Unravelling the Molecular Identification and Antifungal Susceptibility Profiles of Aspergillus spp. Isolated from Chronic Pulmonary Aspergillosis Patients in Jakarta, Indonesia: The Emergence of Cryptic Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aspergillus spp. Isolates
2.2. Molecular Identification
2.3. Antifungal Susceptibility Tests
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Isolate Identification
3.3. Antifungal Susceptibility Profiles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases—Estimate precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull. World Health Organ. 2011, 89, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Wahyuningsih, R.; Adawiyah, R.; Sjam, R.; Prihartono, J.; Ayu, E.; Wulandari, T.; Rozaliyani, A.; Ronny, R.; Imran, D.; Tugiran, M.; et al. Serious fungal disease incidence and prevalence in Indonesia. Mycoses 2021, 64, 1203–1212. [Google Scholar] [CrossRef]
- Setianingrum, F.; Rozaliyani, A.; Syam, R.; Adawiyah, R.; Tugiran, M.; Sari, C.Y.I.; Burhan, E.; Wahyuningsih, R.; Rautemaa-Richardson, R.; Denning, D.W. Evaluation and comparison of automated and manual ELISA for diagnosis of chronic pulmonary aspergillosis (CPA) in Indonesia. Diagn. Microbiol. Infect. Dis. 2020, 98, 15124. [Google Scholar] [CrossRef] [PubMed]
- Setianingrum, F.; Rozaliyani, A.; Adawiyah, R.; Syam, R.; Tugiran, M.; Sari, C.Y.I.; Nandipinto, F.; Ramnath, J.; Arifin, A.R.; Handayani, D.; et al. A prospective longitudinal study of chronic pulmonary aspergillosis in pulmonary tuberculosis in Indonesia (APICAL). Thorax 2021. [Google Scholar] [CrossRef]
- Nguyen, N.T.B.; Le Ngoc, H.; Nguyen, N.V.; Van Dinh, L.; Van Nguyen, H.; Nguyen, H.T.; Denning, D.W. Chronic pulmonary aspergillosis situation among post tuberculosis patients in vietnam: An observational study. J. Fungi 2021, 7, 532. [Google Scholar] [CrossRef] [PubMed]
- Tone, K.; Suzuki, J.; Alshahni, M.M.; Kuwano, K.; Makimura, K. Species-specific detection of medically important aspergilli by a loop-mediated isothermal amplification method in chronic pulmonary aspergillosis. Med. Mycol. 2019, 57, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Page, I.D.; Chakaya, J.; Jabeen, K.; Jude, C.M.; Cornet, M.; Alastruey-izquierdo, A.; Bongomin, F.; Bowyer, P.; Chakrabarti, A.; et al. Case Definition of Chronic Pulmonary Aspergillosis in Resource-Constrained Settings. Emerg. Infect. Dis. 2018, 24, e171312. [Google Scholar] [CrossRef]
- Gautier, M.; Normand, A.C.; Ranque, S. Previously unknown species of Aspergillus. Clin. Microbiol. Infect. 2016, 22, 662–669. [Google Scholar] [CrossRef] [Green Version]
- Balajee, S.A.; Nickle, D.; Varga, J.; Marr, K.A. Molecular Studies Reveal Frequent Misidentification of Aspergillus fumigatus by Morphotyping. Eukaryot. Cell 2006, 5, 1705–1712. [Google Scholar] [CrossRef] [Green Version]
- Tam, E.W.T.; Chen, J.H.K.; Lau, E.C.L.; Ngan, A.H.Y.; Fung, K.S.C.; Lee, K.C.; Lam, C.W.; Yuen, K.Y.; Lau, S.K.P.; Woo, P.C.Y. Misidentification of Aspergillus nomius and Aspergillus tamarii as Aspergillus flavus: Characterization by internal transcribed spacer, β-tubulin, and calmodulin gene sequencing, metabolic fingerprinting, and matrix-assisted laser desorption ionization-ti. J. Clin. Microbiol. 2014, 52, 1153–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozaliyani, A.; Sedono, R.; Sjam, R.; Tugiran, M.; Adawiyah, R.; Setianingrum, F.; Jusuf, A.; Sungkar, S.; Hagen, F.; Meis, J.F.; et al. Molecular typing and antifungal susceptibility study of Aspergillus spp. in intensive care unit (ICU) patients in Indonesia. J. Infect. Dev. Ctries. 2021, 15, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, J.; Kocsub, S.; Visagie, C.M.; Yilmaz, N.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; Frisvad, J.C. Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): An overview of families, genera, subgenera, sections, series and species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef] [PubMed]
- Nedel, W.L.; Pasqualotto, A.C. Treatment of Infections by Cryptic Aspergillus Species. Mycopathologia 2014, 178, 441–445. [Google Scholar] [CrossRef]
- Alastruey-izquierdo, A.; Alcazar-Fuoli, L.; Rivero-Menéndez, O.; Ayats, J.; Castro, C.; García-Rodríguez, J.; Goterris-Bonet, L.; Ibáñez-Martínez, E.; Linares-Sicilia, M.J.; Martin-Gomez, M.T.; et al. Molecular Identification and Susceptibility Testing of Molds Isolated in a Prospective Surveillance of Triazole Resistance in Spain (FILPOP2 Study). Antimicrob. Agents Chemother. 2018, 62, e00358-18. [Google Scholar] [CrossRef] [Green Version]
- Tsang, C.C.; Tang, J.Y.M.; Ye, H.; Xing, F.; Lo, S.K.F.; Xiao, C.; Han, L.; Wu, A.K.L.; Ngan, A.H.Y.; Law, K.C.; et al. Rare/cryptic Aspergillus species infections and importance of antifungal susceptibility testing. Mycoses 2020, 63, 1283–1298. [Google Scholar] [CrossRef]
- Sabino, R.; Gonçalves, P.; Melo, A.M.; Sim, D.; Oliveira, M.; Francisco, M.; Viegas, C.; Carvalho, D.; Martins, C.; Ferreira, T.; et al. Trends on Aspergillus Epidemiology—Perspectives from a National Reference Laboratory Surveillance Program. J. Fungi 2021, 7, 28. [Google Scholar] [CrossRef]
- Bongomin, F.; Moore, C.B.; Masania, R.; Rowbotham, E.; Alastruey-Izquierdo, A.; Novak-Frazer, L.; Richardson, M.D. Sequence analysis of isolates of Aspergillus from patients with chronic and allergic aspergillosis reveals a spectrum of cryptic species. Future Microbiol. 2018, 13, 1557–1563. [Google Scholar] [CrossRef] [Green Version]
- Gontia-Mishra, I.; Tripathi, N.; Tiwari, S. A simple and rapid DNA extraction protocol for filamentous fungi efficient for molecular studies. Indian J. Biotechnol. 2014, 13, 536–539. [Google Scholar]
- White, T.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Glass, N.L.; Donaldson, G.C. Development of Primer Sets Designed for Use with the PCR To Amplify Conserved Genes from Filamentous Ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.-B.; Go, S.-J.; Shin, H.-D.; Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of Aspergillus fumigatus and related species. Mycologia 2005, 97, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Nazaries, L.; Munro, S.; Anderson, I.C.; Campbell, C.D. Use of multiplex terminal restriction fragment length polymorphism for rapid and simultaneous analysis of different components of the soil microbial community. Appl. Environ. Microbiol. 2006, 72, 7278–7285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.B.; Hubka, V.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashtiani, N.M.; Kachuei, R.; Yalfani, R.; Harchegani, A.B.; Nosratabadi, M. Identification of Aspergillus sections Flavi, Nigri, and Fumigati and their differentiation using specific primers. Le Infez. Med. 2017, 25, 127–132. [Google Scholar]
- Yang, R.H.; Su, J.H.; Shang, J.J.; Wu, Y.Y.; Li, Y.; Bao, D.P.; Yao, Y.J. Evaluation of the ribosomal DNA internal transcribed spacer (ITS), specifically ITS1 and ITS2, for the analysis of fungal diversity by deep sequencing. PLoS ONE 2018, 13, e0206428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellemain, E.; Carlsen, T.; Brochmann, C.; Coissac, E.; Taberlet, P.; Kauserud, H. ITS as an environmental DNA barcode for fungi: An in silico approach reveals potential PCR biases. World J. Microbiol. Biotechnol. 2010, 31, 189–999. [Google Scholar] [CrossRef] [Green Version]
- Espinel-Ingroff, A.; Arthington-Skaggs, B.; Iqbal, N.; Ellis, D.; Pfaller, M.A.; Messer, S.; Rinaldi, M.; Fothergill, A.; Gibbs, D.L.; Wang, A. Multicenter evaluation of a new disk agar diffusion method for susceptibility testing of filamentous fungi with voriconazole, posaconazole, itraconazole, amphotericin B, and caspofungin. J. Clin. Microbiol. 2007, 45, 1811–1820. [Google Scholar] [CrossRef] [Green Version]
- Al-Wathiqi, F.; Ahmad, S.; Khan, Z. Molecular identification and antifungal susceptibility profile of Aspergillus flavus isolates recovered from clinical specimens in Kuwait. BMC Infect. Dis. 2013, 13, 126. [Google Scholar] [CrossRef] [Green Version]
- Espinel-Ingroff, A.; Canton, E.; Fothergill, A.; Ghannoum, M.; Johnson, E.; Jones, R.N.; Ostrosky-Zeichner, L.; Schell, W.; Gibbs, D.L.; Wang, A.; et al. Quality control guidelines for amphotericin B, itraconazole, posaconazole, and voriconazole disk diffusion susceptibility tests with nonsupplemented Mueller-Hinton agar (CLSI M51-A document) for nondermatophyte filamentous fungi. J. Clin. Microbiol. 2011, 49, 2568–2571. [Google Scholar] [CrossRef] [Green Version]
- Varga, J.; Frisvad, J.C.; Kocsubé, S.; Brankovics, B.; Tóth, B.; Szigeti, G.; Samson, R.A.; Varga, A.; Frisvad, A. New and revisited species in Aspergillus section Nigri Extrolite analysis. Stud. Mycol. 2011, 69, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Varga, J.; Kevei, F.; Vagvogyi, C.; Vriesema, A.; Croft, H. Double-stranded RNA mycoviruses in section Nigri of the Aspergillus genus. Can. J. Microbiol. 1994, 40, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Treviño-Rangel, R.D.J.; Villanueva-Lozano, H.; Bonifaz, A.; Castañón-Olivares, L.R.; Andrade, A.; Becerril-García, M.A.; Martínez-Reséndez, M.F.; Ayala-Gaytán, J.; Montoya, A.M.; González, G.M. Species distribution and antifungal susceptibility patterns of Aspergillus isolates from clinical specimens and soil samples in Mexico. Med. Mycol. 2021, 59, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Arabatzis, M.; Kambouris, M.; Kyprianou, M.; Chrysaki, A.; Foustoukou, M.; Kanellopoulou, M.; Kondyli, L.; Kouppari, G.; Koutsia-Karouzou, C.; Lebessi, E.; et al. Polyphasic identification and susceptibility to seven antifungals of 102 Aspergillus isolates recovered from immunocompromised hosts in Greece. Antimicrob. Agents Chemother. 2011, 55, 3025–3030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrickx, M.; Beguin, H.; Detandt, M. Genetic re-identification and antifungal susceptibility testing of Aspergillus section Nigri strains of the BCCM/IHEM collection. Mycoses 2012, 55, 148–155. [Google Scholar] [CrossRef] [PubMed]
- D’hooge, E.; Becker, P.; Stubbe, D.; Normand, A.C.; Piarroux, R.; Hendrickx, M. Black aspergilli: A remaining challenge in fungal taxonomy? Med. Mycol. 2019, 57, 773–780. [Google Scholar] [CrossRef]
- Alshehri, B.; Palanisamy, M. Evaluation of molecular identification of Aspergillus species causing fungal keratitis. Saudi J. Biol. Sci. 2020, 27, 751–756. [Google Scholar] [CrossRef]
- Peterson, S.W. Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia 2008, 100, 205–226. [Google Scholar] [CrossRef]
- Hubka, V.; Kolarik, M. β-tubulin paralogue tubC is frequently misidentified as the benA gene in Aspergillus section Nigri taxonomy: Primer specificity testing and taxonomic consequences. Persoonia 2012, 29, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jurjevic, Ž.; Peterson, S.W.; Stea, G.; Solfrizzo, M.; Varga, J.; Hubka, V.; Perrone, G. Two novel species of Aspergillus section Nigri from indoor air. IMA Fungus 2012, 3, 159–173. [Google Scholar] [CrossRef]
- Masih, A.; Singh, P.K.; Kathuria, S.; Agarwal, K.; Meis, J.F.; Chowdhary, A. Identification by molecular methods and matrix-assisted laser desorption ionization-time of flight mass spectrometry and antifungal susceptibility profiles of clinically significant rare aspergillus species in a referral chest hospital in Delhi, India. J. Clin. Microbiol. 2016, 54, 2354–2364. [Google Scholar] [CrossRef] [Green Version]
- Lass-Flörl, C. Susceptibility testing in Aspergillus species complex. Clin. Microbiol. Infect. 2014, 20, 49–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, R.M.; Ismail, D.K.; Elkholy, Y.S. Comparison of e test and disc diffusion methods for susceptibility testing of filamentous fungi; experience of a routine lab. Arch. Clin. Infect. Dis. 2018, 13, e57889. [Google Scholar] [CrossRef] [Green Version]
- Serrano, M.C.; Ramírez, M.; Morilla, D.; Valverde, A.; Chávez, M.; Espinel-Ingroff, A.; Claro, R.; Fernández, A.; Almeida, C.; Martín-Mazuelos, E. A comparative study of the disc diffusion method with the broth microdilution and Etest methods for viroconazole susceptibility testing of Aspergillus spp. J. Antimicrob. Chemother. 2004, 53, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Khare, V.; Kumar, D.; Ahmad, A.; Banerjee, G.; Singh, M. Comparative evaluation of disc diffusion and E-test with broth micro-dilution in Susceptibility testing of amphotericin B, voriconazole and caspofungin against clinical Aspergillus isolates. J. Clin. Diagnostic Res. 2015, 9, DC01–DC04. [Google Scholar] [CrossRef]
- Ashu, E.E.; Korfanty, G.A.; Samarasinghe, H.; Pum, N.; You, M.; Yamamura, D.; Xu, J. Widespread amphotericin B-resistant strains of aspergillus fumigatus in Hamilton, Canada. Infect. Drug Resist. 2018, 11, 1549–1555. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, S.S.; Stchigel, A.M.; Cano, J.; Guarro, J.; Colombo, A.L. In vitro antifungal susceptibility of clinically relevant species belonging to Aspergillus section Flavi. Antimicrob. Agents Chemother. 2013, 57, 1944–1947. [Google Scholar] [CrossRef] [Green Version]
- Diekema, D.; Messer, S.; Hollis, R.; Jones, R.; Pfaller, M. Activities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungi. J. Clin. Microbiol. 2003, 41, 3623–3626. [Google Scholar] [CrossRef] [Green Version]
- Guinea, J.; Peláez, T.; Alcalá, L.; Ruiz-Serrano, M.J.; Bouza, E. Antifungal susceptibility of 596 Aspergillus fumigatus strains isolated from outdoor air, hospital air, and clinical samples: Analysis by site of isolation. Antimicrob. Agents Chemother. 2005, 49, 3495–3497. [Google Scholar] [CrossRef] [Green Version]
- Bongomin, F.; Harris, C.; Hayes, G.; Kosmidis, C.; Denning, D.W. Twelve-month clinical outcomes of 206 patients with chronic pulmonary aspergillosis. PLoS ONE 2018, 13, e0193732. [Google Scholar] [CrossRef] [Green Version]
- van Rhijn, N.; Denning, D.W. Is an azole-resistant Aspergillus hotspot emerging in South-East Asia? Environ. Microbiol. 2021, 23, 7275–7277. [Google Scholar] [CrossRef]
- My, D.N.T.; Van, L.T.; Linh, T.H.K.; Tuyen, N.P.; Phuong, N.T.; Thu Anh, N.; Lan, N.P.H.; Ngoc, N.T.B.; Fisher, M.C.; Rhodes, J.; et al. Unprecedented Prevalence of Azole-Resistant Aspergillus fumigatus Identified in the Environment of Vietnam, with Marked Variability by Land Use Type. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Duong, T.M.N.; Nguyen, P.T.; Van Le, T.; Nguyen, H.L.P.; Nguyen, B.N.T.; Nguyen, B.P.T.; Nguyen, T.A.; Chen, S.C.A.; Barrs, V.R.; Halliday, C.L.; et al. Drug-resistant aspergillus flavus is highly prevalent in the environment of Vietnam: A new challenge for the management of aspergillosis? J. Fungi 2020, 6, 296. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Monteiro, C.; Maia, M.; Faria, M.A.; Lopes, V.; Lameiras, C.; Pinheiro, D. Aspergillus species and antifungals susceptibility in clinical setting in the north of Portugal: Cryptic species and emerging azoles resistance in A. fumigatus. Front. Microbiol. 2018, 9, 656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoran, T.; Sartori, B.; Sappl, L.; Aigner, M.; Sánchez-Reus, F.; Rezusta, A.; Chowdhary, A.; Taj-Aldeen, S.J.; Arendrup, M.C.; Oliveri, S.; et al. Azole-resistance in Aspergillus terreus and related species: An emerging problem or a rare Phenomenon? Front. Microbiol. 2018, 9, 516. [Google Scholar] [CrossRef] [PubMed]
- Chrenkova, V.; Hubka, V.; Cetkovsky, P.; Kouba, M.; Weinbergerova, B.; Lyskova, P.; Hornofova, L.; Hubacek, P. Proven Invasive Pulmonary Aspergillosis in Stem Cell Transplant Recipient Due to Aspergillus sublatus, a Cryptic Species of A. nidulans. Mycopathologia 2018, 183, 423–429. [Google Scholar] [CrossRef]
- Kosmidis, C.; Muldoon, E.G. Challenges in the management of chronic pulmonary aspergillosis. Med. Mycol. 2017, 55, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Campione, E.; Gaziano, R.; Doldo, E.; Marino, D.; Falconi, M.; Iacovelli, F.; Tagliaferri, D.; Pacello, L.; Bianchi, L.; Lanna, C.; et al. Antifungal effect of all-trans retinoic acid against aspergillus fumigatus in vitro and in a pulmonary aspergillosis in vivo model. Antimicrob. Agents Chemother. 2021, 65, e01874-20. [Google Scholar] [CrossRef]
- Cosio, T.; Gaziano, R.; Zuccari, G.; Costanza, G.; Grelli, S.; Di Francesco, P.; Bianchi, L.; Campione, E. Retinoids in fungal infections: From bench to bedside. Pharmaceuticals 2021, 14, 962. [Google Scholar] [CrossRef]
- Snelders, E.; Camps, S.M.T.; Karawajczyk, A.; Schaftenaar, G.; Kema, G.H.J.; van der Lee, H.A.; Klaassen, C.H.; Melchers, W.J.G.; Verweij, P.E. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS ONE 2012, 7, e31801. [Google Scholar] [CrossRef] [Green Version]
- Denning, D.W.; Park, S.; Lass-Florl, C.; Fraczek, M.G.; Kirwan, M.; Gore, R.; Smith, J.; Bueid, A.; Moore, C.B.; Bowyer, P.; et al. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin. Infect. Dis. 2011, 52, 1123–1129. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, J.A.; Manavathu, E.K. Are the TR 46 /Y121F/T289A Mutations in Azole-Resistant Aspergillosis Patient Acquired or Environmental?". Antimicrob. Agents Chemother. 2016, 60, 3259–3260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Linden, J.W.M.; Snelders, E.; Kampinga, G.A.; Rijnders, B.J.A.; Mattsson, E.; Debets-Ossenkopp, Y.J.; Kuijper, E.J.; van Tiel, F.H.; Melchers, W.J.G.; Verweij, P.E. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007-2009. Emerg. Infect. Dis. 2011, 17, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
| All (n = 59) | CPA (n = 29) | Non-CPA (n = 30) | p-Value | Cryptic (n = 16) | Sensu Stricto (n = 43) | p-Value | |
|---|---|---|---|---|---|---|---|
| Section | |||||||
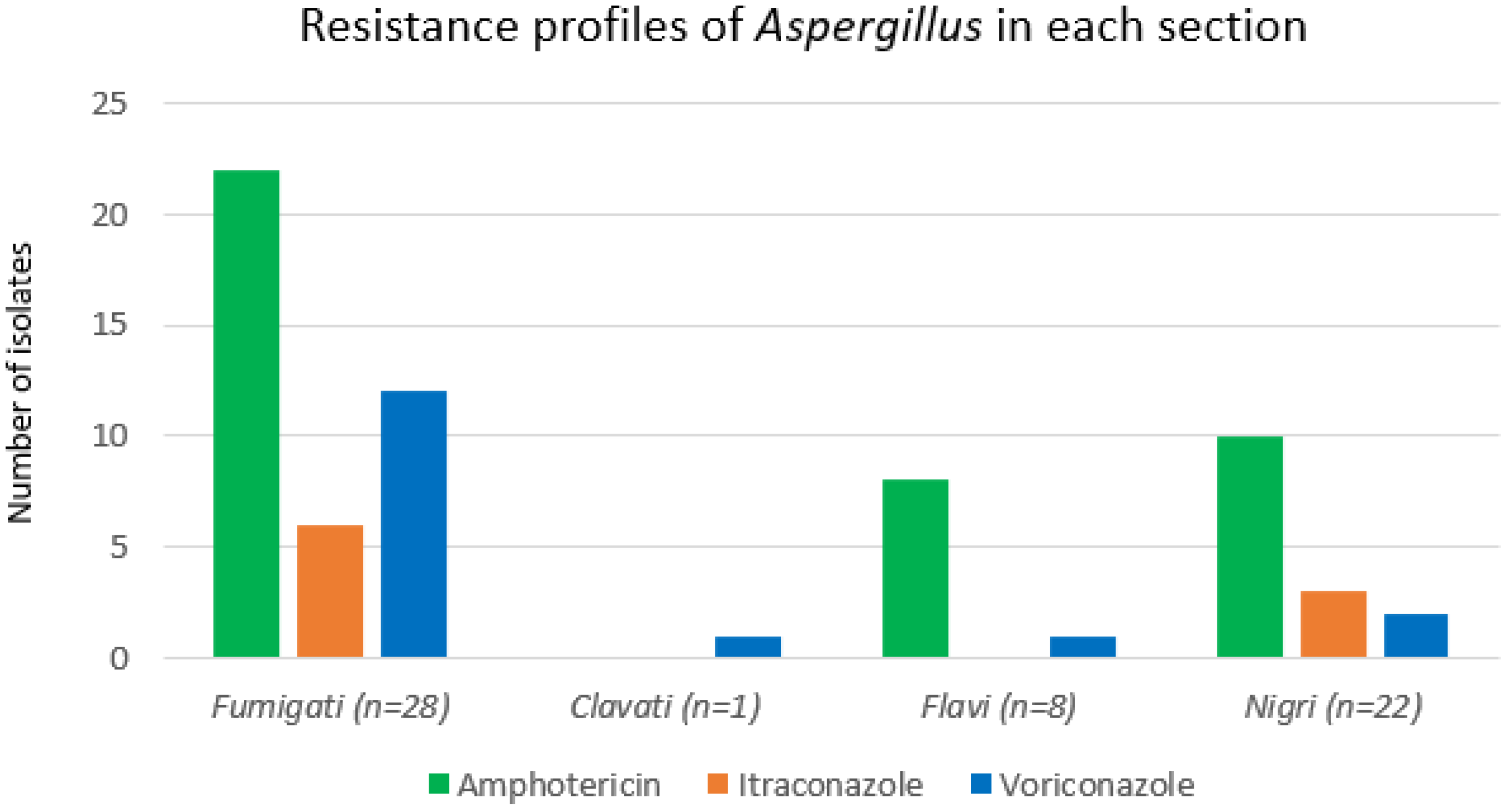
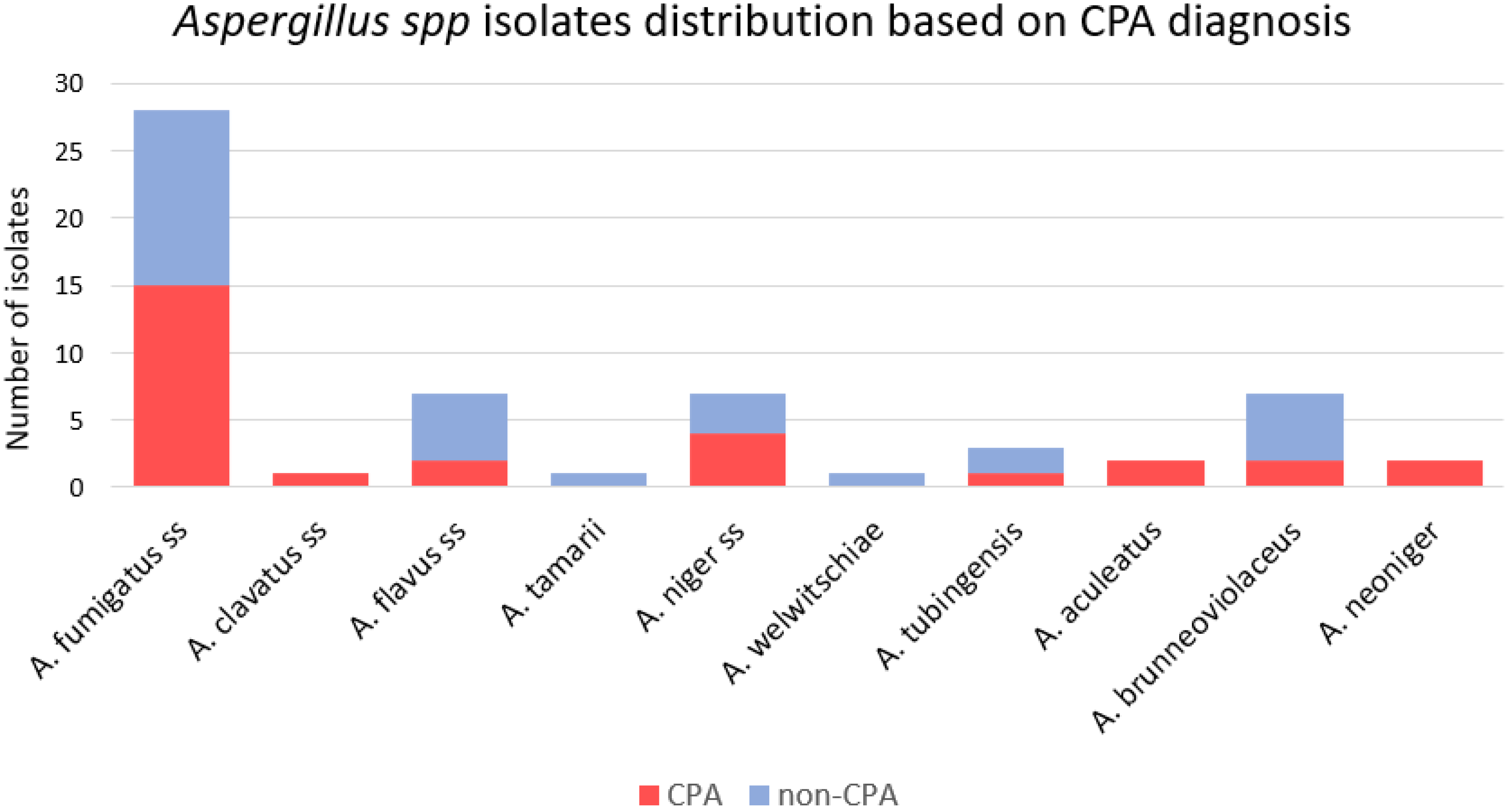
| Fumigati | 28 (49%) | 15 (54%) | 13 (43%) | 0.519 | 0 (0%) | 28 (65%) | <0.005 |
| Clavati | 1 (2%) | 1 (3%) | 0 (0%) | 0.492 | 0 (0%) | 1 (2%) | 1 |
| Flavi | 8 (14%) | 2 (7%) | 6 (20%) | 0.254 | 1 (6%) | 7 (16%) | 0.427 |
| Nigri | 22 (37%) | 11 (38%) | 11 (37%) | 0.920 | 15 (93%) | 7 (16%) | <0.005 |
| Symptoms | |||||||
| Haemoptysis | 36 (61%) | 23 (79%) | 13 (43%) | 0.005 | 12 (75%) | 24 (56%) | 0.236 |
| Massive haemoptysis | 19 (32%) | 12 (41%) | 7 (23%) | 0.170 | 7 (44%) | 12 (28%) | 0.348 |
| Recurrent haemoptysis | 15 (25%) | 11 (38%) | 4 (13%) | 0.039 | 3 (19%) | 12 (28) | 0.738 |
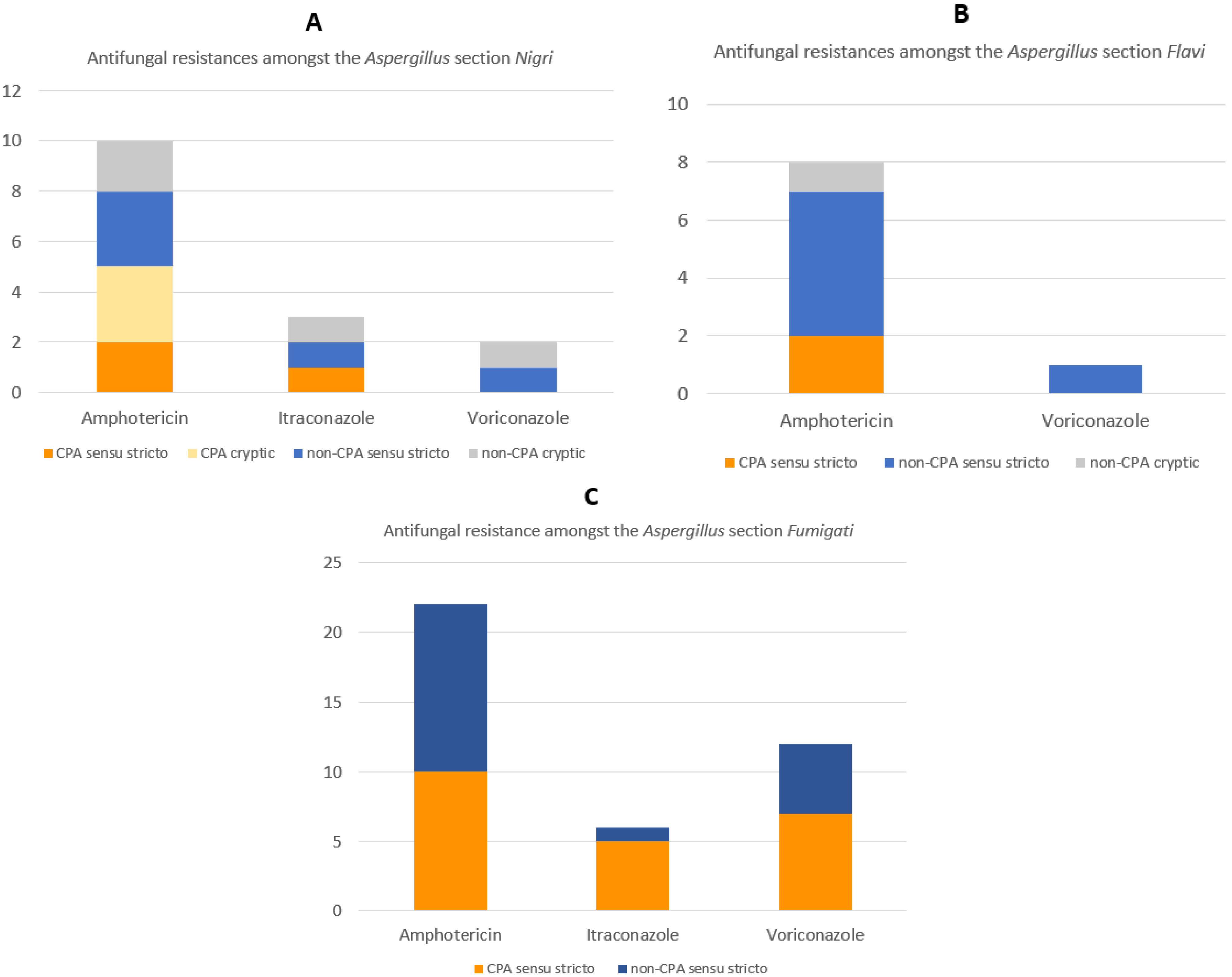
| No | Sections | Sample Code | Final ID | Genes Used for ID | Diagnosis | Amphotericin | Itraconazole | Voriconazole |
|---|---|---|---|---|---|---|---|---|
| 1 | Fumigati | 006-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | CPA | Resistant | Resistant | Susceptible |
| 2 | 012-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | CPA | Resistant | Susceptible | Intermediate | |
| 3 | 013-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Susceptible | Intermediate | |
| 4 | 014-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | CPA | Resistant | Susceptible | Susceptible | |
| 5 | 015-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | CPA | Susceptible | Susceptible | Susceptible | |
| 6 | 018-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Susceptible | Resistant | |
| 7 | 019-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Susceptible | Intermediate | |
| 8 | 020-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | CPA | Resistant | Intermediate | Resistant | |
| 9 | 022-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Susceptible | Resistant | |
| 10 | 023-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | CPA | Resistant | Susceptible | Susceptible | |
| 11 | 025-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Susceptible | Susceptible | |
| 12 | 026-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Susceptible | Intermediate | |
| 13 | 027-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Susceptible | Resistant | |
| 14 | 036-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Susceptible | Intermediate | |
| 15 | 048-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | Non-CPA | Susceptible | Resistant | Intermediate | |
| 16 | 069-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Susceptible | Susceptible | |
| 17 | 080-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Susceptible | Resistant | |
| 18 | 083-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | CPA | Resistant | Susceptible | Susceptible | |
| 19 | 084-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | CPA | Susceptible | Resistant | Resistant | |
| 20 | 085-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Susceptible | Resistant | |
| 21 | 091-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | CPA | Resistant | Resistant | Resistant | |
| 22 | 092-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | CPA | Resistant | Resistant | Resistant | |
| 23 | 094-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | CPA | Susceptible | Susceptible | Resistant | |
| 24 | 097-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | CPA | Resistant | Resistant | Resistant | |
| 25 | 101-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Susceptible | Susceptible | |
| 26 | 103-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | CPA | Resistant | Intermediate | Susceptible | |
| 27 | 109-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | CPA | Intermediate | Intermediate | Susceptible | |
| 28 | 110-BT | A. fumigatus sensu stricto | ITS, BenA, CaM | CPA | Intermediate | Susceptible | Resistant | |
| 29 | Clavati | 064-BT | A. clavatus sensu stricto | ITS, BenA, CaM | CPA | Susceptible | Susceptible | Resistant |
| 30 | Flavi | 052-BT | A. tamarii | CaM | Non-CPA | Resistant | Susceptible | Intermediate |
| 31 | 066-BT | A. flavus sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Susceptible | Susceptible | |
| 32 | 069-BT | A. flavus sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Susceptible | Susceptible | |
| 33 | 071-BT | A. flavus sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Susceptible | Intermediate | |
| 34 | 080-BT | A. flavus sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Susceptible | Susceptible | |
| 35 | 086-BT | A. flavus sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Susceptible | Resistant | |
| 36 | 092-BT | A. flavus sensu stricto | ITS, BenA, CaM | CPA | Resistant | Susceptible | Susceptible | |
| 37 | 103-BT | A. flavus sensu stricto | ITS, BenA, CaM | CPA | Resistant | Susceptible | Intermediate | |
| 38 | Nigri | 057-BT | A. niger sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Resistant | Resistant |
| 39 | 083-BT | A. niger sensu stricto | ITS, BenA, CaM | CPA | Resistant | Susceptible | Susceptible | |
| 40 | 064-BT | A. niger sensu stricto | ITS, BenA, CaM | CPA | Resistant | Resistant | Intermediate | |
| 41 | 074-BT | A. niger sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Susceptible | Susceptible | |
| 42 | 079-BT | A. niger sensu stricto | ITS, BenA, CaM | CPA | Intermediate | Susceptible | Susceptible | |
| 43 | 085-BT | A. niger sensu stricto | ITS, BenA, CaM | Non-CPA | Resistant | Susceptible | Susceptible | |
| 44 | 103-BT | A. niger sensu stricto | ITS, BenA, CaM | CPA | Susceptible | Susceptible | Susceptible | |
| 45 | 076-BT | A. welwitschiae | BenA, CaM | Non-CPA | Susceptible | Susceptible | Resistant | |
| 46 | 099-BT | A. tubingensis | BenA, CaM | Non-CPA | Susceptible | Susceptible | Susceptible | |
| 47 | 101-BT | A. tubingensis | BenA, CaM | CPA | Resistant | Susceptible | Susceptible | |
| 48 | 068-BT | A. brunneoviolaceus | CaM | Non-CPA | Intermediate | Susceptible | Susceptible | |
| 49 | 073-BT | A. aculeatus | ITS, BenA, CaM | CPA | Intermediate | Susceptible | Susceptible | |
| 50 | 100-BT | A. aculeatus | ITS, BenA, CaM | CPA | Resistant | Susceptible | Susceptible | |
| 51 | 060-BT | A. brunneoviolaceus | CaM | CPA | Susceptible | Susceptible | Susceptible | |
| 52 | 006-BT | A. brunneoviolaceus | CaM | CPA | Intermediate | Susceptible | Susceptible | |
| 53 | 061-BT | A. brunneoviolaceus | CaM | Non-CPA | Susceptible | Susceptible | Susceptible | |
| 54 | 062-BT | A. brunneoviolaceus | CaM | Non-CPA | Intermediate | Susceptible | Susceptible | |
| 55 | 069-BT | A. brunneoviolaceus | CaM | Non-CPA | Susceptible | Susceptible | Susceptible | |
| 56 | 098-BT | A. brunneoviolaceus | CaM | Non-CPA | Resistant | Susceptible | Susceptible | |
| 57 | 086-BT | A. tubingensis | ITS, BenA, CaM | Non-CPA | Resistant | Resistant | Intermediate | |
| 58 | 089-BT | A. neoniger | CaM | CPA | Intermediate | Susceptible | Susceptible | |
| 59 | 097-BT | A. neoniger | CaM | CPA | Resistant | Intermediate | Intermediate |
| All (n = 59) | CPA (n = 29) | Non-CPA (n = 30) | p-Value | Cryptic (n = 16) | Sensu Stricto (n = 43) | p-Value | |
|---|---|---|---|---|---|---|---|
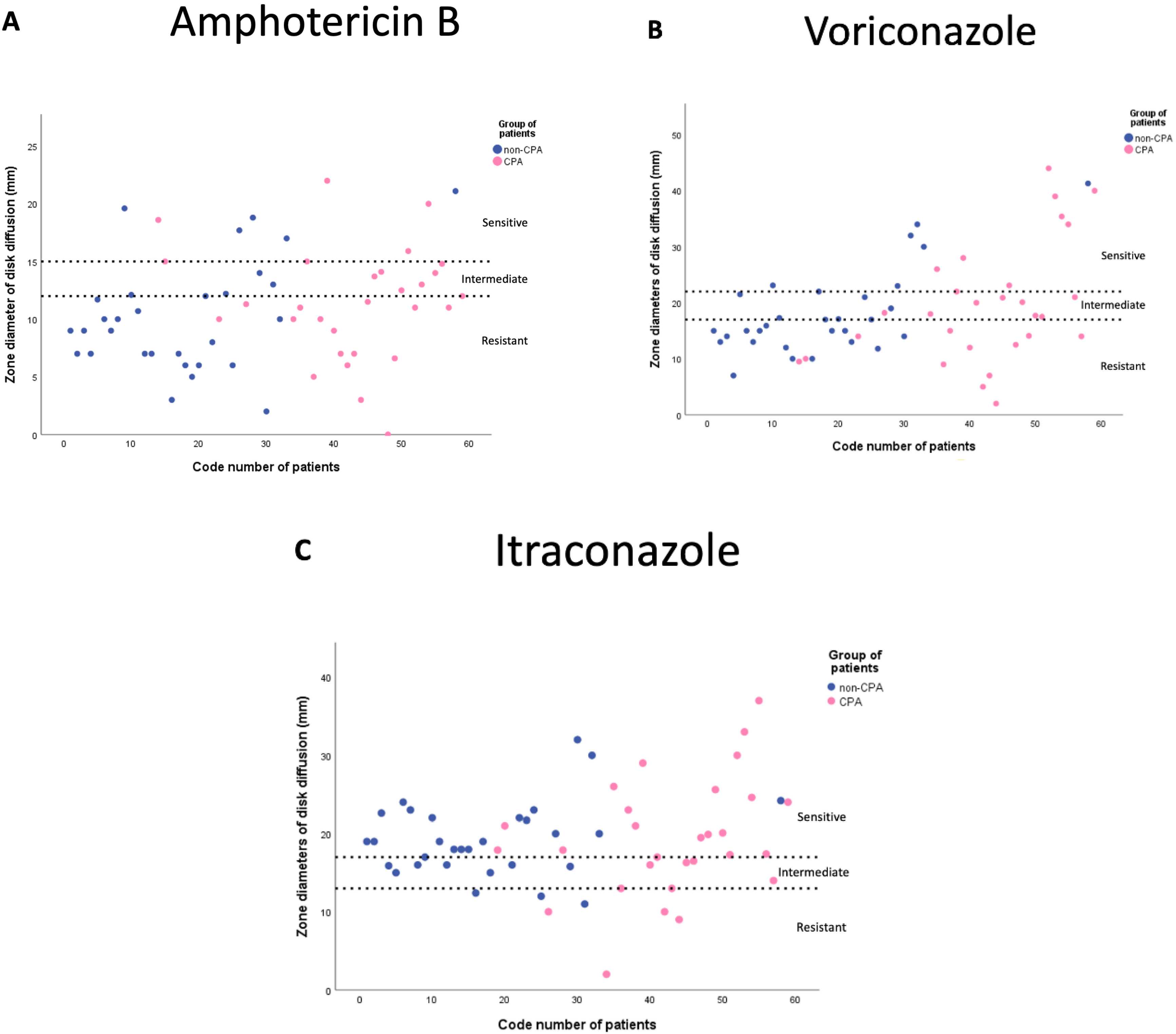
| Amphotericin B | |||||||
| Zone of inhibition (range) | 0–22 | 0–22 | 2–21.1 | 2–21.1 | 0–22 | ||
| Mean of inhibition zone ± SD | 10.8 ± 4.8 | 11.4 ± 4.9 | 10.3 ± 4.8 | 0.381 | 13.9 ± 4.6 | 9.7 ± 4.5 | 0.002 |
| Susceptible | 11 (19%) | 6 (21%) | 5 (17%) | 0.748 | 5 (31%) | 6 (14%) | 0.149 |
| Intermediate | 8 (14%) | 6 (21%) | 2 (7%) | 0.145 | 5 (31%) | 3 (7%) | 0.028 |
| Resistant | 40 (68%) | 17 (59%) | 23 (77%) | 0.170 | 6 (38%) | 34 (79%) | 0.002 |
| Voriconazole | |||||||
| Zone of inhibition (range) | 2–44 | 2–44 | 7–41.3 | 11.8–41.3 | 2–44 | ||
| Mean of inhibition zone ± SD | 18.9 ± 9.2 | 19.6 ± 10.7 | 18.1 ± 7.7 | 0.541 | 26.4 ± 10.4 | 16.1 ± 7.1 | <0.005 |
| Susceptible | 31 (53%) | 17 (59%) | 14 (47%) | 0.358 | 12 (75%) | 19 (44%) | 0.035 |
| Intermediate | 12 (20%) | 4 (14%) | 8 (27%) | 0.333 | 3 (19%) | 9 (21%) | 1 |
| Resistant | 16 (27%) | 8 (28%) | 8 (27%) | 1 | 1 (6%) | 15 (35%) | 0.045 |
| Itraconazole | |||||||
| Zone of inhibition (range) | 2–37 | 2–37 | 11–32 | 11–37 | 2–30 | ||
| Mean of inhibition zone ± SD | 19.3 ± 6.2 | 19.3 ± 7.5 | 19.2 ± 4.8 | 0.939 | 22.7 ± 7.3 | 18 ± 5.3 | 0.009 |
| Susceptible | 46 (78%) | 19 (66%) | 27 (90%) | 0.030 | 14 (88%) | 32 (74%) | 0.481 |
| Intermediate | 4 (7%) | 4 (14%) | 0 (0%) | 0.052 | 1 (6%) | 3 (7%) | 1 |
| Resistant | 9 (15%) | 6 (21%) | 3 (10%) | 0.299 | 1 (6%) | 8 (19%) | 0.421 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozaliyani, A.; Abdullah, A.; Setianingrum, F.; Sjamsuridzal, W.; Wahyuningsih, R.; Bowolaksono, A.; Fatril, A.E.; Adawiyah, R.; Tugiran, M.; Syam, R.; et al. Unravelling the Molecular Identification and Antifungal Susceptibility Profiles of Aspergillus spp. Isolated from Chronic Pulmonary Aspergillosis Patients in Jakarta, Indonesia: The Emergence of Cryptic Species. J. Fungi 2022, 8, 411. https://doi.org/10.3390/jof8040411
Rozaliyani A, Abdullah A, Setianingrum F, Sjamsuridzal W, Wahyuningsih R, Bowolaksono A, Fatril AE, Adawiyah R, Tugiran M, Syam R, et al. Unravelling the Molecular Identification and Antifungal Susceptibility Profiles of Aspergillus spp. Isolated from Chronic Pulmonary Aspergillosis Patients in Jakarta, Indonesia: The Emergence of Cryptic Species. Journal of Fungi. 2022; 8(4):411. https://doi.org/10.3390/jof8040411
Chicago/Turabian StyleRozaliyani, Anna, Asriyani Abdullah, Findra Setianingrum, Wellyzar Sjamsuridzal, Retno Wahyuningsih, Anom Bowolaksono, Ayu Eka Fatril, Robiatul Adawiyah, Mulyati Tugiran, Ridhawati Syam, and et al. 2022. "Unravelling the Molecular Identification and Antifungal Susceptibility Profiles of Aspergillus spp. Isolated from Chronic Pulmonary Aspergillosis Patients in Jakarta, Indonesia: The Emergence of Cryptic Species" Journal of Fungi 8, no. 4: 411. https://doi.org/10.3390/jof8040411
APA StyleRozaliyani, A., Abdullah, A., Setianingrum, F., Sjamsuridzal, W., Wahyuningsih, R., Bowolaksono, A., Fatril, A. E., Adawiyah, R., Tugiran, M., Syam, R., Wibowo, H., Kosmidis, C., & Denning, D. W. (2022). Unravelling the Molecular Identification and Antifungal Susceptibility Profiles of Aspergillus spp. Isolated from Chronic Pulmonary Aspergillosis Patients in Jakarta, Indonesia: The Emergence of Cryptic Species. Journal of Fungi, 8(4), 411. https://doi.org/10.3390/jof8040411









