Interactions between Core Elements of the Botrytis cinerea Circadian Clock Are Modulated by Light and Different Protein Domains
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. In Silico Analysis of the Botrytis cinerea Proteins
2.3. Plasmids and Genetic Constructs
2.4. Protein–Protein Interaction Assays
3. Results
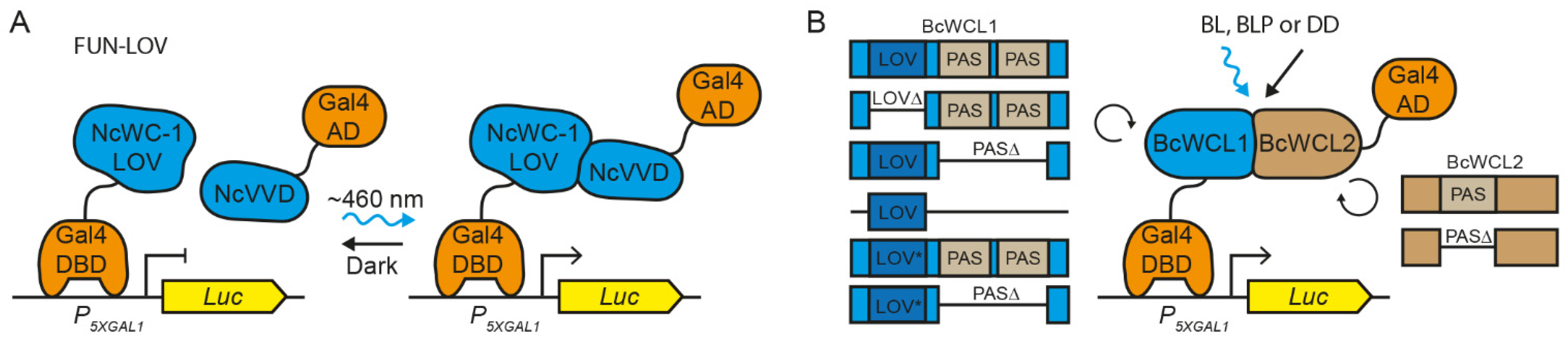
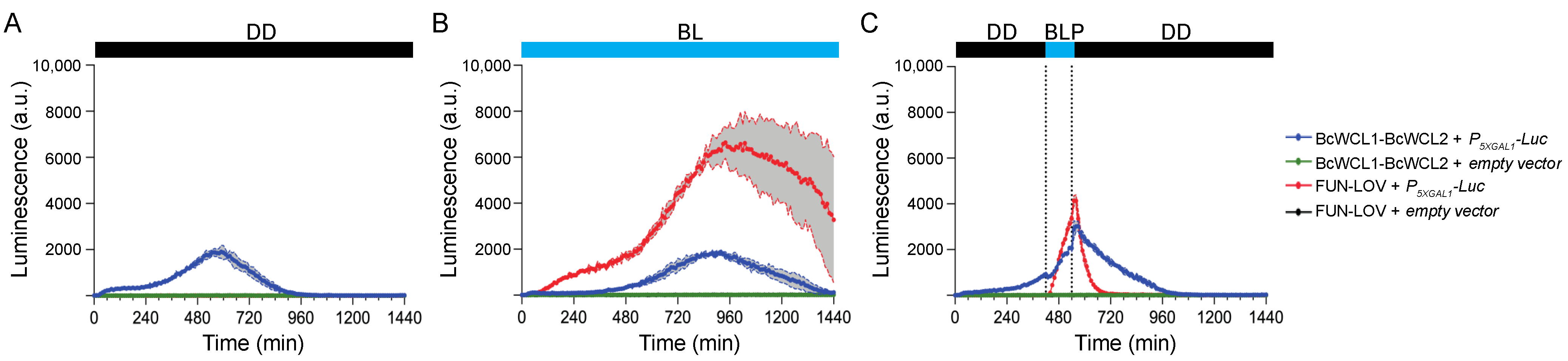
3.1. The Components of the BcWCC Interact in the Presence or Absence of Light
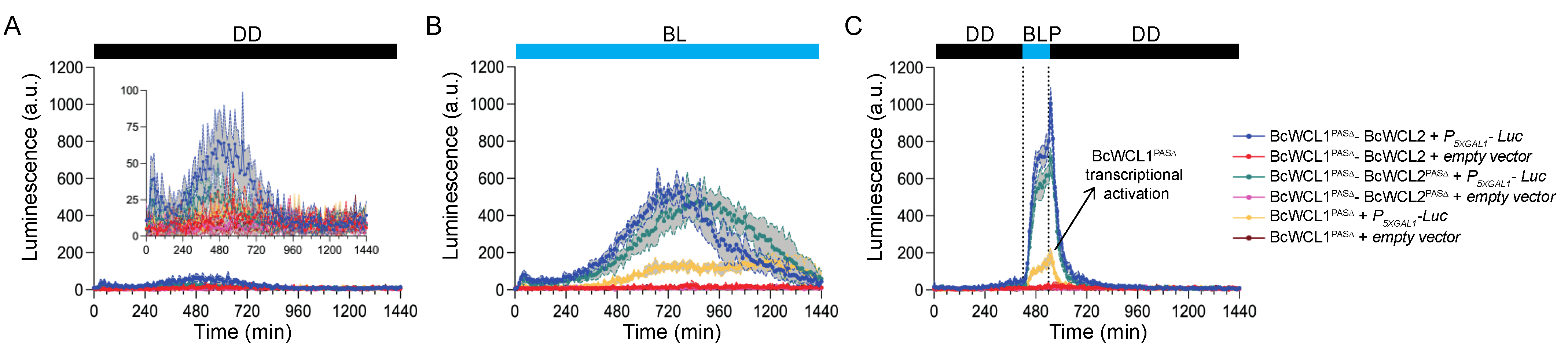
3.2. Different Domains Participate in the Protein–Protein Interaction between BcWCL1 and BcWCL2
3.3. The BcWCL1PAS∆ Protein Responds to Blue-Light Stimulation and Interacts with BcWCL2 or BcWCL2PAS∆
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrera-Estrella, A.; Horwitz, B.A. Looking through the eyes of fungi: Molecular genetics of photoreception. Mol. Microbiol. 2007, 64, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Romero, J.; Hedtke, M.; Kastner, C.; Muller, S.; Fischer, R. Fungi, hidden in soil or up in the air: Light makes a difference. Annu. Rev. Microbiol. 2010, 64, 585–610. [Google Scholar] [CrossRef] [PubMed]
- Corrochano, L.M. Light in the Fungal World: From Photoreception to Gene Transcription and Beyond. Annu. Rev. Genet. 2019, 53, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Fischer, R. Light sensing and responses in fungi. Nat. Rev. Microbiol. 2019, 17, 25–36. [Google Scholar] [CrossRef]
- Schumacher, J. How light affects the life of Botrytis. Fungal Genet. Biol. 2017, 106, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Veloso, J.; van Kan, J.A.L. Many Shades of Grey in Botrytis-Host Plant Interactions. Trends Plant Sci. 2018, 23, 613–622. [Google Scholar] [CrossRef]
- Fraser, D.P.; Hayes, S.; Franklin, K.A. Photoreceptor crosstalk in shade avoidance. Curr. Opin. Plant Biol. 2016, 33, 1–7. [Google Scholar] [CrossRef]
- Cerrudo, I.; Keller, M.M.; Cargnel, M.D.; Demkura, P.V.; de Wit, M.; Patitucci, M.S.; Pierik, R.; Pieterse, C.M.; Ballare, C.L. Low red/far-red ratios reduce Arabidopsis resistance to Botrytis cinerea and jasmonate responses via a COI1-JAZ10-dependent, salicylic acid-independent mechanism. Plant Physiol. 2012, 158, 2042–2052. [Google Scholar] [CrossRef]
- Tan, K.K.; Epton, H.A.S. Effect of light on the growth and sporulation of Botrytis cinerea. Trans. Br. Mycol. Soc. 1973, 61, 145–157. [Google Scholar] [CrossRef]
- Tan, K.K. Complete reversibility of sporulation by near ultraviolet and blue light in Botrytis cinerea. Trans. Br. Mycol. Soc. 1974, 63, 203–205. [Google Scholar] [CrossRef]
- Tan, K.K. Interaction of near-ultraviolet, blue, red, and far-red light in sporulation of Botrytis cinerea. Trans. Br. Mycol. Soc. 1975, 64, 215–222. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kumagai, T.; Oda, Y. Locus of blue and near ultraviolet reversible photoreaction in the stages of conidial development in Botrytis cinerea. J. Gen. Microbiol. 1977, 98, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Oda, Y. Inhibitory Loci of both Blue and near Ultraviolet Lights on Lateral-type Sclerotial Development in Botrytis cinerea. Jpn. J. Phytopathol. 1979, 45, 54–61. [Google Scholar] [CrossRef][Green Version]
- Amselem, J.; Cuomo, C.A.; van Kan, J.A.L.; Viaud, M.; Benito, E.P.; Couloux, A.; Coutinho, P.M.; de Vries, R.P.; Dyer, P.S.; Fillinger, S.; et al. Genomic Analysis of the Necrotrophic Fungal Pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 2011, 7, e1002230. [Google Scholar] [CrossRef]
- Van Kan, J.A.; Stassen, J.H.; Mosbach, A.; Van Der Lee, T.A.; Faino, L.; Farmer, A.D.; Papasotiriou, D.G.; Zhou, S.; Seidl, M.F.; Cottam, E.; et al. A gapless genome sequence of the fungus Botrytis cinerea. Mol. Plant Pathol. 2017, 18, 75–89. [Google Scholar] [CrossRef]
- Canessa, P.; Schumacher, J.; Hevia, M.A.; Tudzynski, P.; Larrondo, L.F. Assessing the Effects of Light on Differentiation and Virulence of the Plant Pathogen Botrytis cinerea: Characterization of the White Collar Complex. PLoS ONE 2014, 8, e84223. [Google Scholar] [CrossRef]
- Heller, J.; Ruhnke, N.; Espino, J.J.; Massaroli, M.; Collado, I.G.; Tudzynski, P. The mitogen-activated protein kinase BcSak1 of Botrytis cinerea is required for pathogenic development and has broad regulatory functions beyond stress response. Mol. Plant Microbe Interact. 2012, 25, 802–816. [Google Scholar] [CrossRef]
- Hu, Y.; He, J.; Wang, Y.; Zhu, P.; Zhang, C.; Lu, R.; Xu, L. Disruption of a phytochrome-like histidine kinase gene by homologous recombination leads to a significant reduction in vegetative growth, sclerotia production, and the pathogenicity of Botrytis cinerea. Physiol. Mol. Plant Pathol. 2014, 85, 25–33. [Google Scholar] [CrossRef]
- Cohrs, K.C.; Schumacher, J. The Two Cryptochrome/Photolyase Family Proteins Fulfill Distinct Roles in DNA Photorepair and Regulation of Conidiation in the Gray Mold Fungus Botrytis cinerea. Appl. Environ. Microbiol. 2017, 83, e00812-17. [Google Scholar] [CrossRef]
- Ballario, P.; Vittorioso, P.; Magrelli, A.; Talora, C.; Cabibbo, A.; Macino, G. White collar-1, a central regulator of blue light responses in Neurospora, is a zinc finger protein. EMBO J. 1996, 15, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Cheng, P.; Yang, Y.; Wang, L.; Gardner, K.H.; Liu, Y. White collar-1, a DNA binding transcription factor and a light sensor. Science 2002, 297, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, A.C.; Liu, Y.; Loros, J.J.; Dunlap, J.C. White Collar-1, a circadian blue light photoreceptor, binding to the frequency promoter. Science 2002, 297, 815–819. [Google Scholar] [CrossRef]
- Glantz, S.T.; Carpenter, E.J.; Melkonian, M.; Gardner, K.H.; Boyden, E.S.; Wong, G.K.; Chow, B.Y. Functional and topological diversity of LOV domain photoreceptors. Proc. Natl. Acad. Sci. USA 2016, 113, E1442–E1451. [Google Scholar] [CrossRef] [PubMed]
- Linden, H.; Macino, G. White collar 2, a partner in blue-light signal transduction, controlling expression of light-regulated genes in Neurospora crassa. EMBO J. 1997, 16, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J. Tools for Botrytis cinerea: New expression vectors make the gray mold fungus more accessible to cell biology approaches. Fungal Genet. Biol. 2012, 49, 483–497. [Google Scholar] [CrossRef]
- Hevia, M.A.; Canessa, P.; Müller-Esparza, H.; Larrondo, L.F. A circadian oscillator in the fungus Botrytis cinerea regulates virulence when infecting Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2015, 112, 8744–8749. [Google Scholar] [CrossRef]
- Hevia, M.A.; Canessa, P.; Larrondo, L.F. Circadian clocks and the regulation of virulence in fungi: Getting up to speed. Semin. Cell Dev. Biol. 2016, 57, 147–155. [Google Scholar] [CrossRef]
- Malzahn, E.; Ciprianidis, S.; Káldi, K.; Schafmeier, T.; Brunner, M. Photoadaptation in Neurospora by Competitive Interaction of Activating and Inhibitory LOV Domains. Cell 2010, 142, 762–772. [Google Scholar] [CrossRef]
- Chen, C.-H.; DeMay, B.S.; Gladfelter, A.S.; Dunlap, J.C.; Loros, J.J. Physical interaction between VIVID and white collar complex regulates photoadaptation in Neurospora. Proc. Natl. Acad. Sci. USA 2010, 107, 16715–16720. [Google Scholar] [CrossRef]
- Hunt, S.M.; Thompson, S.; Elvin, M.; Heintzen, C. VIVID interacts with the WHITE COLLAR complex and FREQUENCY-interacting RNA helicase to alter light and clock responses in Neurospora. Proc. Natl. Acad. Sci. USA 2010, 107, 16709–16714. [Google Scholar] [CrossRef] [PubMed]
- Salinas, F.; Rojas, V.; Delgado, V.; López, J.; Agosin, E.; Larrondo, L.F.; Idnurm, A. Fungal Light-Oxygen-Voltage Domains for Optogenetic Control of Gene Expression and Flocculation in Yeast. mBio 2018, 9, e00626-18. [Google Scholar] [CrossRef] [PubMed]
- Goffeau, A.; Barrell, B.G.; Bussey, H.; Davis, R.W.; Dujon, B.; Feldmann, H.; Galibert, F.; Hoheisel, J.D.; Jacq, C.; Johnston, M.; et al. Life with 6000 genes. Science 1996, 274, 546, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef]
- Cheng, P.; He, Q.; Yang, Y.; Wang, L.; Liu, Y. Functional conservation of light, oxygen, or voltage domains in light sensing. Proc. Natl. Acad. Sci. USA 2003, 100, 5938–5943. [Google Scholar] [CrossRef]
- Schwerdtfeger, C.; Linden, H. VIVID is a flavoprotein and serves as a fungal blue light photoreceptor for photoadaptation. EMBO J. 2003, 22, 4846–4855. [Google Scholar] [CrossRef]
- Wei, D.; Li, M.; Zhang, X.; Xing, L. An improvement of the site-directed mutagenesis Method by combination of megaprimer, one-side PCR and DpnI treatment. Anal. Biochem. 2004, 331, 401–403. [Google Scholar] [CrossRef]
- Oldenburg, K.R.; Vo, K.T.; Michaelis, S.; Paddon, C. Recombination-mediated PCR-directed plasmid construction in vivo in yeast. Nucleic Acids Res. 1997, 25, 451–452. [Google Scholar] [CrossRef]
- Shenoy, A.R.; Visweswariah, S.S. Site-directed mutagenesis using a single mutagenic oligonucleotide and DpnI digestion of template DNA. Anal. Biochem. 2003, 319, 335–336. [Google Scholar] [CrossRef]
- Rienzo, A.; Pascual-Ahuir, A.; Proft, M. The use of a real-time luciferase assay to quantify gene expression dynamics in the living yeast cell. Yeast 2012, 29, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Rojas, V.; Delgado, V.; Salinas, F.; Larrondo, L.F. Modular and Molecular Optimization of a LOV (Light–Oxygen–Voltage)-Based Optogenetic Switch in Yeast. Int. J. Mol. Sci. 2021, 22, 8538. [Google Scholar] [CrossRef] [PubMed]
- Devia, J.; Bastías, C.; Kessi-Pérez, E.I.; Villarroel, C.A.; De Chiara, M.; Cubillos, F.A.; Liti, G.; Martínez, C.; Salinas, F. Transcriptional Activity and Protein Levels of Horizontally Acquired Genes in Yeast Reveal Hallmarks of Adaptation to Fermentative Environments. Front. Genet. 2020, 11, 293. [Google Scholar] [CrossRef]
- Heintzen, C.; Loros, J.J.; Dunlap, J.C. The PAS Protein VIVID Defines a Clock-Associated Feedback Loop that Represses Light Input, Modulates Gating, and Regulates Clock Resetting. Cell 2001, 104, 453–464. [Google Scholar] [CrossRef]
- Zoltowski, B.D.; Schwerdtfeger, C.; Widom, J.; Loros, J.J.; Bilwes, A.M.; Dunlap, J.C.; Crane, B.R. Conformational Switching in the Fungal Light Sensor Vivid. Science 2007, 316, 1054–1057. [Google Scholar] [CrossRef]
- Nakasako, M.; Iwata, T.; Matsuoka, D.; Tokutomi, S. Light-Induced Structural Changes of LOV Domain-Containing Polypeptides from Arabidopsis Phototropin 1 and 2 Studied by Small-Angle X-ray Scattering. Biochemistry 2004, 43, 14881–14890. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Yang, Y.; Wang, L.; He, Q.; Liu, Y. WHITE COLLAR-1, a Multifunctional NeurosporaProtein Involved in the Circadian Feedback Loops, Light Sensing, and Transcription Repression of wc-2. J. Biol. Chem. 2003, 278, 3801–3808. [Google Scholar] [CrossRef]
- Foley, B.J.; Stutts, H.; Schmitt, S.L.; Lokhandwala, J.; Nagar, A.; Zoltowski, B.D. Characterization of a Vivid Homolog in Botrytis cinerea. Photochem. Photobiol. 2018, 94, 985–993. [Google Scholar] [CrossRef]
- Glantz, S.T.; Berlew, E.E.; Jaber, Z.; Schuster, B.S.; Gardner, K.H.; Chow, B.Y. Directly light-regulated binding of RGS-LOV photoreceptors to anionic membrane phospholipids. Proc. Natl. Acad. Sci. USA 2018, 115, E7720–E7727. [Google Scholar] [CrossRef]
- Bodvard, K.; Peeters, K.; Roger, F.; Romanov, N.; Igbaria, A.; Welkenhuysen, N.; Palais, G.; Reiter, W.; Toledano, M.B.; Käll, M.; et al. Light-sensing via hydrogen peroxide and a peroxiredoxin. Nat. Commun. 2017, 8, 14791. [Google Scholar] [CrossRef]
- Chen, C.-H.; Ringelberg, C.S.; Gross, R.H.; Dunlap, J.C.; Loros, J.J. Genome-wide analysis of light-inducible responses reveals hierarchical light signalling in Neurospora. EMBO J. 2009, 28, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.; Simon, A.; Cohrs, K.C.; Viaud, M.; Tudzynski, P. The Transcription Factor BcLTF1 Regulates Virulence and Light Responses in the Necrotrophic Plant Pathogen Botrytis cinerea. PLoS Genet. 2014, 10, e1004040. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Sancar, G.; Dekhang, R.; Sullivan, C.M.; Li, S.; Tag, A.G.; Sancar, C.; Bredeweg, E.L.; Priest, H.D.; McCormick, R.F.; et al. Transcription factors in light and circadian clock signaling networks revealed by genomewide mapping of direct targets for neurospora white collar complex. Eukaryot. Cell 2010, 9, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Brandhoff, B.; Simon, A.; Dornieden, A.; Schumacher, J. Regulation of conidiation in Botrytis cinerea involves the light-responsive transcriptional regulators BcLTF3 and BcREG1. Curr. Genet. 2017, 63, 931–949. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Yañez, C.; Sánchez, E.; Pérez-Lara, G.; Seguel, A.; Camejo, P.Y.; Larrondo, L.F.; Vidal, E.A.; Canessa, P. A comprehensive transcription factor and DNA-binding motif resource for the construction of gene regulatory networks in Botrytis cinerea and Trichoderma atroviride. Comput. Struct. Biotechnol. J. 2021, 19, 6212–6228. [Google Scholar] [CrossRef]
- Ballario, P.; Talora, C.; Galli, D.; Linden, H.; Macino, G. Roles in dimerization and blue light photoresponse of the PAS and LOV domains of Neurospora crassa white collar proteins. Mol. Microbiol. 1998, 29, 719–729. [Google Scholar] [CrossRef]
- Rojas, V.; Salinas, F.; Guzman-Zamora, L.; Romero, A.; Delgado, V.; Larrondo, L.F. Exploiting Fungal Photobiology as a Source of Novel Bio-blocks for Optogenetic Systems. In Genetics and Biotechnology; Benz, J.P., Schipper, K., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 297–318. [Google Scholar]


| Protein | Gene ID | Protein Length (aa) | DNA Binding Domain (aa) | LOV Domain (aa) | LOV Domain Cys (aa) | PAS Domain (aa) |
|---|---|---|---|---|---|---|
| BcWCL1 | Bcin02g07400 | 1137 | 932-984 | 375–493 | 414 | 571–670; 697–791 |
| BcWCL2 | Bcin05g05530 | 509 | 448–500 | - | - | 146–244 |
| NcWC-1 | NCU02356 | 1167 | 928–987 | 389–505 | 428 | 585–684; 705–800 |
| NcWC-2 | NCU00902 | 530 | 462–514 | - | - | 162–255 |
| NcVVD | NCU03967 | 186 | - | 73–182 | 108 | - |
| PHOT1 | AT3G45780 | 996 | - | 485–577 (LOV2) | 512 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas, V.; Salinas, F.; Romero, A.; Larrondo, L.F.; Canessa, P. Interactions between Core Elements of the Botrytis cinerea Circadian Clock Are Modulated by Light and Different Protein Domains. J. Fungi 2022, 8, 486. https://doi.org/10.3390/jof8050486
Rojas V, Salinas F, Romero A, Larrondo LF, Canessa P. Interactions between Core Elements of the Botrytis cinerea Circadian Clock Are Modulated by Light and Different Protein Domains. Journal of Fungi. 2022; 8(5):486. https://doi.org/10.3390/jof8050486
Chicago/Turabian StyleRojas, Vicente, Francisco Salinas, Andrés Romero, Luis F. Larrondo, and Paulo Canessa. 2022. "Interactions between Core Elements of the Botrytis cinerea Circadian Clock Are Modulated by Light and Different Protein Domains" Journal of Fungi 8, no. 5: 486. https://doi.org/10.3390/jof8050486
APA StyleRojas, V., Salinas, F., Romero, A., Larrondo, L. F., & Canessa, P. (2022). Interactions between Core Elements of the Botrytis cinerea Circadian Clock Are Modulated by Light and Different Protein Domains. Journal of Fungi, 8(5), 486. https://doi.org/10.3390/jof8050486






