Promising Antifungal Activity of Encephalartos laurentianus de Wild against Candida albicans Clinical Isolates: In Vitro and In Vivo Effects on Renal Cortex of Adult Albino Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Materials
2.2. Isolation and Identification of C. albicans Isolates
2.3. Animals
2.4. LC-MS/MS for Metabolite Profiling
2.5. In Vitro Antifungal Activity of ELME
2.5.1. Disc Agar Diffusion
2.5.2. Determination of Minimum Inhibitory Concentration (MIC)
2.5.3. Biofilm Formation Assay
2.5.4. Antibiofilm Activity of ELME
- a.
- Scanning electron microscope (SEM)
- b.
- Fluorescent microscope
- c.
- Quantitative real-time polymerase chain reaction (qRT-PCR)
2.6. In Vivo Antifungal Activity of ELME
2.6.1. Experimental Protocol
- Group I (control group): 15 rats each received 1 mL saline (0.9%) by intraperitoneal (IP) injection once daily for 1 week;
- Group II (C. albicans group, C6 isolate, with the concentration of 2 × 105 CFU/mL): 15 rats each received a suspension of C. albicans (0.1 mL) by intravenous (IV) injection in the tail vein once;
- Group III (fluconazole treated group): 15 rats each received a suspension of C. albicans (0.1 mL) by IV injection in the tail vein once. Then, this group received 10 mg/kg fluconazole by IP injection for 1 week;
- Group IV (ELME treated group, 50 mg/kg): 15 rats received a suspension of C. albicans (0.1 mL) by IV injection in the tail vein once. Then, this group received 50 mg/kg ELME by IP injection for 1 week;
- Group V (ELME treated group, 100 mg/kg): 15 rats received a suspension of C. albicans (0.1 mL) by IV injection in the tail vein once. Then, this group received 100 mg/kg ELME by IP injection for 1 week;
- Rats were monitored for 15 days after infection to determine the survival rate. Ether inhalation was used to anesthetize all rats to be sacrificed. Kidney tissues were obtained and processed for histological and immunohistochemical investigations. In addition, fungal tissue burden (number of CFU/g kidney tissues) was calculated.
2.6.2. Histopathological and Immunohistochemical Studies
2.6.3. Morphometric Measurements
2.7. Cytotoxicity MTT Assay
2.8. Statistical Analysis
3. Results
3.1. LC-ESI-MS/MS of ELME
3.1.1. Identification of Flavonoids, Biflavonoids, and Catechins
3.1.2. Identification of Anthocyanidin-O-Glycosides
3.1.3. Identification of Carboxylic or Fatty, or Phenolic Acids Derivatives
3.1.4. Identification of Alkaloids Derivatives
3.1.5. Identification of Coumarins Derivatives
3.1.6. Identification of Other Derivatives
3.2. In Vitro Antifungal Activity of ELME
3.3. In Vitro Antibiofilm Activity of ELME
3.3.1. Biofilm Viability
3.3.2. SEM Examination
3.3.3. Fluorescent Microscope Examination
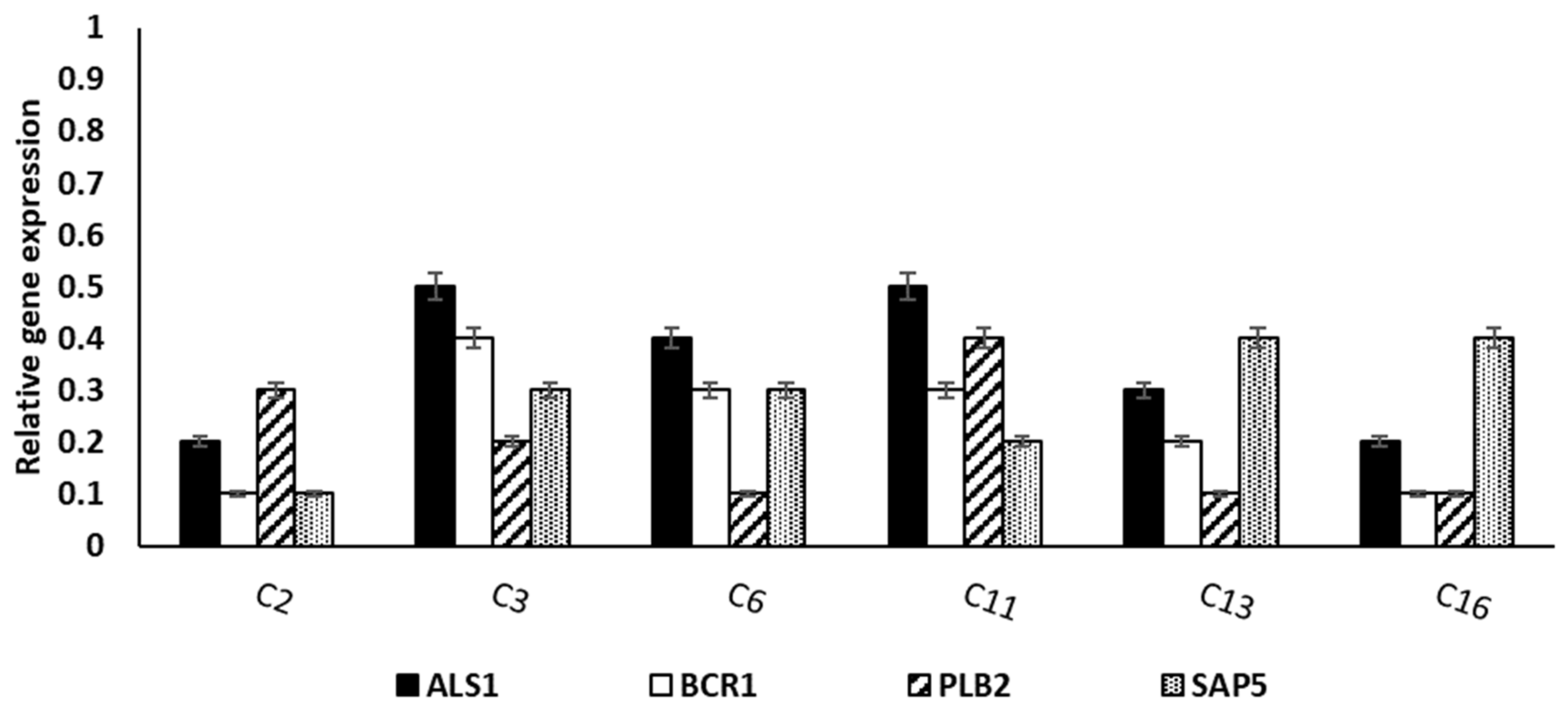
3.3.4. qRT-PCR
3.4. In Vivo Antibiofilm Activity of ELME
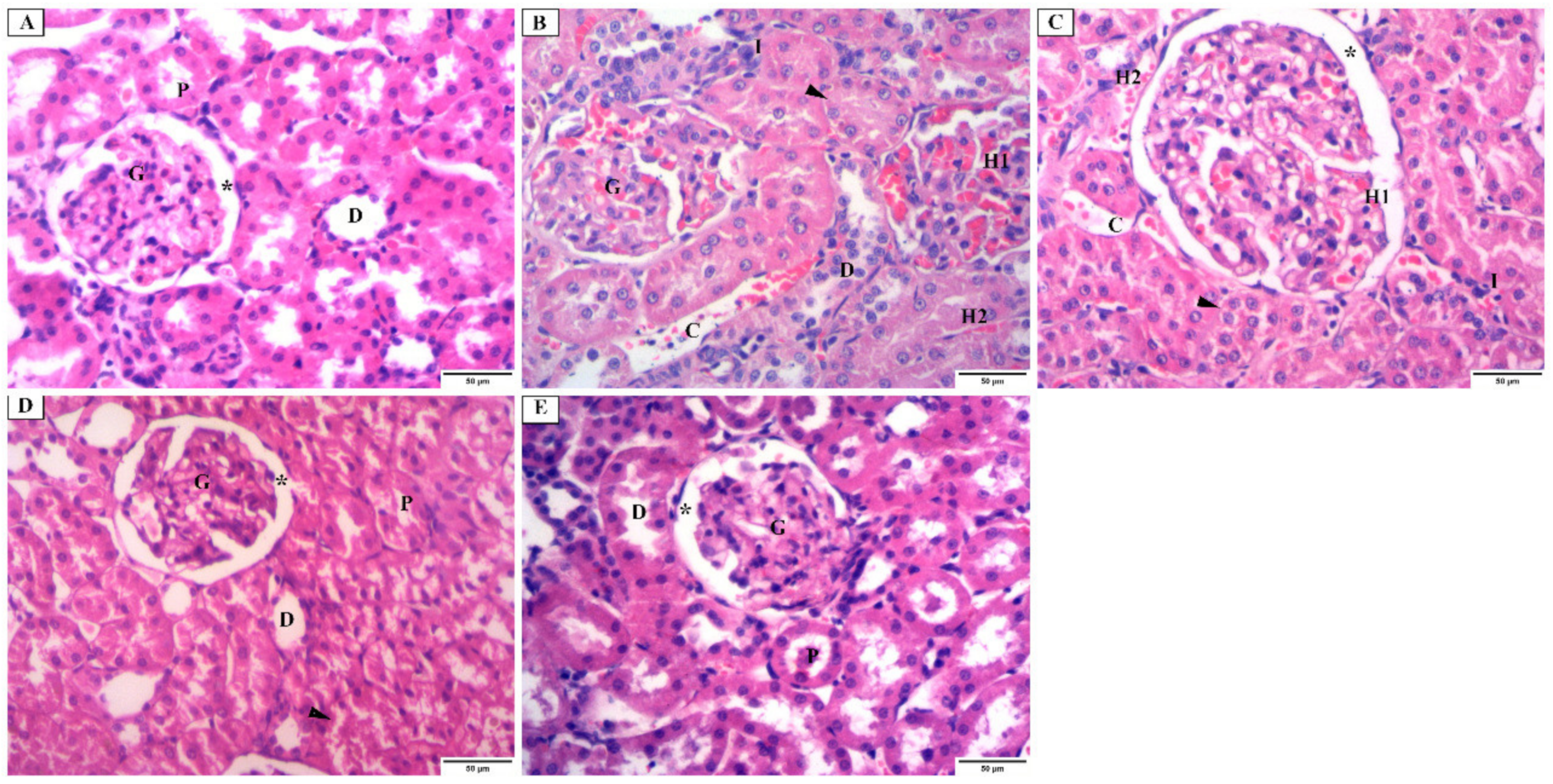
3.4.1. Histopathological Examination Using H&E Stain
3.4.2. Masson Trichrome Stain
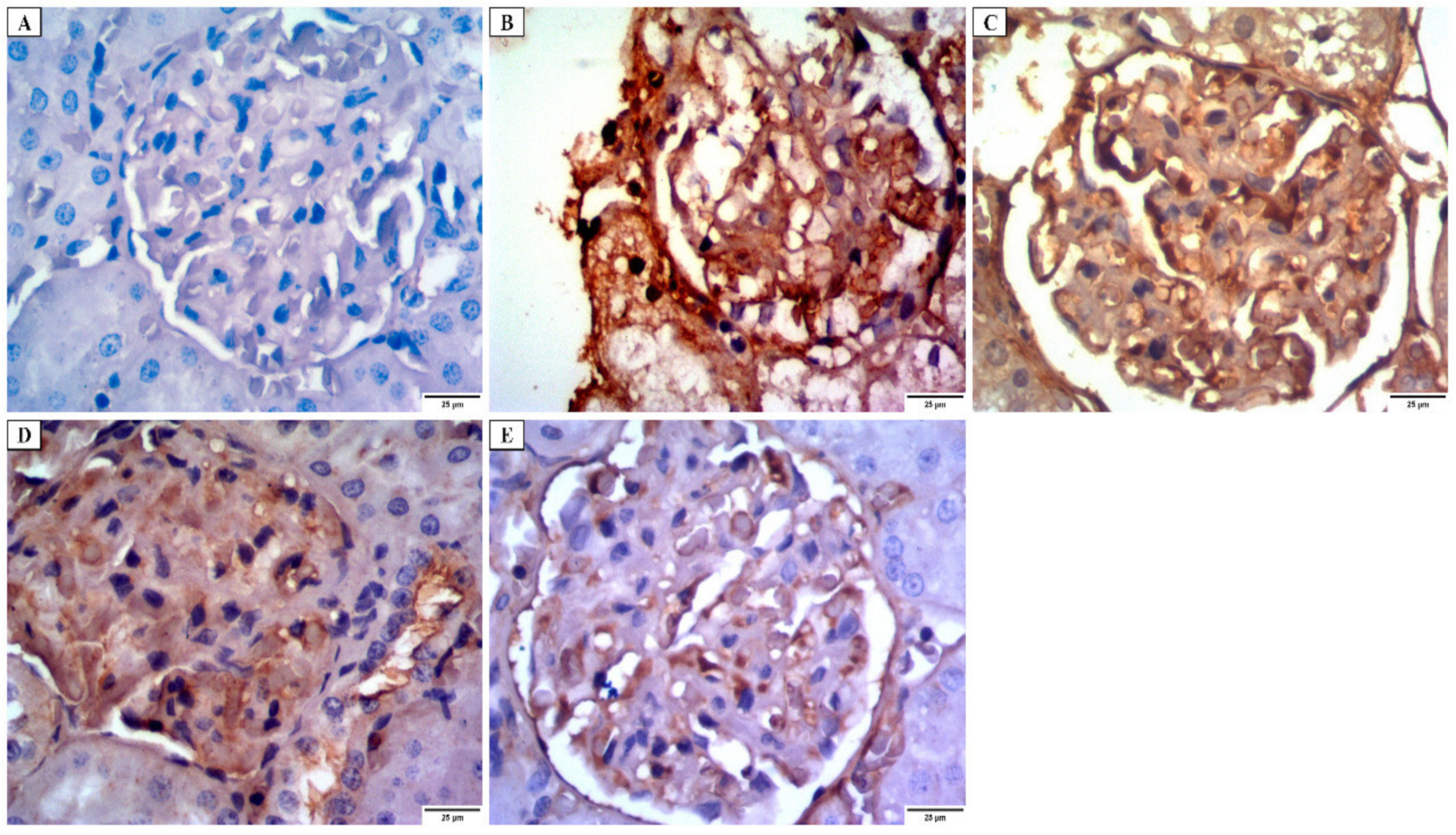
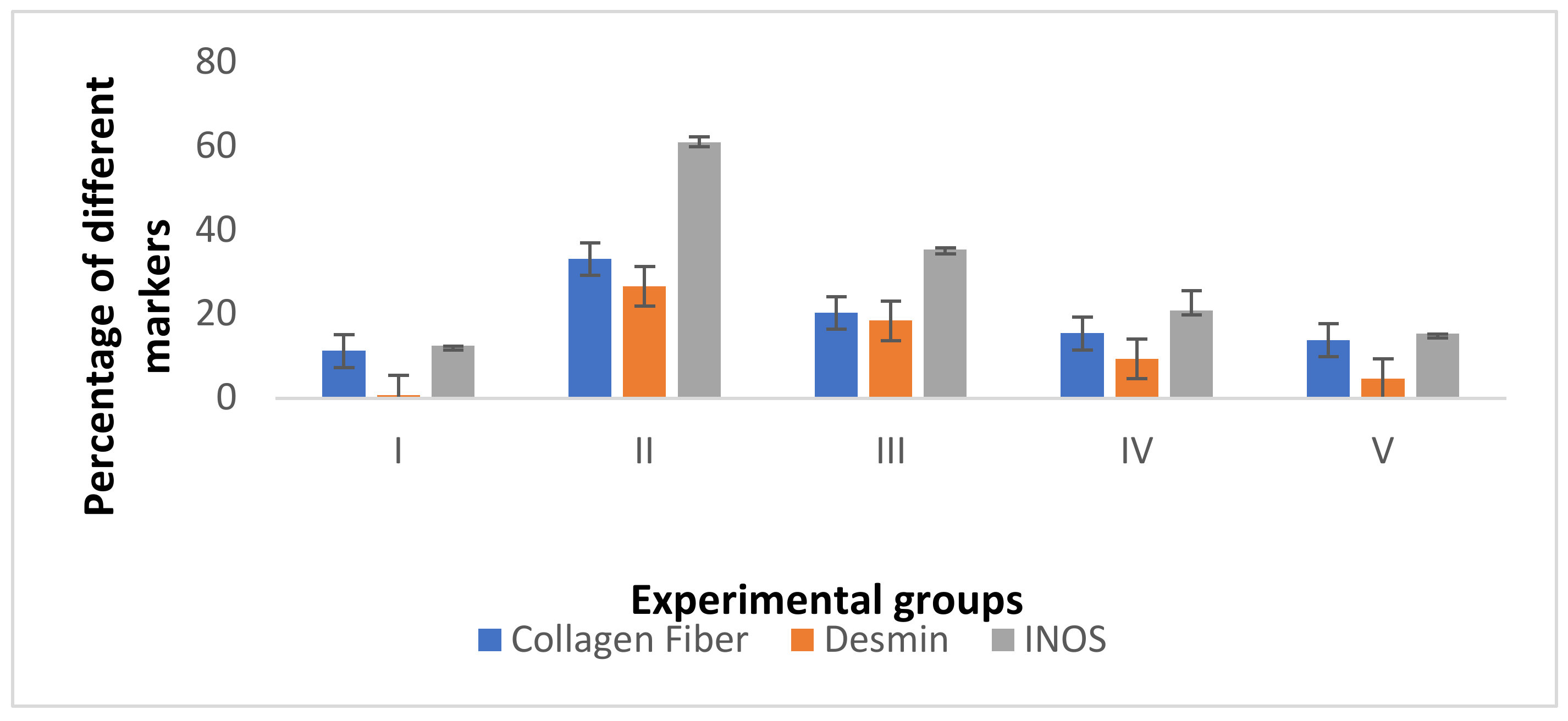
3.4.3. Immunohistochemical Investigations
- a.
- Desmin immunostaining
- b.
- Alkaline phosphatase enzyme immunostaining
- c.
- iNOs immunostaining
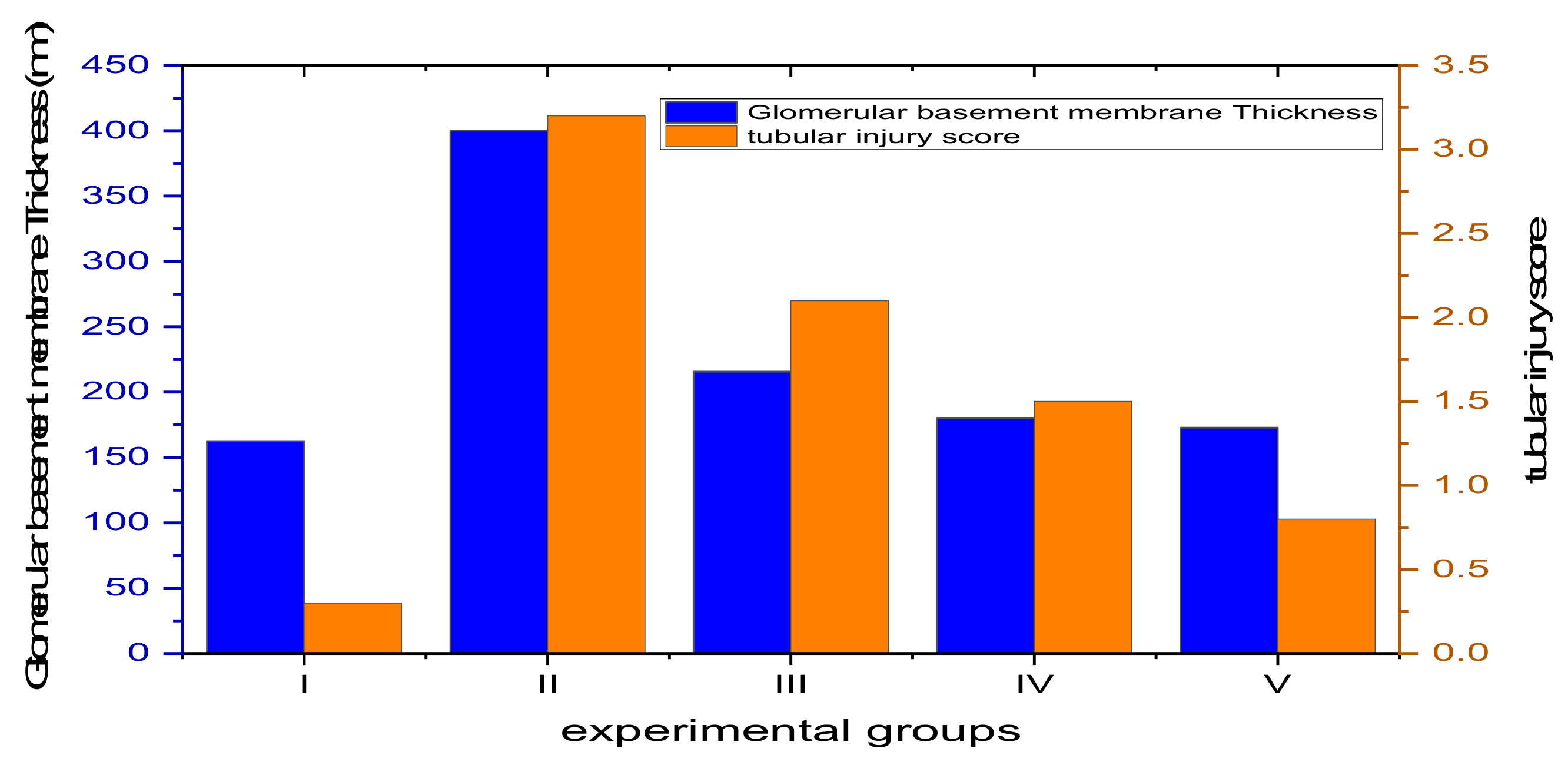
3.4.4. Morphometric Analysis
3.4.5. Counting the CFU/g and Determination of the Survival Rates
3.5. Cytotoxicity MTT Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans—The virulence factors and clinical manifestations of infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Tóth, R.; Gácser, A. Mechanisms of pathogenic Candida species to evade the host complement attack. Front. Cell. Infect. Microbiol. 2020, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Kernien, J.F.; Snarr, B.D.; Sheppard, D.C.; Nett, J.E. The interface between fungal biofilms and innate immunity. Front. Immunol. 2018, 8, 1968. [Google Scholar] [CrossRef]
- Hamdy, R.F.; Zaoutis, T.E.; Seo, S.K. Antifungal stewardship considerations for adults and pediatrics. Virulence 2017, 8, 658–672. [Google Scholar] [CrossRef]
- Sousa, F.; Ferreira, D.; Reis, S.; Costa, P. Current insights on antifungal therapy: Novel nanotechnology approaches for drug delivery systems and new drugs from natural sources. Pharmaceuticals 2020, 13, 248. [Google Scholar] [CrossRef]
- Bhuiyan, F.R.; Howlader, S.; Raihan, T.; Hasan, M. Plants metabolites: Possibility of natural therapeutics against the COVID-19 pandemic. Front. Med. 2020, 7, 444. [Google Scholar] [CrossRef]
- Azari, B.; Zahmatkesh Moghadam, S.; Zarrinfar, H.; Tasbandi, A.; Jamialahmadi, T.; Sahebkar, A. Antifungal Activity of Curcuminoids and Difluorinated Curcumin Against Clinical Isolates of Candida Species. In Natural Products and Human Diseases; Springer: Berlin/Heidelberg, Germany, 2021; pp. 123–129. [Google Scholar]
- Zarrinfar, H.; Behnam, M.; Hatamipour, M.; Sahebkar, A. Antifungal Activities of Curcuminoids and Difluorinated Curcumin Against Clinical Dermatophyte Isolates. In Pharmacological Properties of Plant-Derived Natural Products and Implications for Human Health; Springer: Berlin/Heidelberg, Germany, 2021; pp. 101–107. [Google Scholar]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef]
- Nigussie, D.; Davey, G.; Tufa, T.B.; Brewster, M.; Legesse, B.A.; Fekadu, A.; Makonnen, E. Antibacterial and antifungal activities of Ethiopian medicinal plants: A systematic review. Front. Pharmacol. 2021, 12, 1327. [Google Scholar] [CrossRef]
- Treutlein, J.; Vorster, P.; Wink, M. Molecular relationships in Encephalartos (Zamiaceae, Cycadales) based on nucleotide sequences of nuclear ITS 1&2, rbcL, and genomic ISSR fingerprinting. Plant Biol. 2005, 7, 79–90. [Google Scholar]
- Negm, W.; Abo El-Seoud, K.; Kabbash, A.; El-Aasr, M. Investigation of the Biological Activity Some Gymnosperm Plants Belong to Cycadales Order. J. Adv. Med. Pharm. Res. 2020, 1, 9–13. [Google Scholar] [CrossRef]
- Negm, W.A.; Abo El-Seoud, K.A.; Kabbash, A.; Kassab, A.A.; El-Aasr, M. Hepatoprotective, cytotoxic, antimicrobial and antioxidant activities of Dioon spinulosum leaves Dyer Ex Eichler and its isolated secondary metabolites. Nat. Prod. Res. 2020, 35, 5166–5176. [Google Scholar] [CrossRef] [PubMed]
- Negm, W.A.; El-Kadem, A.H.; Elekhnawy, E.; Attallah, N.G.; Al-Hamoud, G.A.; El-Masry, T.A.; Zayed, A. Wound-Healing Potential of Rhoifolin-Rich Fraction Isolated from Sanguisorba officinalis Roots Supported by Enhancing Re-Epithelization, Angiogenesis, Anti-Inflammatory, and Antimicrobial Effects. Pharmaceuticals 2022, 15, 178. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, B.; Mokhtar, F.A.; El-Masry, T.A.; Elekhnawy, E.; Mostafa, S.A.; Abdelkader, D.H.; Elharty, M.E.; Saleh, A.; Negm, W.A. Antimicrobial Activity of Brassica rapa L. Flowers Extract on Gastrointestinal Tract Infections and Antiulcer Potential against Indomethacin-Induced Gastric Ulcer in Rats Supported by Metabolomics Profiling. J. Inflamm. Res. 2021, 14, 7411. [Google Scholar] [CrossRef]
- Attallah, N.G.M.; Negm, W.A.; Elekhnawy, E.; Elmongy, E.I.; Altwaijry, N.; El-Haroun, H.; El-Masry, T.A.; El-Sherbeni, S.A. Elucidation of Phytochemical Content of Cupressus macrocarpa Leaves: In Vitro and In Vivo Antibacterial Effect against Methicillin-Resistant Staphylococcus aureus Clinical Isolates. Antibiotics 2021, 10, 890. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; 20th Informational Supplement; CLSI Document; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Elekhnawy, E.; Sonbol, F.; Abdelaziz, A.; Elbanna, T. An investigation of the impact of triclosan adaptation on Proteus mirabilis clinical isolates from an Egyptian university hospital. Braz. J. Microbiol. 2021, 52, 927–937. [Google Scholar] [CrossRef]
- El-Ganiny, A.M.; Kamel, H.A.; Yossef, N.E.; Mansour, B.; El-Baz, A.M. Repurposing pantoprazole and haloperidol as efflux pump inhibitors in azole resistant clinical Candida albicans and non-albicans isolates. Saudi Pharm. J. 2022, 30, 245–255. [Google Scholar] [CrossRef]
- Elekhnawy, E.; Negm, W.A.; El-Aasr, M.; Kamer, A.A.; Alqarni, M.; Batiha, G.E.-S.; Obaidullah, A.J.; Fawzy, H.M. Histological assessment, anti-quorum sensing, and anti-biofilm activities of Dioon spinulosum extract: In vitro and in vivo approach. Sci. Rep. 2022, 12, 180. [Google Scholar] [CrossRef]
- Attallah, N.G.; Negm, W.A.; Elekhnawy, E.; Altwaijry, N.; Elmongy, E.I.; El-Masry, T.A.; Alturki, E.A.; Yousef, D.A.; Y Shoukheba, M. Antibacterial Activity of Boswellia sacra Flueck. Oleoresin Extract against Porphyromonas gingivalis Periodontal Pathogen. Antibiotics 2021, 10, 859. [Google Scholar] [CrossRef]
- Pu, Y.; Liu, A.; Zheng, Y.; Ye, B. In vitro damage of Candida albicans biofilms by chitosan. Exp. Ther. Med. 2014, 8, 929–934. [Google Scholar] [CrossRef]
- Li, H.; Gong, H.; Qi, Y.; Li, J.; Ji, X.; Sun, J.; Tian, R.; Bao, H.; Song, X.; Chen, Q. In vitro and in vivo antifungal activities and mechanism of heteropolytungstates against Candida species. Sci. Rep. 2017, 7, 16942. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, J.A. Histological and histochemical methods: Theory and practice. Shock 1999, 12, 479. [Google Scholar]
- Pollock, L.; Rampling, D.; Greenwald, S.; Malone, M. Desmin expression in rhabdomyosarcoma: Influence of the desmin clone and immunohistochemical method. J. Clin. Pathol. 1995, 48, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Ramos-Vara, J.A.; Kiupel, M.; Baszler, T.; Bliven, L.; Brodersen, B.; Chelack, B.; West, K.; Czub, S.; Del Piero, F.; Dial, S. Suggested guidelines for immunohistochemical techniques in veterinary diagnostic laboratories. J. Vet. Diagn. Investig. 2008, 20, 393–413. [Google Scholar] [CrossRef]
- Chen, J.; Ren, J.; Loo, W.T.; Hao, L.; Wang, M. Lysyl oxidases expression and histopathological changes of the diabetic rat nephron. Mol. Med. Rep. 2018, 17, 2431–2441. [Google Scholar] [CrossRef]
- White, S. Methods of evidence-based medicine and decision analysis. In Basic & Clinical Biostatistics, 5th ed.; McGraw-Hill Education: New York, NY, USA, 2020. [Google Scholar]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- De Barros, P.P.; Rossoni, R.D.; Garcia, M.T.; de Lima Kaminski, V.; Loures, F.V.; Fuchs, B.B.; Mylonakis, E.; Junqueira, J.C. The Anti-Biofilm Efficacy of Caffeic Acid Phenethyl Ester (CAPE) In Vitro and a Murine Model of Oral Candidiasis. Front. Cell. Infect. Microbiol. 2021, 11, 700305. [Google Scholar] [CrossRef]
- Bonifácio, B.V.; Vila, T.V.M.; Masiero, I.F.; da Silva, P.B.; da Silva, I.C.; de Oliveira Lopes, É.; Ramos, M.A.D.S.; de Souza, L.P.; Vilegas, W.; Pavan, F.R.; et al. Antifungal Activity of a Hydroethanolic Extract from Astronium urundeuva Leaves against Candida albicans and Candida glabrata. Front. Microbiol. 2019, 10, 2642. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, S.; Tewari, R.; Bhatt, V.P.; Sharma, J.; Maurya, I.K. Phytochemical analysis and mode of action against Candida glabrata of Paeonia emodi extracts. J. Mycol. Med. 2018, 28, 443–451. [Google Scholar] [CrossRef]
- Diba, A.; Alizadeh, F. In vitro and in vivo antifungal activity of Allium hirtifolium and Allium sativum. Avicenna J. Phytomed. 2018, 8, 465. [Google Scholar]
- Alalwan, H.; Rajendran, R.; Lappin, D.F.; Combet, E.; Shahzad, M.; Robertson, D.; Nile, C.J.; Williams, C.; Ramage, G. The anti-adhesive effect of curcumin on Candida albicans biofilms on denture materials. Front. Microbiol. 2017, 8, 659. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Zago, C.E.; Silva, S.; Sanitá, P.V.; Barbugli, P.A.; Dias, C.M.I.; Lordello, V.B.; Vergani, C.E. Dynamics of biofilm formation and the interaction between Candida albicans and methicillin-susceptible (MSSA) and -resistant Staphylococcus aureus (MRSA). PLoS ONE 2015, 10, e0123206. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Petropoulos, S.; Ferreira, I.C. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre-and post-harvest conditions: A review. Food Chem. 2016, 211, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Abu-Darwish, M.; Cabral, C.; Gonçalves, M.; Cavaleiro, C.; Cruz, M.; Zulfiqar, A.; Khan, I.; Efferth, T.; Salgueiro, L. Chemical composition and biological activities of Artemisia judaica essential oil from southern desert of Jordan. J. Ethnopharmacol. 2016, 191, 161–168. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K.-Y. Hedera rhombea inhibits the biofilm formation of Candida, thereby increases the susceptibility to antifungal agent, and reduces infection. PLoS ONE 2021, 16, e0258108. [Google Scholar] [CrossRef]
- Song, Z.; Papanicolaou, N.; Dean, S.; Bing, Z. Localized candidiasis in kidney presented as a mass mimicking renal cell carcinoma. Case Rep. Infect. Dis. 2012, 2012, 953590. [Google Scholar] [CrossRef][Green Version]
- Khan, S.H.; Younus, H.; Allemailem, K.S.; Almatroudi, A.; Alrumaihi, F.; Alruwetei, A.M.; Alsahli, M.A.; Khan, A.; Khan, M.A. Potential of methylglyoxal-conjugated chitosan nanoparticles in treatment of fluconazole-resistant Candida albicans infection in a murine model. Int. J. Nanomed. 2020, 15, 3681. [Google Scholar] [CrossRef]
- Ayoub, I.M.; Korinek, M.; Hwang, T.-L.; Chen, B.-H.; Chang, F.-R.; El-Shazly, M.; Singab, A.N.B. Probing the antiallergic and anti-inflammatory activity of biflavonoids and dihydroflavonols from Dietes bicolor. J. Nat. Prod. 2018, 81, 243–253. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Souto, A.L.; Tavares, J.F.; Da Silva, M.S.; Diniz, M.D.F.F.M.; Athayde-Filho, D.; Filgueiras, P.; Barbosa Filho, J.M. Anti-inflammatory activity of alkaloids: An update from 2000 to 2010. Molecules 2011, 16, 8515–8534. [Google Scholar] [CrossRef] [PubMed]
- Sui, G.; Li, T.; Zhang, B.; Wang, R.; Hao, H.; Zhou, W. Recent advances on synthesis and biological activities of aurones. Bioorg. Med. Chem. 2021, 29, 115895. [Google Scholar] [CrossRef] [PubMed]
- Grover, J.; Jachak, S.M. Coumarins as privileged scaffold for anti-inflammatory drug development. RSC Adv. 2015, 5, 38892–38905. [Google Scholar] [CrossRef]
- Su, X.; Zhang, J.; Wang, H.; Xu, J.; He, J.; Liu, L.; Zhang, T.; Chen, R.; Kang, J. Phenolic acid profiling, antioxidant, and anti-inflammatory activities, and miRNA regulation in the polyphenols of 16 blueberry samples from China. Molecules 2017, 22, 312. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-W.; Wei, W.; Yang, X.-W.; Zhang, Y.-B.; Xu, W.; Yang, Y.-F.; Zhong, G.-Y.; Liu, H.-N.; Yang, S.-L. Anti-inflammatory phenolic acid esters from the roots and rhizomes of Notopterygium incisium and their permeability in the human Caco-2 monolayer cell model. Molecules 2017, 22, 935. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J. Anti-inflammatory activity of capsaicin and dihydrocapsaicin through heme oxygenase-1 induction in raw264. 7 macrophages. J. Food Biochem. 2014, 38, 381–387. [Google Scholar] [CrossRef]
- Uddin, M.J.; Xu, S.; Crews, B.C.; Aleem, A.M.; Ghebreselasie, K.; Banerjee, S.; Marnett, L.J. Harmaline analogs as substrate-selective cyclooxygenase-2 inhibitors. ACS Med. Chem. Lett. 2020, 11, 1881–1885. [Google Scholar] [CrossRef]
- Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal models of inflammation for screening of anti-inflammatory drugs: Implications for the discovery and development of phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 84367. [Google Scholar] [CrossRef]
- Jin, Y.-S. Recent advances in natural antifungal flavonoids and their derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 126589. [Google Scholar] [CrossRef]
- Buchmann, D.; Schultze, N.; Borchardt, J.; Böttcher, I.; Schaufler, K.; Guenther, S. Synergistic antimicrobial activities of epigallocatechin gallate, myricetin, daidzein, gallic acid, epicatechin, 3-hydroxy-6-methoxyflavone and genistein combined with antibiotics against ESKAPE pathogens. J. Appl. Microbiol. 2022, 132, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tang, X.; Liu, T.; Peng, F.; Zhou, Q.; Luo, H.; He, M.; Xue, W. Antimicrobial evaluation of myricetin derivatives containing benzimidazole skeleton against plant pathogens. Fitoterapia 2021, 149, 104804. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A review on flavonoid apigenin: Dietary intake, ADME, antimicrobial effects, and interactions with human gut microbiota. BioMed Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Liu, M.; Fu, Y.; Zhang, J.; Liu, W.; Li, J.; Li, X.; Li, Y.; Wang, T. Antimicrobial mechanism of luteolin against Staphylococcus aureus and Listeria monocytogenes and its antibiofilm properties. Microb. Pathog. 2020, 142, 104056. [Google Scholar] [CrossRef] [PubMed]
- Alejo-Armijo, A.; Glibota, N.; Frías, M.P.; Altarejos, J.; Gálvez, A.; Ortega-Morente, E.; Salido, S. Antimicrobial and antibiofilm activities of procyanidins extracted from laurel wood against a selection of foodborne microorganisms. Int. J. Food Sci. Technol. 2017, 52, 679–686. [Google Scholar] [CrossRef]
- Oliveira, H.; Correia, P.; Bessa, L.J.; Guimarães, M.; Gameiro, P.; Freitas, V.D.; Mateus, N.; Cruz, L.; Fernandes, I. Cyanidin-3-Glucoside Lipophilic Conjugates for Topical Application: Tuning the Antimicrobial Activities with Fatty Acid Chain Length. Processes 2021, 9, 340. [Google Scholar] [CrossRef]
- Negm, W.A.; El-Aasr, M.; Kamer, A.A.; Elekhnawy, E. Investigation of the Antibacterial Activity and Efflux Pump Inhibitory Effect of Cycas thouarsii R. Br. Extract against Klebsiella pneumoniae Clinical Isolates. Pharmaceuticals 2021, 14, 756. [Google Scholar] [CrossRef]
- Elmongy, E.I.; Negm, W.A.; Elekhnawy, E.; El-Masry, T.A.; Attallah, N.G.; Altwaijry, N.; Batiha, G.E.-S.; El-Sherbeni, S.A. Antidiarrheal and Antibacterial Activities of Monterey Cypress Phytochemicals: In Vivo and In Vitro Approach. Molecules 2022, 27, 346. [Google Scholar] [CrossRef]
- Patel, J. Synthesis, characterization and antimicrobial screening of some furofused coumarines. Int. J. Adv. Res. Dev. 2018, 3, 1–8. [Google Scholar]
- Widelski, J.; Luca, S.V.; Skiba, A.; Chinou, I.; Marcourt, L.; Wolfender, J.-L.; Skalicka-Wozniak, K. Isolation and antimicrobial activity of coumarin derivatives from fruits of Peucedanum luxurians Tamamsch. Molecules 2018, 23, 1222. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, X.; Xie, X.; Laster, K.V.; Pang, M.; Liu, K.; Hwang, J.; Kim, D.J. Harmaline isolated from Peganum harmala suppresses growth of esophageal squamous cell carcinoma through targeting mTOR. Phytother. Res. 2021, 35, 6377–6388. [Google Scholar] [CrossRef]
- Qaisar, U. Peganum harmala: A rich source of antimicrobial agents. Int. J. Biotech Trends Technol. 2019, 9, 1–10. [Google Scholar] [CrossRef]








| Peak NO | Identification | Error | R.T. (min.) | m/z | Adduct Ion | Formula | MS/MS |
|---|---|---|---|---|---|---|---|
| 1 | f.a Citraconic acid | −2.8 | 1.006 | 129.02 | [M − H]− | C5H6O4 | 84.99, 85.02, 129.01 |
| 2 | a Rosmarinic acid | 6.0 | 1.009 | 359.11 | [M − H]− | C18H16O8 | 181.04, 329.09, 359.07, 359.11 |
| 3 | a Malic acid | −4.7 | 1.331 | 133.10 | [M − H]− | C4H6O5 | 133.01 |
| 4 | t 3-Amino-1,2,4-triazole | −0.6 | 1.392 | 85.027 | [M + H]+ | C2H4N4 | 71.95, 85.02 |
| 5 | a 3-(4-hydroxyphenyl)prop-2-enoic acid | −0.5 | 1. 393 | 165.07 | [M + H]+ | C9H8O3 | 61.02, 85.02, 166.08 |
| 6 | s Resveratrol | 6.4 | 1.470 | 229.15 | [M + H]+ | C14H12O3 | 58.06, 60.08, 70.06, 229.15 |
| 7 | ak Harmaline | 0.6 | 1.561 | 215.05 | [M + H]+ | C13H14N2O | 72.08, 156.10, 169.13, 215.05 |
| 8 | ak Nicotinic acid | −0.9 | 1.693 | 124.03 | [M + H]+ | C6H5NO2 | 53.03, 78.03, 80.04, 124.03 |
| 9 | ak 5-Hydroxyindoleacetic acid | 0.8 | 1.3058 | 192.10 | [M + H]+ | C10H9NO3 | 148.07, 177.07, 192.10 |
| 10 | f.a Linoleic acid | 0.6 | 2.290 | 281.10 | [M + H]+ | C18H32O2 | 123.07, 151.04, 165.09, 281.05 |
| 11 | f.g Acacetin-7-O-neohesperidoside | −8.1 | 4.305 | 593.28 | [M + H]+ | C28H32O14 | 593.25 |
| 12 | f.g Kaempferol-3-Glucuronide | 10.1 | 5.108 | 461.16 | [M − H]− | C21H18O12 | 188.93, 239.09, 256.92, 324.90, 392.89, 461.15 |
| 13 | b Procyanidin B1 | 1.5 | 5.115 | 579.14 | [M + H]+ | C30H26O12 | 123.04, 127.03, 135.04, 579.14 |
| 14 | a Chlorogenic acid | 1.3 | 5.534 | 355.07 | [M + H]+ | C16H18O9 | 135.04, 163.04, 355.07 |
| 15 | co 6,7-dihydroxycoumarin | −0.4 | 5.439 | 179.03 | [M + H]+ | C9H6O4 | 51.02, 77.03 179.03 |
| 16 | f.g Isorhamnetin-3-O-rutinoside | −2.1 | 5.465 | 623.22 | [M − H]− | C28H32O16 | 315.03, 532.91, 579.15, 623.17 |
| 17 | c (+)-3 3′ 4′ 5 7-Pentahydroxyflavan | 3.6 | 5.574 | 291.08 | [M + H]+ | C15H14O6 | 111.04, 119.04, 123.04, 291.08 |
| 18 | f.g Baicalein-7-O-glucuronide | 4.2 | 5.575 | 447.16 | [M + H]+ | C21H18O11 | 125.13, 129.07, 135.04, 269.06, 273.08, 447.13 |
| 19 | f.g Luteolin-8-C-glucoside | −12.6 | 5.656 | 449.14 | [M + H]+ | C21H20O11 | 139.07, 449.12 |
| 20 | f.g Syringetin-3-O-galactoside | −5.2 | 5.693 | 507.17 | [M − H2O − H]− | C23H24O13 | 112.98, 138.02, 163.06, 218.95, 286.93, 307.10, 354.92, 507.16 |
| 21 | f.g Phlorizin | 2.7 | 5.707 | 435.22 | [M − H]− | C21H24O10 | 389.21, 433.83, 435.20, 435.23 |
| 22 | ak Dihydrocapsaicin | 3.3 | 5.974 | 308.18 | [M + H]+ | C18H29NO3 | 123.04, 131.04, 137.05, 149.05 |
| 23 | b Procyanidin B2 | 0.6 | 6.161 | 579.18 | [M + H]+ | C30H26O12 | 112.02, 579.18 |
| 24 | f.g Quercetin-4′-glucoside | −1.3 | 7.061 | 465.10 | [M + H]+ | C21H20O12 | 69.03, 115.04, 289.04, 303.04, 465.09 |
| 25 | f Myricetin | −1.5 | 7.063 | 317.06 | [M − H]− | C21H24O10 | 112.98, 155.03, 165.98, 180.96, 194.99, 274.04, 287.08, 302.07 |
| 26 | an Cyanidin-3-glucoside | 1.0 | 7.109 | 449.10 | [M]+ | C21H21O11 | 85.01, 117.02, 147.02, 201.02, 287.05, 449.10 |
| 27 | f.g Luteolin-3′,7-di-O-glucoside | −0.4 | 7.227 | 611.15 | [M + H]+ | C27H30O16 | 135.04, 148.10, 271.06, 273.06, 611.15 |
| 28 | f.g Daidzein-8-C-glucoside | −0.5 | 7.279 | 417.13 | [M + H]+ | C21H20O9 | 180.06, 399.21, 417.13 |
| 29 | f.g Isoquercitrin | −2.1 | 7.283 | 463.11 | [M − H]− | C21H20O12 | 48.17, 462.90 |
| 30 | an Petunidin-3-O-beta-glucopyranoside | 0.9 | 7.567 | 479.11 | [M]+ | C22H23O12 | 165.09, 211.12, 285.08, 299.10, 302.04, 479.11 |
| 31 | b Procyanidin C1 | 0.9 | 7.772 | 865.21 | [M-H]- | C45H38O18 | 11.46, 865.20 |
| 32 | f.g apigenin-7-O-glucoside | 3.5 | 7.891 | 433.11 | [M + H]+ | C21H20O10 | 119.04, 141.07, 148.11, 135.02, 229.04, 270.22, 433.11 |
| 33 | f.g Acacetin-7-O-rutinoside | 8.0 | 7.870 | 593.17 | [M + H]+ | C28H32O14 | 520.20, 575.32, 593.17 |
| 34 | tp Sabinene | 0.4 | 8.068 | 137.05 | [M + H]+ | C10H16 | 55.05, 66.04, 68.74, 79.05, 94.04, 137.05 |
| 35 | an Peonidine-3-O-glucoside chloride | −6.1 | 8.101 | 463.12 | [M]+ | C22H23O11 | 73.05,167.99, 197.47, 234.10, 258.04, 281.08, 286.04, 301.06, 309.47, 342.01, 399.15, 463.12 |
| 36 | co Daphnetin | −0.8 | 8.202 | 179.03 | [M + H]+ | C9H6O4 | 77.03, 104.99, 123.00, 133.02, 135.04, 151.038 |
| 37 | f.g Diosmin | −0.8 | 8.762 | 609.17 | [M + H]+ | C28H32O15 | 265.11, 303.11, 609.16 |
| 38 | au Maritimetin-6-O-glucoside | 0.8 | 8.827 | 449.16 | [M + H]+ | C21H20O11 | 74.09, 133.02,135.04, 257.04, 285.03, 360.05, 375.07, 388.05, 403.07, 417.09, 434.09, 449.12 |
| 39 | f.g Rhoifolin | 0.7 | 8.974 | 577.26 | [M − H]− | C27H30O14 | 576.81, 532.90, 269.10 |
| 40 | f.g Apigenin-6-C-glucoside -7-O-glucoside | −2.0 | 9.027 | 595.37 | [M + H]+ | C21H20O12 | 165.02, 177.06, 285.09, 303.05 |
| 41 | f 3′ 4′ 5 7-tetrahydroxyflavanone | 0.0 | 9.760 | 289.07 | [M + H]+ | C15H12O6 | 117.03, 121.06, 135.04, 139.03, 145.02, 153.01, 163.03, 179.03, 181.06, 289.07 |
| 42 | f Luteolin | 8.1 | 10.002 | 287.04 | [M + H]+ | C15H10O6 | 67.01, 77.04, 287.04 |
| 43 | an Cyanidin-3, 5-di-O-glucoside | −1.2 | 10.357 | 611.22 | [M]+ | C27H31O16 | 215.06, 266.99, 309.06, 355.06, 449.13, 594.23, 611.22 |
| 44 | f.g Luteolin-4′-O-glucoside | −3.9 | 10.370 | 449.14 | [M + H]+ | C21H20O11 | 147.04, 153.06, 167.02, 287.05, 287.10, 449.14 |
| 45 | f.g Rutin | 1.7 | 10.842 | 609.15 | [M − H]− | C27H30O16 | 609.15 |
| 46 | f Naringenin | 0.9 | 11.086 | 273.07 | [M + H]+ | C15H12O5 | 67.04, 111.08, 119.03, 125.10, 129.07, 135.01. 273.07 |
| 47 | f 3 5 7-trihydroxy-4′-methoxyflavone | 0.7 | 11.278 | 301.10 | [M + H]+ | C16H12O6 | 181.06, 215.07, 223.07, 258.08, 273.11, 301.11 |
| 48 | f 3′-Methoxy-4′,5,7-trihydroxyflavonol | 0.4 | 11.329 | 317.06 | [M + H]+ | C16H12O7 | 129.97, 137.02, 168.00, 245.04, 263.21, 274.04, 287.09, 302.04, 317.06 |
| 49 | ak Caffeine | −1.9 | 11.857 | 195.13 | [M + H]+ | C8H10N4O2 | 195.13 |
| 50 | f Formononetin | 5.9 | 12.010 | 269.07 | [M + H]+ | C16H12O4 | 137.02, 225.06, 254.06, 269.07 |
| 51 | f 4′,5-dihydroxy-7-methoxyflavone | −2.6 | 12.613 | 287.09 | [M + H]+ | C16H14O5 | 137.02, 145.08, 167.03, 175.07, 287.09 |
| 52 | f Acacetin | 1.0 | 12.776 | 285.08 | [M + H]+ | C16H12O5 | 128.06, 207.06, 241.04, 242.05, 270.05, 285.07 |
| 53 | f Apigenin | 0.8 | 12.788 | 271.09 | [M + H]+ | C15H10O5 | 65.04, 67.01, 68.99, 89.03, 109.02, 115.05, 153.02, 163.04, 253.14, 271.09 |
| 54 | f 4′,5,7-Trihydroxyflavonol | 0.9 | 14.059 | 287.09 | [M + H]+ | C15H10O6 | 67.02, 91.05, 111.04, 119.04, 124.01, 147.04, 167.03, 287.09 |
| 55 | f (+-)-Taxifolin | −0.1 | 14.290 | 305.13 | [M + H]+ | C15H12O7 | 305.13 |
| 56 | a Methyl dihydrojasmonate | −9.7 | 14.433 | 227.16 | [M + H]+ | C13H22O3 | 79.05, 95.08, 167.14, 195.14, 227.14 |
| 57 | f 3′ 4′ 5 7-tetrahydroxyflavanone | −1.4 | 14.553 | 289.18 | [M + H]+ | C15H12O6 | 271.17, 289.18 |
| 58 | f.g Apigenin 8-C-glucoside | −6.3 | 14.961 | 433.12 | [M + H]+ | C21H20O10 | 135.03, 391.09, 433.13 |
| 59 | ak Capsaicin | 0 | 15.293 | 306.20 | [M + H]+ | C18H27NO3 | 108.04, 126.02, 137.06, 153.12, 306.20 |
| 60 | f.g Quercetin-3-Arabinoside | −6.8 | 15.368 | 435.14 | [M + H]+ | C20H18O11 | 135.04, 240.04, 271.06, 389.10, 435.14 |
| 61 | an Cyanidin-3-O-(2″-O-β -xylopyranosyl-β-glucooside) | 8.6 | 17.917 | 581.13 | [M]+ | C26H29O15 | 107.04, 133.06, 135.04, 153.01, 297.07, 581.14 |
| 62 | c (-)-Epicatechin | 2.5 | 18.280 | 291.07 | [M + H]+ | C15H14O6 | 81.07, 135.05, 275.05, 291.07 |
| 63 | co Esculin | −2.2 | 18.380 | 341.19 | [M + H]+ | C15H16O9 | 112.07, 121.14, 131.04, 139.08, 161.06, 165.08, 179.12, 180.13, 287.24, 341.19 |
| 64 | f 3 3′ 4′ 5-tetrahydroxy-7-methoxyflavone | −4.7 | 20.178 | 317.11 | [M + H]+ | C16H12O7 | 105.07, 129.07, 215.18, 267.20, 299.20, 317.11 |
| Biofilm Forming Ability | No. of Isolates before Treatment with ELME | No. of Isolates after Treatment with ELME |
|---|---|---|
| Non-biofilm forming | 4 | 6 |
| Weak biofilm-forming | 2 | 6 |
| Moderate biofilm-forming | 6 | 3 |
| Strong biofilm-forming | 4 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negm, W.A.; El-Aasr, M.; Attia, G.; Alqahtani, M.J.; Yassien, R.I.; Abo Kamer, A.; Elekhnawy, E. Promising Antifungal Activity of Encephalartos laurentianus de Wild against Candida albicans Clinical Isolates: In Vitro and In Vivo Effects on Renal Cortex of Adult Albino Rats. J. Fungi 2022, 8, 426. https://doi.org/10.3390/jof8050426
Negm WA, El-Aasr M, Attia G, Alqahtani MJ, Yassien RI, Abo Kamer A, Elekhnawy E. Promising Antifungal Activity of Encephalartos laurentianus de Wild against Candida albicans Clinical Isolates: In Vitro and In Vivo Effects on Renal Cortex of Adult Albino Rats. Journal of Fungi. 2022; 8(5):426. https://doi.org/10.3390/jof8050426
Chicago/Turabian StyleNegm, Walaa A., Mona El-Aasr, Ghada Attia, Moneerah J. Alqahtani, Rania Ibrahim Yassien, Amal Abo Kamer, and Engy Elekhnawy. 2022. "Promising Antifungal Activity of Encephalartos laurentianus de Wild against Candida albicans Clinical Isolates: In Vitro and In Vivo Effects on Renal Cortex of Adult Albino Rats" Journal of Fungi 8, no. 5: 426. https://doi.org/10.3390/jof8050426
APA StyleNegm, W. A., El-Aasr, M., Attia, G., Alqahtani, M. J., Yassien, R. I., Abo Kamer, A., & Elekhnawy, E. (2022). Promising Antifungal Activity of Encephalartos laurentianus de Wild against Candida albicans Clinical Isolates: In Vitro and In Vivo Effects on Renal Cortex of Adult Albino Rats. Journal of Fungi, 8(5), 426. https://doi.org/10.3390/jof8050426









