Unveiling a Novel Role of Cdc42 in Pyruvate Metabolism Pathway to Mediate Insecticidal Activity of Beauveria bassiana
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Strains and Culture Conditions
2.2. Recognition and Bioinformatic Analysis of Cdc42 in B. Bassiana
2.3. Generation of cdc42 Mutants
2.4. Phenotypic Experiments
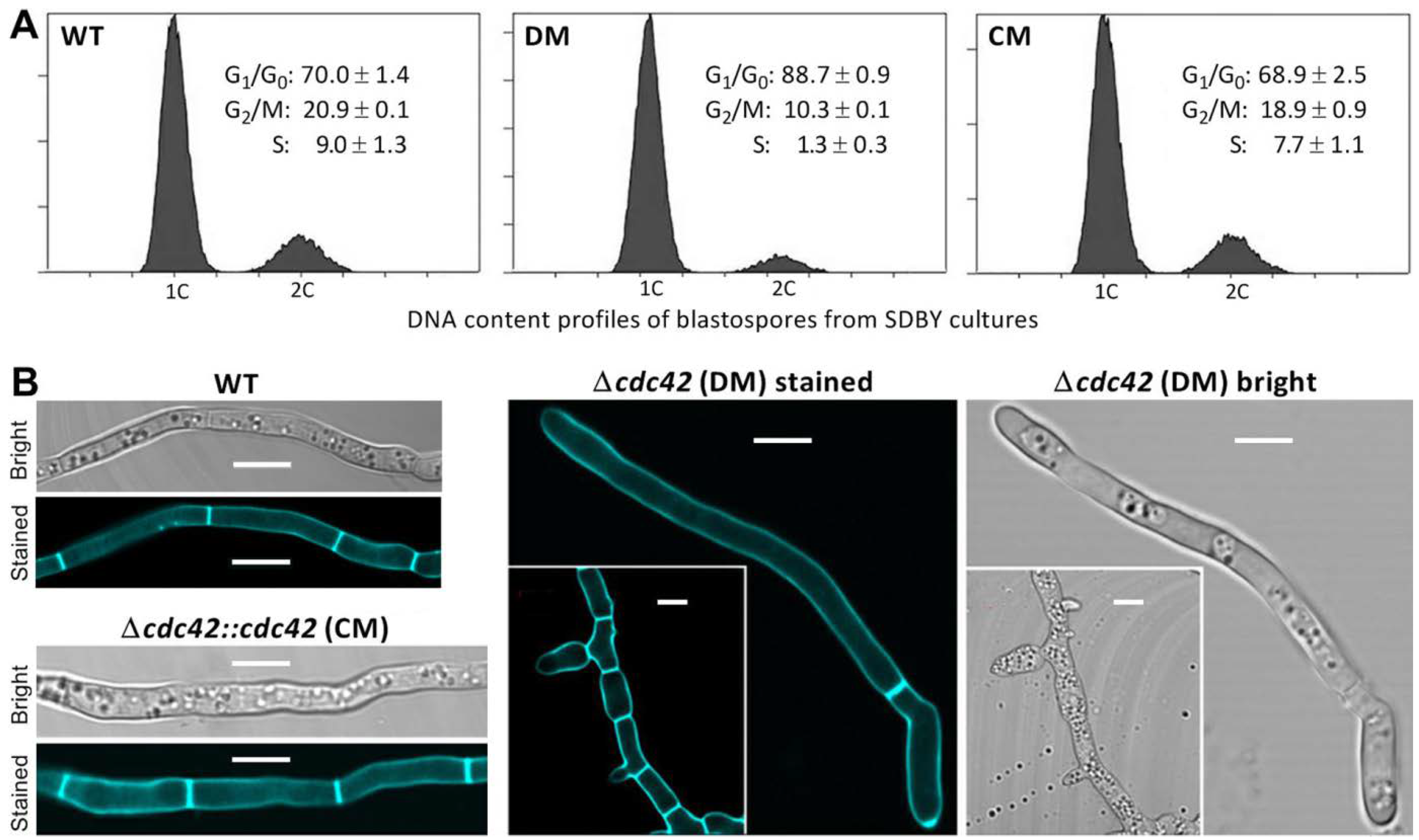
2.5. Examination of Cell Cycle and Division
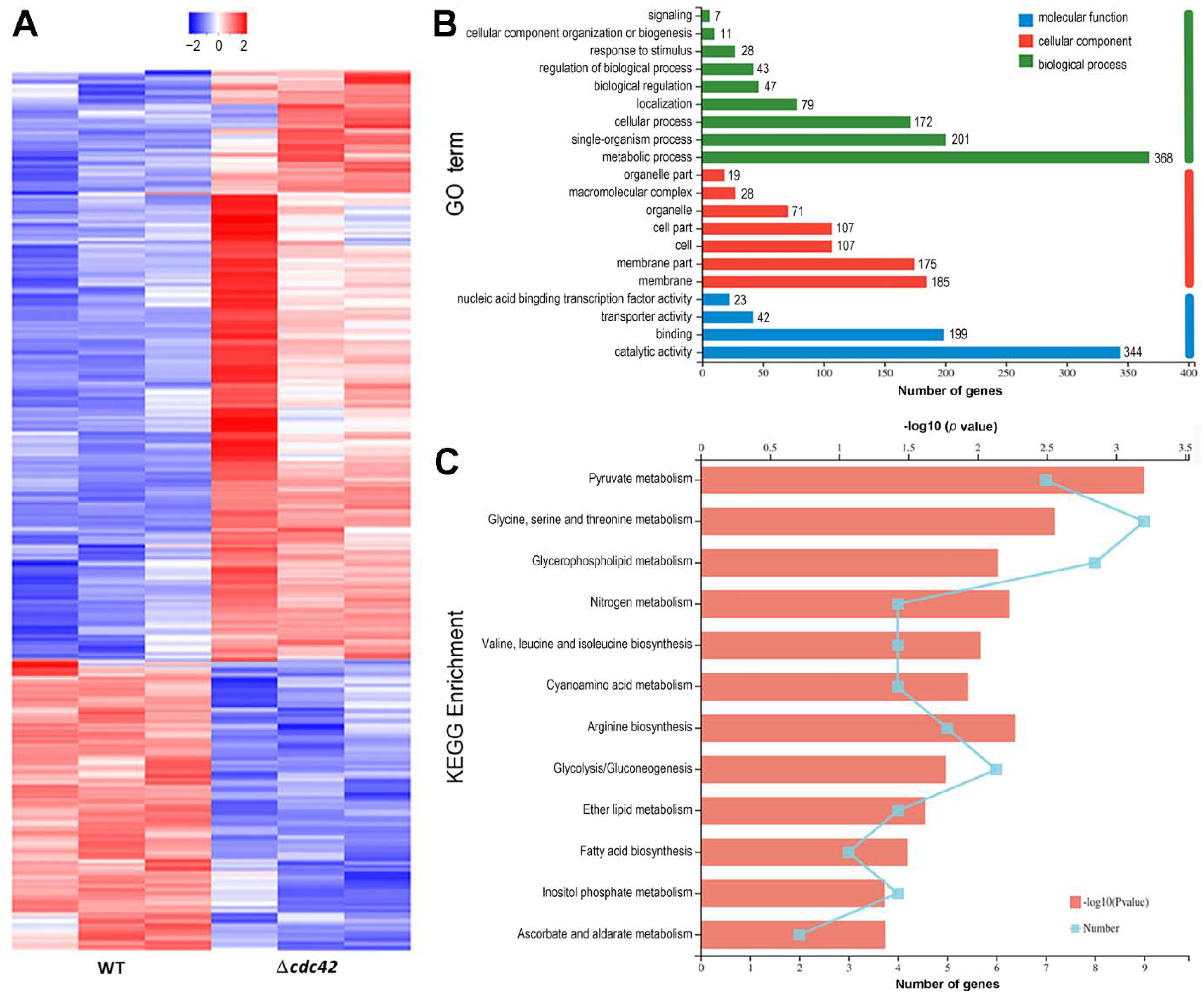
2.6. Transcriptomic Analysis
2.7. Assessments of Protein and ATP Levels Associated with TCA Cycle
2.8. Assessments of Beauvericin Level and Pr1 Family Protease Activity
3. Results
3.1. Recognition of Orthologous Cdc42 in B. Bassiana
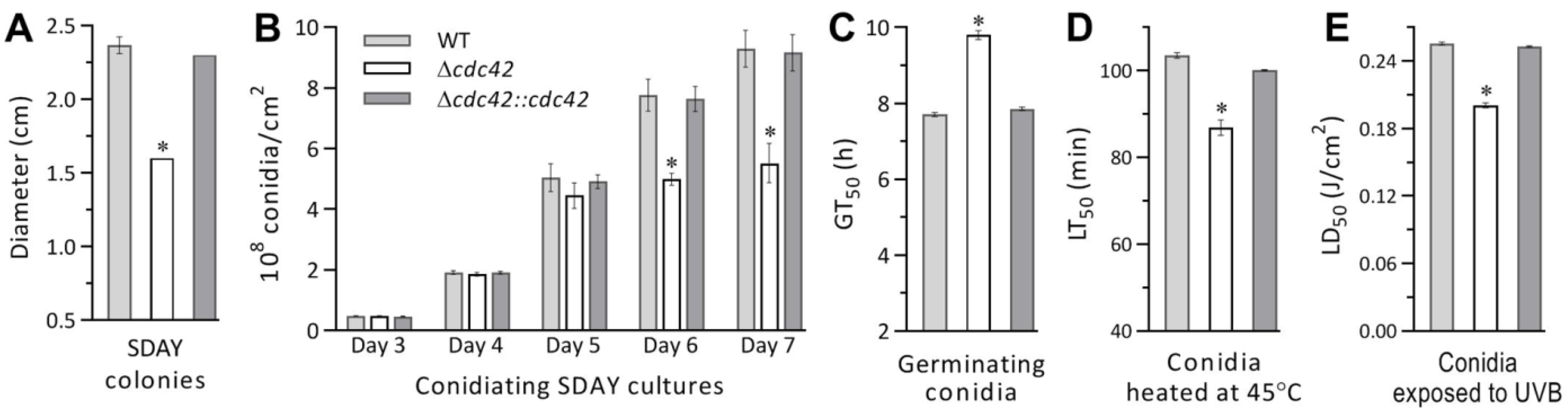
3.2. Impact of cdc42 Deletion on Radial Growth, Conidiation, and Conidial Quality
3.3. Impact of cdc42 Deletion on Hyphal Septum Formation and Morphology
3.4. Indispensability of cdc42 for Fungal Insect Pathogencity
3.5. Transcriptomic Insight into an Indispensability of cdc42 for Fungal Insect Pathogencity
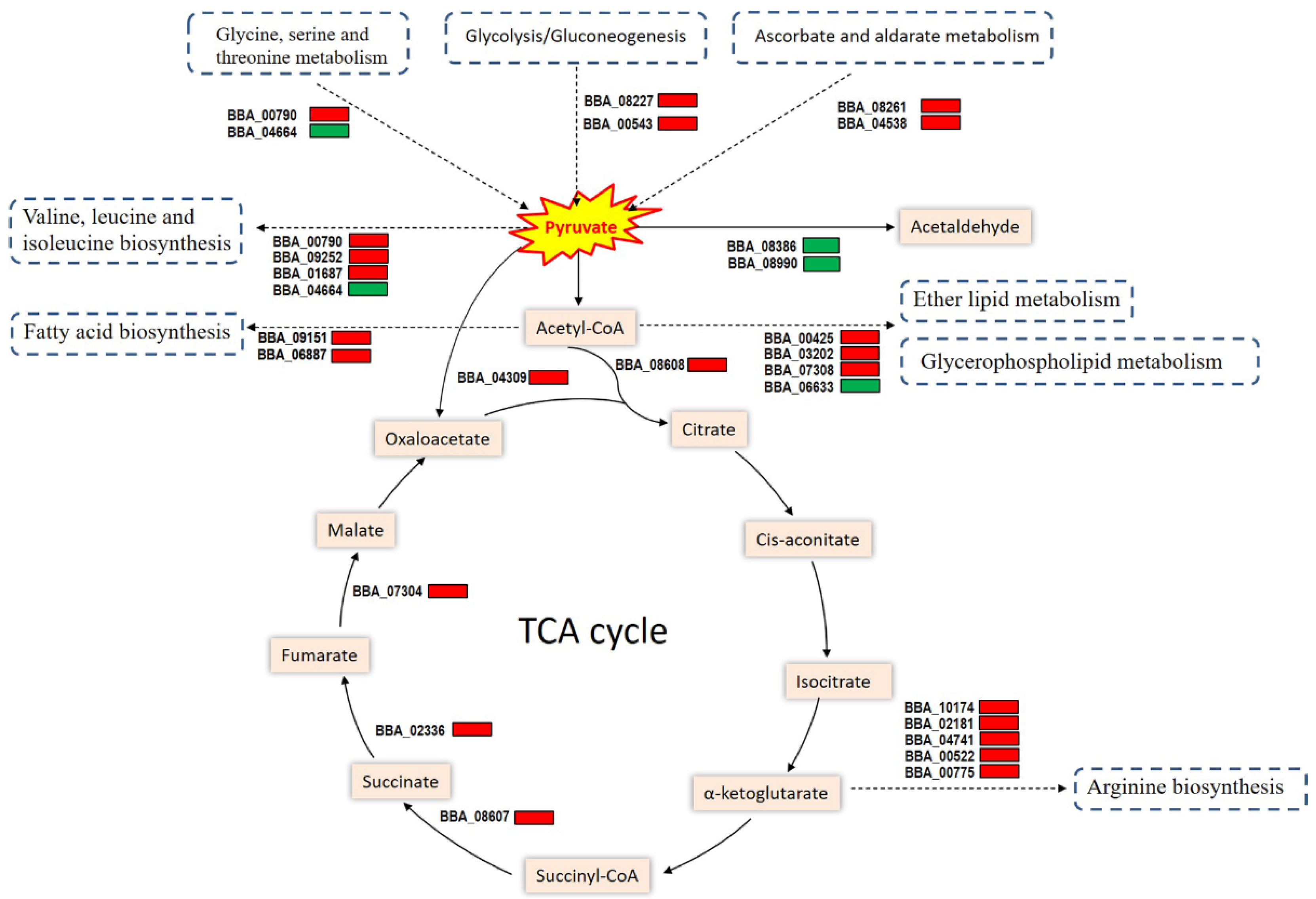
3.6. Link of Cdc42 to Pyruvate Metabolism and Related Pathways
3.7. Validated Role of Cdc42 in Pyruvate Metabolism, TCA Cycle, and Virulence Maintenance
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.S.; Feng, M.G. Advances in fundamental and applied studies in China of fungal biocontrol agents for use against arthropod pests. Biol. Control 2014, 68, 129–135. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A.; Keyhani, N.O. Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects 2013, 4, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.; Santi, L.; Corrêa, A.; Silva, L.; Bresciani, F.R.; Schrank, A.; Vainstein, M.H. The entomopathogen Metarhizium anisopliae can modulate the secretion of lipolytic enzymes in response to different substrates including components of arthropod cuticle. Fungal Biol. 2010, 114, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.G.; Jin, F.; Fan, Y.H.; Zhang, Y.J.; Bidochka, M.J.; Leger, R.; Yan, P. Expressing a fusion protein with protease and chitinase activities increases the virulence of the insect pathogen Beauveria bassiana. J. Invertebr. Pathol. 2009, 102, 155–159. [Google Scholar] [CrossRef]
- Fan, Y.H.; Fang, W.G.; Guo, S.J.; Pei, X.Q.; Zhang, Y.J.; Xiao, Y.H.; Li, D.M.; Jin, K.; Bidochka, M.J.; Pei, Y. Increased insect virulence in Beauveria bassiana strains overexpressing an engineered chitinase. Appl. Environ. Microbiol. 2007, 73, 295–302. [Google Scholar] [CrossRef]
- Fang, W.G.; Leng, B.; Xiao, Y.H.; Jin, K.; Ma, J.C.; Fan, Y.H.; Feng, J.; Yang, X.Y.; Zhang, Y.J.; Pei, Y. Cloning of Beauveria bassiana chitinase gene Bbchit1 and its application to improve fungal strain virulence. Appl. Environ. Microbiol. 2005, 71, 363–370. [Google Scholar] [CrossRef]
- Gao, B.J.; Mou, Y.N.; Tong, S.M.; Ying, S.H.; Feng, M.G. Subtilisin-like Pr1 proteases marking the evolution of pathogenicity in a wide-spectrum insect-pathogenic fungus. Virulence 2020, 11, 365–380. [Google Scholar] [CrossRef]
- Nilanonta, C.; Isaka, M.; Kittakoop, P.; Trakulnaleamsai, S.; Tanticharoen, M.; Thebtaranonth, Y. Precursor-directed biosynthesis of beauvericin analogs by the insect pathogenic fungus Paecilomyces tenuipes BCC 1614. Tetrahedron 2002, 58, 3355–3360. [Google Scholar] [CrossRef]
- Xu, Y.; Orozco, R.; Wijeratne, E.M.; Gunatilaka, A.A.; Stock, S.P.; Molnar, I. Biosynthesis of the cyclooligomer depsipeptide beauvericin, a virulence factor of the entomopathogenic fungus Beauveria bassiana. Chem. Biol. 2008, 15, 898–907. [Google Scholar] [CrossRef]
- Cai, Q.; Wang, J.J.; Fu, B.; Ying, S.H.; Feng, M.G. Gcn5-dependent histone H3 acetylation and gene activity is required for the asexual development and virulence of Beauveria bassiana. Environ. Microbiol. 2018, 20, 1484–1497. [Google Scholar] [CrossRef]
- He, P.H.; Dong, W.X.; Chu, X.L.; Feng, M.G.; Ying, S.H. The cellular proteome is affected by a gelsolin (BbGEL1) during morphological transitions in aerobic surface versus liquid growth in the entomopathogenic fungus Beauveria bassiana. Environ. Microbiol. 2016, 18, 4153–4169. [Google Scholar] [CrossRef]
- Zhang, A.X.; Mouhoumed, A.Z.; Tong, S.M.; Ying, S.H.; Feng, M.G. BrlA and AbaA govern virulence-required dimorphic switch, conidiation, and pathogenicity in a fungal insect pathogen. mSystems 2019, 4, e00140–e00519. [Google Scholar] [CrossRef]
- Zhang, L.B.; Feng, M.G. Antioxidant enzymes and their contributions tobiological control potential offungal insect pathogens. Appl. Microbiol. Biotechnol. 2018, 102, 4995–5004. [Google Scholar] [CrossRef]
- Ying, S.H.; Feng, M.G. Insight into vital role of autophagy in sustaining biological control potential of fungal pathogens against pest insects and nematodes. Virulence 2019, 10, 429–437. [Google Scholar] [CrossRef]
- Tong, S.M.; Feng, M.G. Insights into regulatory roles of MAPK-cascaded pathways in multiple stress responses and life cycles of insect and nematode mycopathogens. Appl. Microbiol. Biotechnol. 2019, 103, 577–587. [Google Scholar] [CrossRef]
- Tong, S.M.; Feng, M.G. Phenotypic and molecular insights into heat tolerance of formulated cells as active ingredients of fungal insecticides. Appl. Microbiol. Biotechnol. 2020, 104, 5711–5724. [Google Scholar] [CrossRef]
- Tong, S.M.; Feng, M.G. Molecular basis and regulatory mechanisms underlying fungal insecticides’ resistance to solar ultraviolet irradiation. Pest Manag. Sci. 2022, 78, 30–42. [Google Scholar] [CrossRef]
- Fang, W.G.; Zhang, Y.J.; Yang, X.Y.; Zheng, X.L.; Hui, D.; Li, Y.; Pei, Y. Agrobacterium tumefaciens-mediated transformation of Beauveria bassiana using an herbicide resistance gene as a selection marker. J. Invertebr. Pathol. 2004, 85, 18–24. [Google Scholar] [CrossRef]
- Adams, A.E.; Johnson, D.I.; Longnecker, R.M.; Sloat, B.F.; Pringle, J.R. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J. Cell Biol. 1990, 111, 131–142. [Google Scholar] [CrossRef]
- Vlahou, G.; Rivero, F. Rho GTPase signaling in Dictyostelium discoideum: Insights from the genome. Eur. J. Cell Biol. 2006, 85, 947–959. [Google Scholar] [CrossRef]
- Perez, P.; Rincon, S.A. Rho GTPases: Regulation of cell polarity and growth in yeasts. Biochem. J. 2010, 426, 243–253. [Google Scholar] [CrossRef]
- Raudaskoski, M.; Kothe, E.; Fowler, T.J.; Jung, E.M.; Horton, J.S. Ras and Rho small G Proteins: Insights from the Schizophyllum commune genome sequence and comparisons to other fungi. Biotechnol. Genet. Eng. 2012, 28, 61–100. [Google Scholar] [CrossRef]
- Johnson, D.I.; Pringle, J.R. Molecular characterization of CDC42, a Saccharomyces cerevisiae gene involved in the development of cell polarity. J. Cell Biol. 1990, 111, 143–152. [Google Scholar] [CrossRef]
- Ziman, M.; O’Brien, J.M.; Ouellette, L.A.; Church, W.R.; Johnson, D.I. Mutational analysis of CDC42Sc, a Saccharomyces cerevisiae gene that encodes a putative GTP-binding protein involved in the control of cell polarity. Mol. Cell. Biol. 1991, 11, 3537–3544. [Google Scholar]
- Kozma, R.; Sarner, S.; Ahmed, S.; Lim, L. Rho family GTPases and neuronal growth cone remodelling: Relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol. Cell. Biol. 1997, 17, 1201–1211. [Google Scholar] [CrossRef]
- Adamo, J.E. Yeast Cdc42 functions at a late step in exocytosis, specifically during polarized growth of the emerging bud. J. Cell Biol. 2001, 155, 581–592. [Google Scholar] [CrossRef]
- Richman, T.J.; Sawyer, M.M.; Johnson, D.I. The Cdc42p GTPase is involved in a G2/M morphogenetic checkpoint regulating the apical-isotropic switch and nuclear division in yeast. J. Biol. Chem. 1999, 274, 16861–16870. [Google Scholar] [CrossRef]
- Miller, P.J.; Johnson, D.I. Cdc42p GTPase is involved in controlling polarized cell growth in Schizosaccharomyces pombe. Mol. Cell. Biol. 1994, 14, 1075–1083. [Google Scholar]
- Woods, B.; Kuo, C.C.; Wu, C.F.; Zyla, T.R.; Lew, D.J. Polarity establishment requires localized activation of Cdc42. J. Cell Biol. 2015, 211, 19–26. [Google Scholar] [CrossRef]
- Cid, V.J.; Adamiková, L.; Sánchez, M.; Molina, M.; Nombela, C. Cell cycle control of septin ring dynamics in the budding yeast. Microbiology 2001, 147, 1437–1450. [Google Scholar] [CrossRef]
- Zhao, Z.S.; Leung, T.; Manser, E.; Lim, L. Pheromone signalling inSaccharomyces cerevisiae requires the small GTP-binding protein Cdc42p and its activator CDC24. Mol. Cell. Biol. 1995, 15, 5246–5257. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.P.; Tepass, U. Cdc42 and vesicle trafficking in polarized cells. Traffic 2010, 11, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Virag, A.; Lee, M.P.; Si, H.; Harris, S.D. Regulation of hyphal morphogenesis by cdc42 and rac1 homologues in Aspergillus nidulans. Mol. Microbiol. 2007, 66, 1579–1596. [Google Scholar] [CrossRef]
- Boyce, K.J.; Hynes, M.J.; Andrianopoulos, A. The CDC42 homolog of the dimorphic fungus Penicillium marneffei is required for correct cell polarization during growth but not development. J. Bacteriol. 2001, 183, 3447–3457. [Google Scholar] [CrossRef]
- Ushinsky, S.C.; Harcus, D.; Ash, J.; Dignard, D.; Marcil, A.; Morchhauser, J.; Thomas, D.Y.; Whiteway, M.; Leberer, E. CDC42 is required for polarized growth in human pathogen Candida albicans. Eukaryot. Cell 2002, 1, 95–104. [Google Scholar] [CrossRef]
- Scheffer, J.; Chen, C.; Heidrich, P.; Dickman, M.B.; Tudzynski, P. A CDC42 homologue in Claviceps purpurea is involved in vegetative differentiation and is essential for pathogenicity. Eukaryot. Cell 2005, 4, 1228–1238. [Google Scholar] [CrossRef][Green Version]
- Kokkelink, L.; Minz, A.; Al-Masri, M.; Giesbert, S.; Barakat, R.; Sharon, A.; Tudzynski, P. The small GTPase BcCdc42 affects nuclear division, germination and virulence of the gray mold fungus Botrytis cinerea. Fungal Genet. Biol. 2011, 48, 1012–1019. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Liang, Y.; Wang, Y.; Tian, C. A Cdc42 homolog in Colletotrichum gloeosporioides regulates morphological development and is required for ROS-mediated plant infection. Curr. Genet. 2018, 64, 1153–1169. [Google Scholar] [CrossRef]
- Jiang, S.S.; Yin, Y.P.; Song, Z.Y.; Zhou, G.L.; Wang, Z.K. RacA and Cdc42 regulate polarized growth and microsclerotium formation in the dimorphic fungus Nomuraea rileyi. Res. Microbiol. 2014, 165, 233–242. [Google Scholar] [CrossRef]
- Zhang, L.B.; Qiu, T.T.; Huang, Z.H.; Ye, X.Y.; Guan, Y. Transcriptomic analysis of sur7-mediated response of Beauveria bassiana to different nutritional conditions. FEMS Microbiol. Lett. 2021, 368, fnab003. [Google Scholar] [CrossRef]
- Xiao, G.H.; Ying, S.H.; Zheng, P.; Wang, Z.L.; Zhang, S.W.; Xie, X.Q.; Shang, Y.F.; St Leger, R.J.; Zhao, G.P.; Wang, C.S.; et al. Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci. Rep. 2012, 2, 483. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.Q.; Li, F.; Ying, S.H.; Feng, M.G. Additive contributions of two manganese-cored superoxide dismutases (MnSODs) to antioxidation, UV tolerance and virulence of Beauveria bassiana. PLoS ONE 2012, 7, e30298. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.Q.; Guan, Y.; Ying, S.H.; Feng, M.G. Differentiated functions of Ras1 and Ras2 proteins in regulating the germination, growth, conidiation, multi-stress tolerance and virulence of Beauveria bassiana. Environ. Microbiol. 2013, 15, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Wang, D.Y.; Ying, S.H.; Feng, M.G. A novel Ras GTPase (Ras3) regulates conidiation, multi-stress tolerance and virulence by acting upstream of hog1 signaling pathway in Beauveria bassiana. Fungal Genet. Biol. 2015, 82, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Wang, J.; Ying, S.H.; Hu, Y.; Feng, M.G. Mas5, a homologue of bacterial DnaJ, is indispensable for the host infection and environmental adaptation of a filamentous fungal insect pathogen. Environ. Microbiol. 2016, 18, 1037–1047. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, Z.Y.; Chen, J.S.; Liu, W.D.; Ke, H.Y.; Zhou, J.; Lu, G.D.; Darvill, A.G.; Albersheim, P.; Wu, S.C.; et al. A Cdc42 ortholog is required for penetration and virulence of Magnaporthe grisea. Fungal Genet. Biol. 2009, 46, 450–460. [Google Scholar] [CrossRef]
- Saeed, F.A. Production of pyruvate by Candida albicans: Proposed role in virulence. FEMS Microbiol. Lett. 2000, 190, 35–38. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Y.; Wang, D.; Lin, X.; Li, X.; Lv, C.; Wang, D.; Zhang, L. Unveiling a Novel Role of Cdc42 in Pyruvate Metabolism Pathway to Mediate Insecticidal Activity of Beauveria bassiana. J. Fungi 2022, 8, 394. https://doi.org/10.3390/jof8040394
Guan Y, Wang D, Lin X, Li X, Lv C, Wang D, Zhang L. Unveiling a Novel Role of Cdc42 in Pyruvate Metabolism Pathway to Mediate Insecticidal Activity of Beauveria bassiana. Journal of Fungi. 2022; 8(4):394. https://doi.org/10.3390/jof8040394
Chicago/Turabian StyleGuan, Yi, Donghuang Wang, Xiaofeng Lin, Xin Li, Chao Lv, Dingyi Wang, and Longbin Zhang. 2022. "Unveiling a Novel Role of Cdc42 in Pyruvate Metabolism Pathway to Mediate Insecticidal Activity of Beauveria bassiana" Journal of Fungi 8, no. 4: 394. https://doi.org/10.3390/jof8040394
APA StyleGuan, Y., Wang, D., Lin, X., Li, X., Lv, C., Wang, D., & Zhang, L. (2022). Unveiling a Novel Role of Cdc42 in Pyruvate Metabolism Pathway to Mediate Insecticidal Activity of Beauveria bassiana. Journal of Fungi, 8(4), 394. https://doi.org/10.3390/jof8040394





