Therapeutic Potential of Green Synthesized Gold Nanoparticles Using Extract of Leptadenia hastata against Invasive Pulmonary Aspergillosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Plant Extract
2.2. Green Synthesis of LH-AuNPs
2.3. Characterization of LH-AuNPs
2.4. Microorganism and Culture Conditions
2.5. Antifungal Susceptibility Test
2.6. Pigment Inhibition
2.7. Time–Kill Curve Studies
2.8. Morphological Modifications
2.8.1. Scanning Electron Microscopy (SEM)
2.8.2. Transmission Electron Microscopy (TEM)
2.9. In Vivo Experiment Design
2.10. Measurement of Fungal Burden in Lung Tissue
2.11. Measurement of Gliotoxin
2.12. Cell Culture and Cytotoxicity Assay
2.13. Histological Study
2.14. Biochemical Assays
2.15. Statistical Analysis
3. Results
3.1. Biosynthesis and Characterization of LH-AuNPs
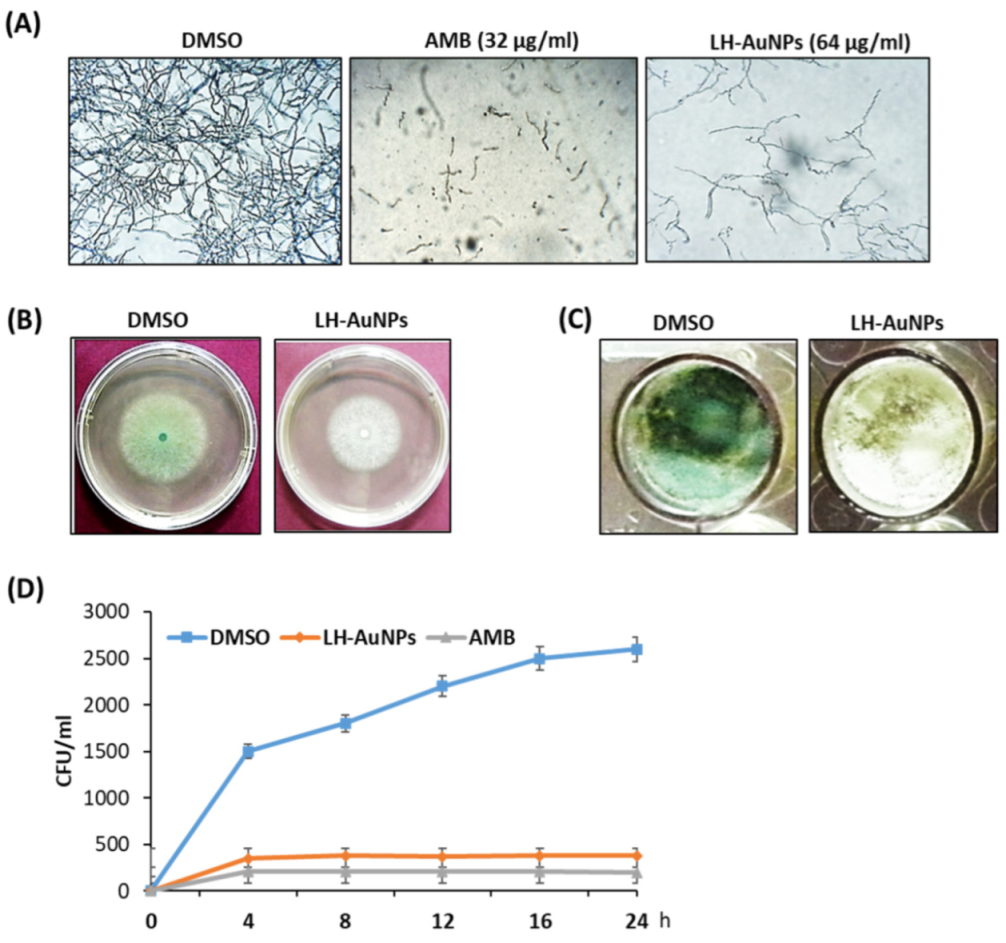
3.2. In Vitro Antifungal Activity of LH-AuNPs against A. fumigatus
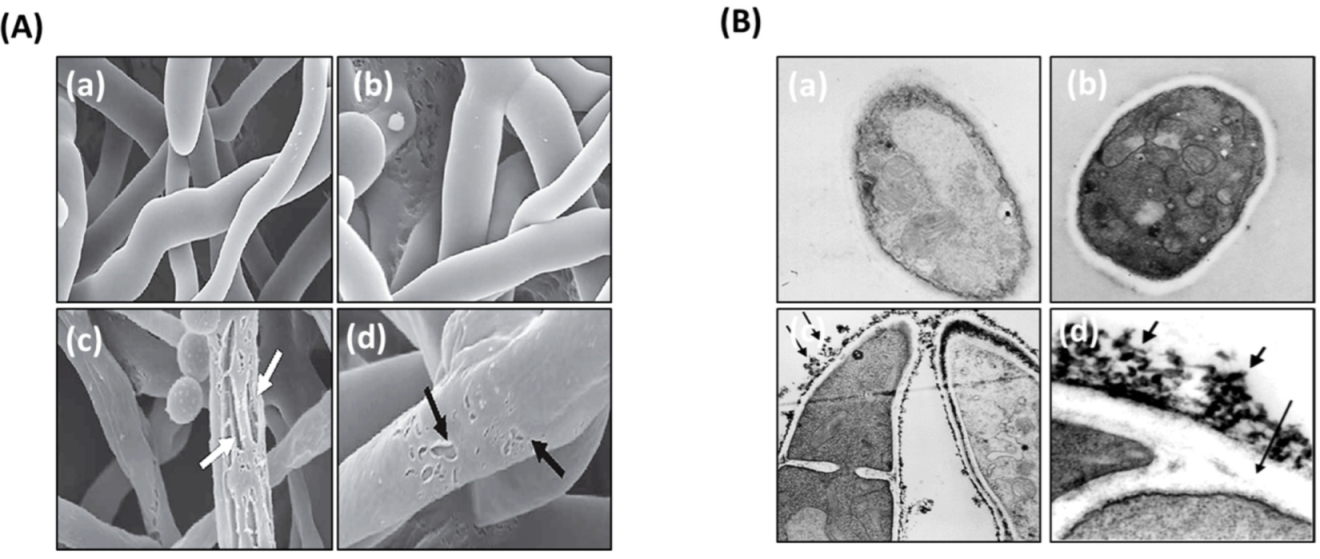
3.3. Ultrastructural Analysis of the Interaction between LH-AuNPs and A. fumigatus Cells Using TEM and SEM
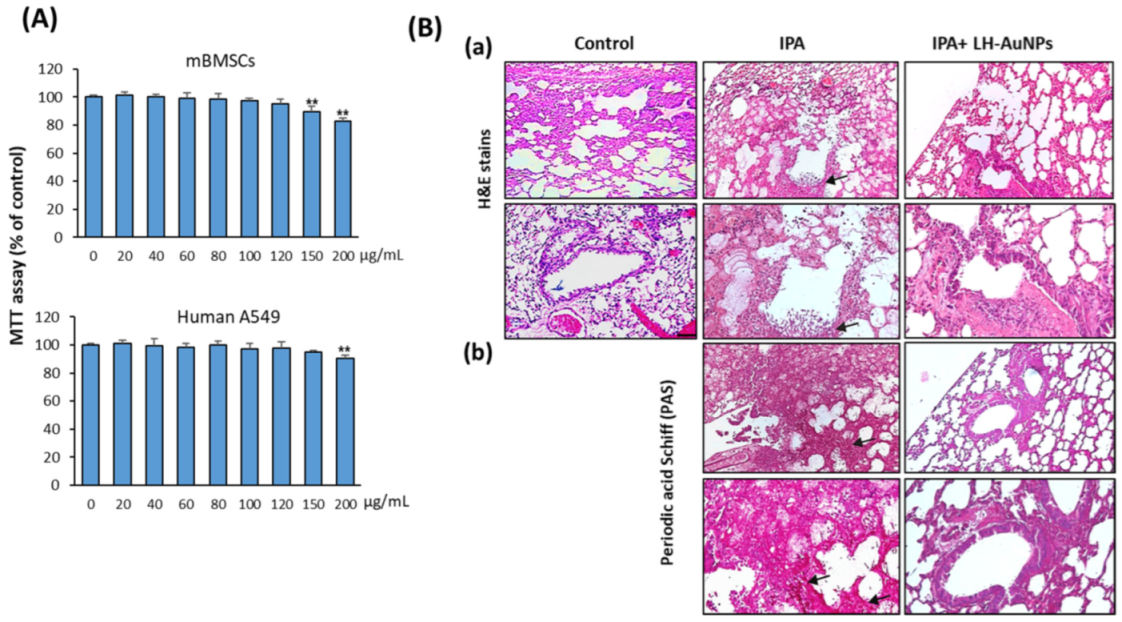
3.4. LH-AuNPs Show No In Vitro Cell Toxicity on Animal and Human Cells
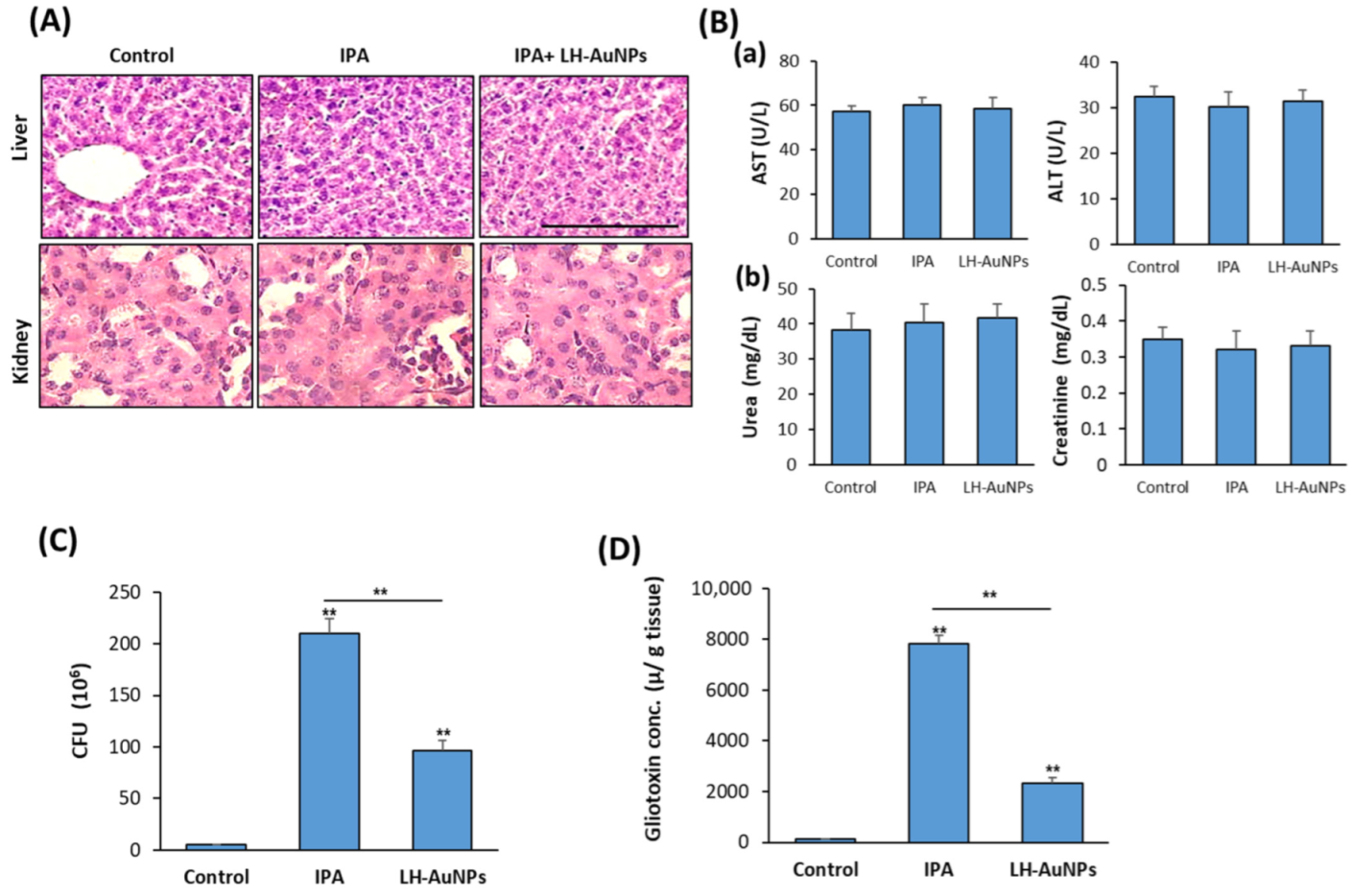
3.5. LH-AuNPs Effectively Repair Lung Tissue Damage in IPA Mice without Any In Vivo Toxicity
3.6. LH-AuNPs Excert Significant Reduction of A. fumigatus Colonization and Gliotoxin Production
3.7. LH-AuNPs Significantly Reduce Inflammation and Oxidative Stress in IPA Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zarrinfar, H.; Mirhendi, H.; Fata, A.; Khodadadi, H.; Kordbacheh, P. Detection of Aspergillus flavus and A. fumigatus in Bronchoalveolar Lavage Specimens of Hematopoietic Stem Cell Transplants and Hematological Malignancies Patients by Real-Time Polymerase Chain Reaction, Nested PCR and Mycological Assays. Jundishapur J. Microbiol. 2015, 8, e13744. [Google Scholar] [PubMed]
- Kashefi, E.; Seyedi, S.J.; Zomorodian, K.; Zare Shahrabadi, Z.; Zarrinfar, H. Successful treatment of pulmonary aspergillosis due to Aspergillus fumigatus in a child affected by systemic lupus erythematosus: A case report from Northeastern Iran. Clin. Case Rep. 2021, 9, e04248. [Google Scholar] [CrossRef] [PubMed]
- Seidler, M.J.; Salvenmoser, S.; Müller, F.M. Aspergillus fumigatus forms biofilms with reduced antifungal drug susceptibility on bronchial epithelial cells. Antimicrob. Agents Chemother. 2008, 52, 4130–4136. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, Q.; Huang, J.; Li, L. Risk factors for invasive pulmonary aspergillosis and hospital mortality in acute-on-chronic liver failure patients: A retrospective-cohort study. Int. J. Med. Sci. 2013, 10, 1625–1631. [Google Scholar] [CrossRef]
- Groll, A.H.; Piscitelli, S.C.; Walsh, T.J. Clinical pharmacology of systemic antifungal agents: A comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv. Pharmacol. 1998, 44, 343–500. [Google Scholar]
- Jenks, J.D.; Hoenigl, M. Treatment of Aspergillosis. J. Fungi 2018, 4, 98. [Google Scholar] [CrossRef]
- Correa-Royero, J.; Tangarife Castaño, V.; Durán, D.; Stashenko, E.; Mesa, A. In vitro antifungal activity and cytotoxic effect of essential oils and extracts of medicinal and aromatic plants against Candida krusei and Aspergillus fumigatus. Rev. Bras. De Farmacogn. 2010, 20, 734. [Google Scholar] [CrossRef]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Pissuwan, D.; Niidome, T.; Cortie, M.B. The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J. Control. Release Off. J. Control. Release Soc. 2011, 149, 65–71. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Sibuyi, N.R.S.; Moabelo, K.L.; Fadaka, A.O.; Meyer, S.; Onani, M.O.; Madiehe, A.M.; Meyer, M. Multifunctional Gold Nanoparticles for Improved Diagnostic and Therapeutic Applications: A Review. Nanoscale Res. Lett. 2021, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ma, X.-L.; Gu, Y.; Huang, H.; Zhang, G.-W. Green Synthesis of Metallic Nanoparticles and Their Potential Applications to Treat Cancer. Front. Chem. 2020, 8, 799. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.M.; Abdallah, B.M. Effective Inhibition of Invasive Pulmonary Aspergillosis by Silver Nanoparticles Biosynthesized with Artemisia sieberi Leaf Extract. Nanomaterials 2021, 12, 51. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Ali, E.M. Green Synthesis of Silver Nanoparticles Using the Lotus lalambensis Aqueous Leaf Extract and Their Anti-Candidal Activity against Oral Candidiasis. ACS Omega 2021, 6, 8151–8162. [Google Scholar] [CrossRef]
- Freiberger, C.E.; Vanderjagt, D.J.; Pastuszyn, A.; Glew, R.S.; Mounkaila, G.; Millson, M.; Glew, R.H. Nutrient content of the edible leaves of seven wild plants from Niger. Plant Foods Hum. Nutr. 1998, 53, 57–69. [Google Scholar] [CrossRef]
- Sena, L.P.; Vanderjagt, D.J.; Rivera, C.; Tsin, A.T.; Muhamadu, I.; Mahamadou, O.; Millson, M.; Pastuszyn, A.; Glew, R.H. Analysis of nutritional components of eight famine foods of the Republic of Niger. Plant Foods Hum. Nutr. 1998, 52, 17–30. [Google Scholar] [CrossRef]
- Mansurah, A.; Salisu Maiwada, A.; Sa’id, I.; Jafar Musa, M.A. Isolation and Characterization of a Potential Angiotensin-Converting Enzyme Inhibitory Peptide from the Leaves of Leptadenia hastata (Asclepiadaceae). Malays. J. Appl. Sci. 2017, 2, 35–47. [Google Scholar]
- Malgwi, S.A.; Zango, M.K.; Mbaya, A.W.; Dennis, G.; Kyari, F.; Sanda, K.A.; Balami, S.B.; Bwala, A.D. Anti-trypanosomal activity of crude root extract of Leptadenia hastata (Pers) dec.ne in Wistar rats infected with Trypanosoma brucei brucei and associated hematological changes. J. Adv. Vet. Anim. Res. 2019, 6, 241–246. [Google Scholar] [CrossRef]
- Umaru, I. Antifungal Activity of Leptadenia hastata (Pers) Decne Leaves Extract. Int. J. Pure Appl. Biosci. 2017, 5, 14–18. [Google Scholar] [CrossRef]
- Umaru, I. Phytochemical, antifungal and antibacterial potential of Leptadenia hastata stem-bark extract. MOJ Toxicol. 2018, 4, 263–268. [Google Scholar]
- Manavathu, E.K.; Cutright, J.L.; Loebenberg, D.; Chandrasekar, P.H. A comparative study of the in vitro susceptibilities of clinical and laboratory-selected resistant isolates of Aspergillus spp. to amphotericin B, itraconazole, voriconazole and posaconazole (SCH 56592). J. Antimicrob. Chemother. 2000, 46, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Meletiadis, J.; Meis, J.F.; Mouton, J.W.; Donnelly, J.P.; Verweij, P.E. Comparison of NCCLS and 3-(4,5-dimethyl-2-Thiazyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) methods of in vitro susceptibility testing of filamentous fungi and development of a new simplified method. J. Clin. Microbiol. 2000, 38, 2949–2954. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Thakur, R.; Chaudhury, A. An in vitro study of the antifungal activity of silver/chitosan nanoformulations against important seed borne pathogens. Int. J. Sci. Technol. Res. 2012, 1, 83–86. [Google Scholar]
- Achar, P.N.; Quyen, P.; Adukwu, E.C.; Sharma, A.; Msimanga, H.Z.; Nagaraja, H.; Sreenivasa, M.Y. Investigation of the Antifungal and Anti-Aflatoxigenic Potential of Plant-Based Essential Oils against Aspergillus flavus in Peanuts. J. Fungi 2020, 6, 383. [Google Scholar] [CrossRef]
- Sbaraglia, G.; D’Errico, P.; Serafini, S.; Vecchiarelli, L.; Perito, S. Pathogenicity of various species of Candida in mice immunodepressed with cyclophosphamide. Boll. Della Soc. Ital. Di Biol. Sper. 1984, 60, 1421–1426. [Google Scholar]
- Fahmy, S.R.; Ali, E.M.; Ahmed, N.S. Therapeutic effect of Sepia ink extract against invasive pulmonary aspergillosis in mice. J. Basic Appl. Zool. 2014, 67, 196–204. [Google Scholar] [CrossRef]
- Botelho, D.; Leo, B.F.; Massa, C.; Sarkar, S.; Tetley, T.; Chung, K.F.; Chen, S.; Ryan, M.P.; Porter, A.; Atochina-Vasserman, E.N.; et al. Exposure to Silver Nanospheres Leads to Altered Respiratory Mechanics and Delayed Immune Response in an in Vivo Murine Model. Front. Pharmacol. 2018, 9, 213. [Google Scholar] [CrossRef]
- Boudra, H.; Morgavi, D. Mycotoxin risk evaluation in feeds contamined by Aspergillus fumigatus. Anim. Feed Sci. Technol. 2005, 120, 113–123. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Alzahrani, A.M.; Abdel-Moneim, A.M.; Ditzel, N.; Kassem, M. A simple and reliable protocol for long-term culture of murine bone marrow stromal (mesenchymal) stem cells that retained their in vitro and in vivo stemness in long-term culture. Biol. Proced. Online 2019, 21, 3. [Google Scholar] [CrossRef]
- Abdallah, B.M. Ali EM: 5′-hydroxy Auraptene stimulates osteoblast differentiation of bone marrow-derived mesenchymal stem cells via a BMP-dependent mechanism. J. Biomed. Sci. 2019, 26, 51. [Google Scholar] [CrossRef] [PubMed]
- Teranishi, Y.; Tanaka, A.; Osumi, M.; Fukui, S. Catalase Activities of Hydrocarbon-utilizing Candida Yeasts. Agric. Biol. Chem. 1974, 38, 1213–1220. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Das, T.; Mishra, S.; Nag, S.; Saha, K.D. Green-synthesized gold nanoparticles from black tea extract enhance the chemosensitivity of doxorubicin in HCT116 cells via a ROS-dependent pathway. RSC Adv. 2022, 12, 8996–9007. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef]
- Ahn, S.; Singh, P.; Jang, M.; Kim, Y.J.; Castro-Aceituno, V.; Simu, S.Y.; Kim, Y.J.; Yang, D.C. Gold nanoflowers synthesized using Acanthopanacis cortex extract inhibit inflammatory mediators in LPS-induced RAW264.7 macrophages via NF-κB and AP-1 pathways. Colloids Surf. B Biointerfaces 2018, 162, 398–404. [Google Scholar] [CrossRef]
- Das, R.K.; Babu, P.J.; Gogoi, N.; Sharma, P.; Bora, U. Microwave-Mediated Rapid Synthesis of Gold Nanoparticles Using Calotropis procera Latex and Study of Optical Properties. ISRN Nanomater. 2012, 2012, 650759. [Google Scholar] [CrossRef]
- Salunke, G.R.; Ghosh, S.; Santosh Kumar, R.J.; Khade, S.; Vashisth, P.; Kale, T.; Chopade, S.; Pruthi, V.; Kundu, G.; Bellare, J.R.; et al. Rapid efficient synthesis and characterization of silver, gold, and bimetallic nanoparticles from the medicinal plant Plumbago zeylanica and their application in biofilm control. Int. J. Nanomed. 2014, 9, 2635–2653. [Google Scholar]
- Shervani, Z.; Taisuke, Y.; Ifuku, S.; Saimoto, H.; Morimoto, M. Preparation of Gold Nanoparticles Loaded Chitin Nanofiber Composite. Adv. Nanopart. 2012, 1, 71–78. [Google Scholar] [CrossRef]
- Shakibaie, M.; Forootanfar, H.; Mollazadeh-Moghaddam, K.; Bagherzadeh, Z.; Nafissi-Varcheh, N.; Shahverdi, A.R.; Faramarzi, M.A. Green synthesis of gold nanoparticles by the marine microalga Tetraselmis suecica. Biotechnol. Appl. Biochem. 2010, 57, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Meng, Y.; Zhu, H.; Hu, Y.; Xu, C.P.; Chao, X.; Li, W.; Li, C.; Pan, C. Green Synthesized Gold Nanoparticles Using Viola betonicifolia Leaves Extract: Characterization, Antimicrobial, Antioxidant, and Cytobiocompatible Activities. Int. J. Nanomed. 2021, 16, 7319–7337. [Google Scholar] [CrossRef] [PubMed]
- Thanighaiarassu, R.R.; Sivamai, P.; Devika, R.; Nambikkairaj, B. Green Synthesis of Gold Nanoparticles Characterization by using Plant Essential Oil Menthapiperita and their Antifungal Activity against Human Pathogenic Fungi. J. Nanomed. Nanotechnol. 2014, 5, 1. [Google Scholar]
- Al-Radadi, N.S. Facile one-step green synthesis of gold nanoparticles (AuNp) using licorice root extract: Antimicrobial and anticancer study against HepG2 cell line. Arab. J. Chem. 2021, 14, 102956. [Google Scholar] [CrossRef]
- Carmona, E.M.; Limper, A.H. Overview of Treatment Approaches for Fungal Infections. Clin. Chest Med. 2017, 38, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Umaru, I.; Ahmad, F.; Wakawa, H.; Aduwamai, U.; Umaru, K. Antifungal Potential of Leptadenia Hastata Against Some Pathogenic Fungi. Am. J. Biochem. Biotechnol. 2018, 14, 57–60. [Google Scholar] [CrossRef][Green Version]
- Bayala, B.; Maria, H.; Ouédraogo, A.; Keiler, A.; Tamboura, H. Leptadenia hastata Pers. (Decne) a Promising Source for Natural Compounds in Biomedical Applications. Am. J. Drug Discov. Dev. 2018, 8, 1–10. [Google Scholar]
- Imam, I.U.; Salihu Abdallah, M.; Ali, M. Antibacterial Activity of Leptadenia Hastata Leaves Extracts against Some Gastro-Intestinal Isolates. Arch. Biomed. Eng. Biotechnol. 2019, 1, 1–15. [Google Scholar]
- Haruna, A.; Mann, A.; Ogbadoyi, E.O. Phytochemical composition and antitrypanosomal activity of the leaf extract of Leptadenia hastata (Pers) Decne. Bayero J. Pure Appl. Sci. 2018, 10, 292. [Google Scholar] [CrossRef]
- Gutiérrez, J.A.; Caballero, S.; Díaz, L.A.; Guerrero, M.A.; Ruiz, J.; Ortiz, C.C. High Antifungal Activity against Candida Species of Monometallic and Bimetallic Nanoparticles Synthesized in Nanoreactors. ACS Biomater. Sci. Eng. 2018, 4, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Hamad, K.; Mahmoud, N.; Al-Dabash, S.; Abd Al-Samad, L.; Abdallah, M.; Al-Bakri, A. Fluconazole conjugated-gold nanorods as an antifungal nanomedicine with low cytotoxicity against human dermal fibroblasts. RSC Adv. 2020, 10, 25889–25897. [Google Scholar] [CrossRef]
- Tharwat, N.; Al-Bedak, O.; Hamouda, R.; Shreif, R.; Mounir, R.; Sami, A. Antifungal effect of gold nanoparticles on fungi isolated from onychomycosis patients. Al-Azhar J. Pharm. Sci. 2019, 60, 26–42. [Google Scholar]
- Belozerskaya, T.; Gessler, N.; Aver‘yanov, A. Melanin Pigments of Fungi; Springer: Cham, Switzerland, 2015; pp. 1–29. [Google Scholar]
- Pihet, M.; Vandeputte, P.; Tronchin, G.; Renier, G.; Saulnier, P.; Georgeault, S.; Mallet, R.; Chabasse, D.; Symoens, F.; Bouchara, J.-P. Melanin is an essential component for the integrity of the cell wall of Aspergillus fumigatus conidia. BMC Microbiol. 2009, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, E.S. Pathogenic roles for fungal melanins. Clin. Microbiol. Rev. 2000, 13, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Nizet, V. Color me bad: Microbial pigments as virulence factors. Trends Microbiol. 2009, 17, 406–413. [Google Scholar] [CrossRef]
- Li, J.J.; Hartono, D.; Ong, C.N.; Bay, B.H.; Yung, L.Y. Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials 2010, 31, 5996–6003. [Google Scholar] [CrossRef]
- Ng, C.-T.; Li, J.; Gurung, R.; Hande, P.; Ong, C.-N.; Bay, B.-H.; Yung, L.-Y. Toxicological profile of small airway epithelial cells exposed to gold nanoparticles. Exp. Biol. Med. 2013, 238, 1355–1361. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef]
- Patra, H.K.; Banerjee, S.; Chaudhuri, U.; Lahiri, P.; Dasgupta, A.K. Cell selective response to gold nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 111–119. [Google Scholar] [CrossRef]
- Bailly, A.L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.A. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci. Rep. 2019, 9, 12890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nie, X.; Ji, Y.; Liu, Y.; Wu, X.; Chen, C.; Fang, X. Quantitative Biokinetics and Systemic Translocation of Various Gold Nanostructures Are Highly Dependent on Their Size and Shape. J. Nanosci. Nanotechnol. 2014, 14, 4124–4138. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, R.; Yang, R.; Liu, J.; Tang, J.; Liu, Y.; Li, J.; Chai, Z.; Chen, C. Size- and surface chemistry-dependent pharmacokinetics and tumor accumulation of engineered gold nanoparticles after intravenous administration. Met. Integr. Biometal Sci. 2015, 7, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Hof, H.; Kupfahl, C. Gliotoxin in Aspergillus fumigatus: An example that mycotoxins are potential virulence factors. Mycotoxin Res. 2009, 25, 123–131. [Google Scholar] [CrossRef]
- Gayathri, L.; Akbarsha, M.A.; Ruckmani, K. In vitro study on aspects of molecular mechanisms underlying invasive aspergillosis caused by gliotoxin and fumagillin, alone and in combination. Sci. Rep. 2020, 10, 14473. [Google Scholar] [CrossRef]
- Amarsaikhan, N.; Tsoggerel, A.; Hug, C.; Templeton, S.P. The Metabolic Cytokine Adiponectin Inhibits Inflammatory Lung Pathology in Invasive Aspergillosis. J. Immunol. 2019, 203, 956–963. [Google Scholar] [CrossRef]
- Schelenz, S.; Smith, D.A.; Bancroft, G.J. Cytokine and chemokine responses following pulmonary challenge with Aspergillus fumigatus: Obligatory role of TNF-alpha and GM-CSF in neutrophil recruitment. Med. Mycol. 1999, 37, 183–194. [Google Scholar] [CrossRef]
- Heinekamp, T.; Schmidt, H.; Lapp, K.; Pähtz, V.; Shopova, I.; Köster-Eiserfunke, N.; Krüger, T.; Kniemeyer, O.; Brakhage, A.A. Interference of Aspergillus fumigatus with the immune response. Semin. Immunopathol. 2015, 37, 141–152. [Google Scholar] [CrossRef]
- Al-Bader, N.; Sheppard, D.C. Aspergillosis and stem cell transplantation: An overview of experimental pathogenesis studies. Virulence 2016, 7, 950–966. [Google Scholar] [CrossRef]
- Zhu, S.; Jiang, X.; Boudreau, M.D.; Feng, G.; Miao, Y.; Dong, S.; Wu, H.; Zeng, M.; Yin, J.-J. Orally administered gold nanoparticles protect against colitis by attenuating Toll-like receptor 4- and reactive oxygen/nitrogen species-mediated inflammatory responses but could induce gut dysbiosis in mice. J. Nanobiotechnol. 2018, 16, 86. [Google Scholar] [CrossRef]
- Haupenthal, D.; Possato, J.C.; Zaccaron, R.P.; Mendes, C.; Rodrigues, M.S.; Nesi, R.T.; Pinho, R.A.; Feuser, P.E.; Machado-de-Ávila, R.A.; Comim, C.M.; et al. Effects of chronic treatment with gold nanoparticles on inflammatory responses and oxidative stress in Mdx mice. J. Drug Target 2020, 28, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Park, H.H. Pleiotropic functions of antioxidant nanoparticles for longevity and medicine. Adv. Colloid Interface Sci. 2013, 201–202, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.-I.; Byun, M.-S.; Jue, D.-M. Gold compound auranofin inhibits IκB kinase (IKK) by modifying Cys-179 of IKKβ subunit. Exp. Mol. Med. 2003, 35, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Zysman, M.; Elgrabli, D.; Murayama, T.; Haruta, M.; Lanone, S.; Ishida, T.; Boczkowski, J. Anti-inflammatory effect of gold nanoparticles supported on metal oxides. Sci. Rep. 2021, 11, 23129. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, M.J. Nano-gold displayed anti-inflammatory property via NF-kB pathways by suppressing COX-2 activity. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1149–1158. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, B.M.; Ali, E.M. Therapeutic Potential of Green Synthesized Gold Nanoparticles Using Extract of Leptadenia hastata against Invasive Pulmonary Aspergillosis. J. Fungi 2022, 8, 442. https://doi.org/10.3390/jof8050442
Abdallah BM, Ali EM. Therapeutic Potential of Green Synthesized Gold Nanoparticles Using Extract of Leptadenia hastata against Invasive Pulmonary Aspergillosis. Journal of Fungi. 2022; 8(5):442. https://doi.org/10.3390/jof8050442
Chicago/Turabian StyleAbdallah, Basem M., and Enas M. Ali. 2022. "Therapeutic Potential of Green Synthesized Gold Nanoparticles Using Extract of Leptadenia hastata against Invasive Pulmonary Aspergillosis" Journal of Fungi 8, no. 5: 442. https://doi.org/10.3390/jof8050442
APA StyleAbdallah, B. M., & Ali, E. M. (2022). Therapeutic Potential of Green Synthesized Gold Nanoparticles Using Extract of Leptadenia hastata against Invasive Pulmonary Aspergillosis. Journal of Fungi, 8(5), 442. https://doi.org/10.3390/jof8050442





