [Google Scholar] [CrossRef]
- Chen, J.C.; Wong, D.; Rabi, S.; Worswick, S.; DeClerck, B.; Gibb, J. All That Coughs Is Not COVID-19: A Delayed Diagnosis of Disseminated Coccidioidomycosis Following Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Open Forum Infect. Dis. 2021, 8, ofab246. [Google Scholar] [CrossRef]
- Nielsen, M.C.; Reynoso, D.; Ren, P. The Brief Case: A Fatal Case of SARS-CoV-2 Coinfection with Coccidioides in Texas—Another Challenge We Face. Burnham, C.A.D., Ed. J. Clin. Microbiol. 2021, 59, e00163-21. [Google Scholar] [CrossRef]
- Ko, J.; Lee, M.M. A Case of Disseminated Coccidioidomycosis in a Patient with a Prolonged Course of COVID-19 Pneumonia. In TP98. TP098 FUNGUS AMONG-US-RARE FUNGAL CASE REPORTS; American Thoracic Society International Conference Abstracts; American Thoracic Society: New York, NY, USA, 2021; p. A3997. [Google Scholar] [CrossRef]
- Mathew, J.; Cherukuri, S.V.; Dihowm, F. SARS-CoV-2 with concurrent coccidioidomycosis complicated by refractory pneumothorax in a Hispanic male: A case report and literature review. World J. Respirol. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Patel, B.; Jarrett, B.; Bixby, B. Diagnostic Error and Cognitive Bias in the Era of COVID-19: Don’t Forget About Endemic Diseases. Chest 2020, 158, A541–A542. [Google Scholar] [CrossRef]
- Krauth, D.S.; Jamros, C.M.; Rivard, S.C.; Olson, N.H.; Maves, R.C. Accelerated Progression of Disseminated Coccidioidomycosis Following SARS-CoV-2 Infection: A Case Report. Mil. Med. 2021, 186, usab132. [Google Scholar] [CrossRef]
- Heaney, A.K.; Head, J.R.; Broen, K.; Click, K.; Taylor, J.; Balmes, J.R.; Zelner, J.; Remais, J.V. Coccidioidomycosis and COVID-19 Co-Infection, United States, 2020. Emerg. Infect. Dis. 2021, 27, 1266–1273. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, X.; Zhao, H.; Wang, H.; Zhao, R.; Sheng, J. Host susceptibility to severe COVID-19 and establishment of a host risk score: Findings of 487 cases outside Wuhan. Crit. Care. 2020, 24, 108. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team. Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019—United States, 12 February–28 March 2020. Morb. Mortal. Wkly. Rep. 2020, 69. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, ciaa248. [Google Scholar] [CrossRef]
- Beaman, L.; Benjamini, E.; Pappagianis, D. Activation of macrophages by lymphokines: Enhancement of phagosome-lysosome fusion and killing of Coccidioides immitis. Infect. Immun. 1983, 39, 1201–1207. [Google Scholar] [CrossRef]
- Benedict, K.; Williams, S.; Beekmann, S.E.; Polgreen, P.M.; Jackson, B.R.; Toda, M. Testing Practices for Fungal Respiratory Infections and SARS-CoV-2 among Infectious Disease Specialists, United States. J. Fungi. 2021, 7, 605. [Google Scholar] [CrossRef]
- Olson, G.; Davis, A.M. Diagnosis and Treatment of Adults With Community-Acquired Pneumonia. JAMA 2020, 323, 885. [Google Scholar] [CrossRef]
- Valdivia, L.; Nix, D.; Wright, M.; Lindberg, E.; Fagan, T.; Lieberman, D.; Stoffer, T.; Ampel, N.M.; Galgiani, J.N. Coccidioidomycosis as a Common Cause of Community-acquired Pneumonia. Emerg. Infect. Dis. 2006, 12, 958–962. [Google Scholar] [CrossRef]
- Chi, G.C.; Benedict, K.; Beer, K.D.; Jackson, B.R.; McCotter, O.; Xie, F.; Lawrence, J.M.; Tartof, S.Y. Antibiotic and antifungal treatment among persons with confirmed coccidioidomycosis—Southern California, 2011. Med. Mycol. 2020, 58, 411–413. [Google Scholar] [CrossRef]
- Chen, S.; Erhart, L.M.; Anderson, S.; Komatsu, K.; Park, B.; Chiller, T.; Sunenshine, R. Coccidioidomycosis: Knowledge, attitudes, and practices among healthcare providers—Arizona, 2007. Med. Mycol. 2011, 49, 649–656. [Google Scholar] [CrossRef]
- Pu, J.; Donovan, F.M.; Ellingson, K.; Leroy, G.; Stone, J.; Bedrick, E.; Galgiani, J.N. Clinician Practice Patterns That Result in the Diagnosis of Coccidioidomycosis Before or During Hospitalization. Clin. Infect. Dis. 2021, 73, e1587–e1593. [Google Scholar] [CrossRef]
- Malo, J.; Holbrook, E.; Zangeneh, T.; Strawter, C.; Oren, E.; Robey, I.; Erickson, H.; Chahal, R.; Durkin, M.; Thompson, C.; et al. Enhanced Antibody Detection and Diagnosis of Coccidioidomycosis with the MiraVista IgG and IgM Detection Enzyme Immunoassay. J. Clin. Microbiol. 2017, 55, 893–901. [Google Scholar] [CrossRef]
- Malo, J.; Holbrook, E.; Zangeneh, T.; Strawter, C.; Oren, E.; Robey, I.; Erickson, H.; Carranza-Chahal, R.; Durkin, M.; Thompson, C.; et al. Comparison of three anti-coccidioides antibody enzyme immunoassay kits for the diagnosis of coccidioidomycosis. Med. Mycol. 2020, 58, 774–778. [Google Scholar] [CrossRef]
- Grys, T.E.; Brighton, A.; Chang, Y.H.; Liesman, R.; Bolster LaSalle, C.; Blair, J.E. Comparison of two FDA-cleared EIA assays for the detection of Coccidioides antibodies against a composite clinical standard. Med. Mycol. 2019, 57, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Kuberski, T.; Herrig, J.; Pappagianis, D. False-Positive IgM Serology in Coccidioidomycosis. J. Clin. Microbiol. 2010, 48, 2047–2049. [Google Scholar] [CrossRef] [PubMed]
- Donovan, F.M.; Ramadan, F.A.; Khan, S.A.; Bhaskara, A.; Lainhart, W.D.; Narang, A.T.; Mosier, J.M.; Ellingson, K.D.; Bedrick, E.J.; Saubolle, M.A.; et al. Comparison of a Novel Rapid Lateral Flow Assay to Enzyme Immunoassay Results for Early Diagnosis of Coccidioidomycosis. Clin. Infect. Dis. 2021, 73, e2746–e2753. [Google Scholar] [CrossRef] [PubMed]
- Huppert, M. Serology of coccidioidomycosis. Mycopathol. Mycol. Appl. 1970, 41, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.E.; Coakley, B.; Santelli, A.C.; Hentz, J.G.; Wengenack, N.L. Serologic testing for symptomatic coccidioidomycosis in immunocompetent and immunosuppressed hosts. Mycopathologia 2006, 162, 317–324. [Google Scholar] [CrossRef]
- Mendoza, N.; Blair, J.E. The Utility of Diagnostic Testing for Active Coccidioidomycosis in Solid Organ Transplant Recipients. Am. J. Transplant. 2013, 13, 1034–1039. [Google Scholar] [CrossRef]
- Durkin, M.; Connolly, P.; Kuberski, T.; Myers, R.; Kubak, B.M.; Bruckner, D.; Pegues, D.; Wheat, L.J. Diagnosis of Coccidioidomycosis with Use of the Coccidioides Antigen Enzyme Immunoassay. Clin. Infect. Dis. 2008, 47, e69–e73. [Google Scholar] [CrossRef]
- Galgiani, J.N.; Grace, G.M.; Lundergan, L.L. New Serologic Tests for Early Detection of Coccidioidomycosis. J. Infect. Dis. 1991, 163, 671–674. [Google Scholar] [CrossRef]
- Kassis, C.; Durkin, M.; Holbrook, E.; Myers, R.; Wheat, L. Advances in Diagnosis of Progressive Pulmonary and Disseminated Coccidioidomycosis. Clin. Infect. Dis. 2021, 72, 968–975. [Google Scholar] [CrossRef]
- Wheat, L.J.; Knox, K.S.; Hage, C.A. Approach to the Diagnosis of Histoplasmosis, Blastomycosis and Coccidioidomycosis. Curr Curr. Treat. Options Infect. Dis. 2014, 6, 337–351. [Google Scholar] [CrossRef]
- Dizon, D.; Mitchell, M.; Dizon, B.; Libke, R.; Peterson, M.W. The utility of real-time polymerase chain reaction in detecting Coccidioides immitis among clinical specimens in the Central California San Joaquin Valley. Med. Mycol. 2019, 57, 688–693. [Google Scholar] [CrossRef]
- Vucicevic, D.; Blair, J.E.; Binnicker, M.J.; McCullough, A.E.; Kusne, S.; Vikram, H.R.; Parish, J.M.; Wengenack, N.L. The utility of Coccidioides polymerase chain reaction testing in the clinical setting. Mycopathologia 2010, 170, 345–351. [Google Scholar] [CrossRef]
- Thompson, G.R.; Bays, D.J.; Johnson, S.M.; Cohen, S.H.; Pappagianis, D.; Finkelman, M.A. Serum (1→3)-β-d-Glucan Measurement in Coccidioidomycosis. J. Clin. Microbiol. 2012, 50, 3060–3062. [Google Scholar] [CrossRef]
- Caceres, D.H.; Chiller, T.; Lindsley, M.D. Immunodiagnostic Assays for the Investigation of Fungal Outbreaks. Mycopathologia 2020, 185, 867–880. [Google Scholar] [CrossRef]
- Durkin, M.; Estok, L.; Hospenthal, D.; Crum-Cianflone, N.; Swartzentruber, S.; Hackett, E.; Wheat, L.J. Detection of Coccidioides Antigenemia following Dissociation of Immune Complexes. Clin. Vaccine Immunol. 2009, 16, 1453–1456. [Google Scholar] [CrossRef]
- Messina, J.A.; Maziarz, E.K.; Galgiani, J.; Truong, J.T.; Htoo, A.K.; Heidari, A.; Johnson, R.H.; Narang, A.T.; Donovan, F.M.; Ewell, M.; et al. A randomized, double-blind, placebo-controlled clinical trial of fluconazole as early empiric treatment of coccidioidomycosis pneumonia (Valley Fever) in adults presenting with community-acquired pneumonia in endemic areas (FLEET-Valley Fever). Contemp. Clin. Trials. Commun. 2021, 24, 100851. [Google Scholar] [CrossRef]
- Sugar, A.M.; Alsip, S.G.; Galgiani, J.N.; Graybill, J.R.; Dismukes, W.E.; Cloud, G.A.; Craven, P.C.; Stevens, D.A. Pharmacology and toxicity of high-dose ketoconazole. Antimicrob. Agents Chemother. 1987, 31, 1874–1878. [Google Scholar] [CrossRef]
- Pont, A.; Graybill, J.R.; Craven, P.C.; Galgiani, J.N.; Dismukes, W.E.; Reitz, R.E.; Stevens, D.A. High-Dose Ketoconazole Therapy and Adrenal and Testicular Function in Humans. Arch. Intern. Med. 1984, 144, 2150–2153. [Google Scholar] [CrossRef]
- Arndt, C.A.S.; Walsh, T.J.; McCully, C.L.; Balis, F.M.; Pizzo, P.A.; Poplack, D.G. Fluconazole Penetration into Cerebrospinal Fluid: Implications for Treating Fungal Infections of the Central Nervous System. J. Infect. Dis. 1988, 157, 178–180. [Google Scholar] [CrossRef]
- Brewer, A.C.; Huber, J.T.; Girardo, M.E.; Kosiorek, H.E.; Burns, M.W.; Stewart, T.D.; Blair, J.E. Cutaneous effects associated with fluconazole in patients treated for coccidioidomycosis. Int. J. Dermatol. 2019, 58, 250–253. [Google Scholar] [CrossRef]
- Thompson, G.R.; Barker, B.M.; Wiederhold, N.P. Large-Scale Evaluation of In Vitro Amphotericin B, Triazole, and Echinocandin Activity against Coccidioides Species from U.S. Institutions. Antimicrob. Agents Chemother. 2017, 61, e02634-16. [Google Scholar] [CrossRef]
- Galgiani, J.N. Comparison of Oral Fluconazole and Itraconazole for Progressive, Nonmeningeal Coccidioidomycosis: A Randomized, Double-Blind Trial. Ann. Intern. Med. 2000, 133, 676. [Google Scholar] [CrossRef]
- Hoffmann, W.J.; McHardy, I.; Thompson, G.R., III. Itraconazole induced hypertension and hypokalemia: Mechanistic evaluation. Mycoses 2018, 61, 337–339. [Google Scholar] [CrossRef]
- Lestner, J.M.; Roberts, S.A.; Moore, C.B.; Howard, S.J.; Denning, D.W.; Hope, W.W. Toxicodynamics of Itraconazole: Implications for Therapeutic Drug Monitoring. Clin. Infect. Dis. 2009, 49, 928–930. [Google Scholar] [CrossRef]
- Sharkey, P.K.; Rinaldi, M.G.; Dunn, J.F.; Hardin, T.C.; Fetchick, R.J.; Graybill, J.R. High-dose itraconazole in the treatment of severe mycoses. Antimicrob. Agents Chemother. 1991, 35, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Vikram, H.R.; Kusne, S.; Seville, M.T.; Blair, J.E. Treatment of Refractory Coccidioidomycosis With Voriconazole or Posaconazole. Clin. Infect. Dis. 2011, 53, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Cortez, K.J.; Walsh, T.J.; Bennett, J.E. Successful Treatment of Coccidioidal Meningitis with Voriconazole. Clin. Infect. Dis. 2003, 36, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Freifeld, A.; Proia, L.; Andes, D.; Baddour, L.M.; Blair, J.; Spellberg, B.; Arnold, S.; Lentnek, A.; Wheat, L.J. Voriconazole Use for Endemic Fungal Infections. Antimicrob. Agents Chemother. 2009, 53, 1648–1651. [Google Scholar] [CrossRef] [PubMed]
- Epaulard, O.; Villier, C.; Ravaud, P.; Chosidow, O.; Blanche, S.; Mamzer-Bruneel, M.; Thiebaut, A.; Leccia, M.; Lortholary, O. A Multistep Voriconazole-Related Phototoxic Pathway May Lead to Skin Carcinoma: Results From a French Nationwide Study. Clin. Infect. Dis. 2013, 57, e182–e188. [Google Scholar] [CrossRef]
- Haylett, A.K.; Felton, S.; Denning, D.W.; Rhodes, L.E. Voriconazole-induced photosensitivity: Photobiological assessment of a case series of 12 patients. Br. J. Dermatol. 2013, 168, 179–185. [Google Scholar] [CrossRef]
- Williams, K.; Mansh, M.; Chin-Hong, P.; Singer, J.; Arron, S.T. Voriconazole-Associated Cutaneous Malignancy: A Literature Review on Photocarcinogenesis in Organ Transplant Recipients. Clin. Infect. Dis. 2014, 58, 997–1002. [Google Scholar] [CrossRef]
- Pham, A.N.; Bubalo, J.S.; Lewis, J.S., II. Comparison of posaconazole serum concentrations from haematological cancer patients on posaconazole tablet and oral suspension for treatment and prevention of invasive fungal infections. Mycoses 2016, 59, 226–233. [Google Scholar] [CrossRef]
- Anstead, G.M.; Corcoran, G.; Lewis, J.; Berg, D.; Graybill, J.R. Refractory Coccidioidomycosis Treated with Posaconazole. Clin. Infect. Dis. 2005, 40, 1770–1776. [Google Scholar] [CrossRef]
- Catanzaro, A.; Cloud, G.A.; Stevens, D.A.; Levine, B.E.; Williams, B.L.; Johnson, R.H.; Rendon, A.; Mirels, L.F.; Lutz, J.E.; Holloway, M.; et al. Safety, Tolerance, and Efficacy of Posaconazole Therapy in Patients with Nonmeningeal Disseminated or Chronic Pulmonary Coccidioidomycosis. Clin. Infect. Dis. 2007, 45, 562–568. [Google Scholar] [CrossRef]
- Ruping, M.J.G.T.; Albermann, N.; Ebinger, F.; Burckhardt, I.; Beisel, C.; Muller, C.; Vehreschild, J.J.; Kochanek, M.; Fatkenheuer, G.; Bangard, C.; et al. Posaconazole concentrations in the central nervous system. J. Antimicrob. Chemother. 2008, 62, 1468–1470. [Google Scholar] [CrossRef]
- Schein, R.; Homans, J.; Larsen, R.A.; Neely, M. Posaconazole for Chronic Refractory Coccidioidal Meningitis. Clin. Infect. Dis. 2011, 53, 1252–1254. [Google Scholar] [CrossRef]
- González, G.M.; Tijerina, R.; Najvar, L.K.; Bocanegra, R.; Rinaldi, M.; Loebenberg, D.; Graybill, J.R. In Vitro and In Vivo Activities of Posaconazole against Coccidioides immitis. Antimicrob. Agents Chemother. 2002, 46, 1352–1356. [Google Scholar] [CrossRef]
- Donnelley, M.A.; Zhu, E.S.; Thompson, G.R. Isavuconazole in the treatment of invasive aspergillosis and mucormycosis infections. Infect. Drug Resist. 2016, 9, 79–86. [Google Scholar] [CrossRef]
- Thompson, G.R.; Rendon, A.; Ribeiro dos Santos, R.; Queiroz-Telles, F.; Ostrosky-Zeichner, L.; Azie, N.; Maher, R.; Lee, M.; Kovanda, L.; Engelhardt, M.; et al. Isavuconazole Treatment of Cryptococcosis and Dimorphic Mycoses. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 63, 356–362. [Google Scholar] [CrossRef]
- Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): A phase 3, randomised-controlled, non-inferiority trial Elsevier Enhanced Reader. Lancet 2015, 387, 760–769. [CrossRef]
- Hamill, R.J. Amphotericin B Formulations: A Comparative Review of Efficacy and Toxicity. Drugs 2013, 73, 919–934. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Ostrosky-Zeichner, L.; Marr, K.A.; Rex, J.H.; Cohen, S.H. Amphotericin B: Time for a New “Gold Standard”. Clin. Infect. Dis. 2003, 37, 415–425. [Google Scholar] [CrossRef]
- Stevens, D.A.; Shatsky, S.A. Intrathecal amphotericin in the management of coccidioidal meningitis. Semin. Respir. Infect. 2001, 16, 263–269. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, S.L.; Chiller, T. Update on the Epidemiology, Diagnosis, and Treatment of Coccidioidomycosis. J. Fungi 2022, 8, 666. https://doi.org/10.3390/jof8070666
Williams SL, Chiller T. Update on the Epidemiology, Diagnosis, and Treatment of Coccidioidomycosis. Journal of Fungi. 2022; 8(7):666. https://doi.org/10.3390/jof8070666
Chicago/Turabian StyleWilliams, Samantha L., and Tom Chiller. 2022. "Update on the Epidemiology, Diagnosis, and Treatment of Coccidioidomycosis" Journal of Fungi 8, no. 7: 666. https://doi.org/10.3390/jof8070666
APA StyleWilliams, S. L., & Chiller, T. (2022). Update on the Epidemiology, Diagnosis, and Treatment of Coccidioidomycosis. Journal of Fungi, 8(7), 666. https://doi.org/10.3390/jof8070666





