Targeted Delivery of Antifungal Liposomes to Rhizopus delemar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Culture Conditions
2.2. Liposome Preparations and Fluorescent Tagging of Dectin-1
2.3. Liposome Binding Activity
2.4. Liposome Inhibition Activity
2.5. Data Management
3. Results
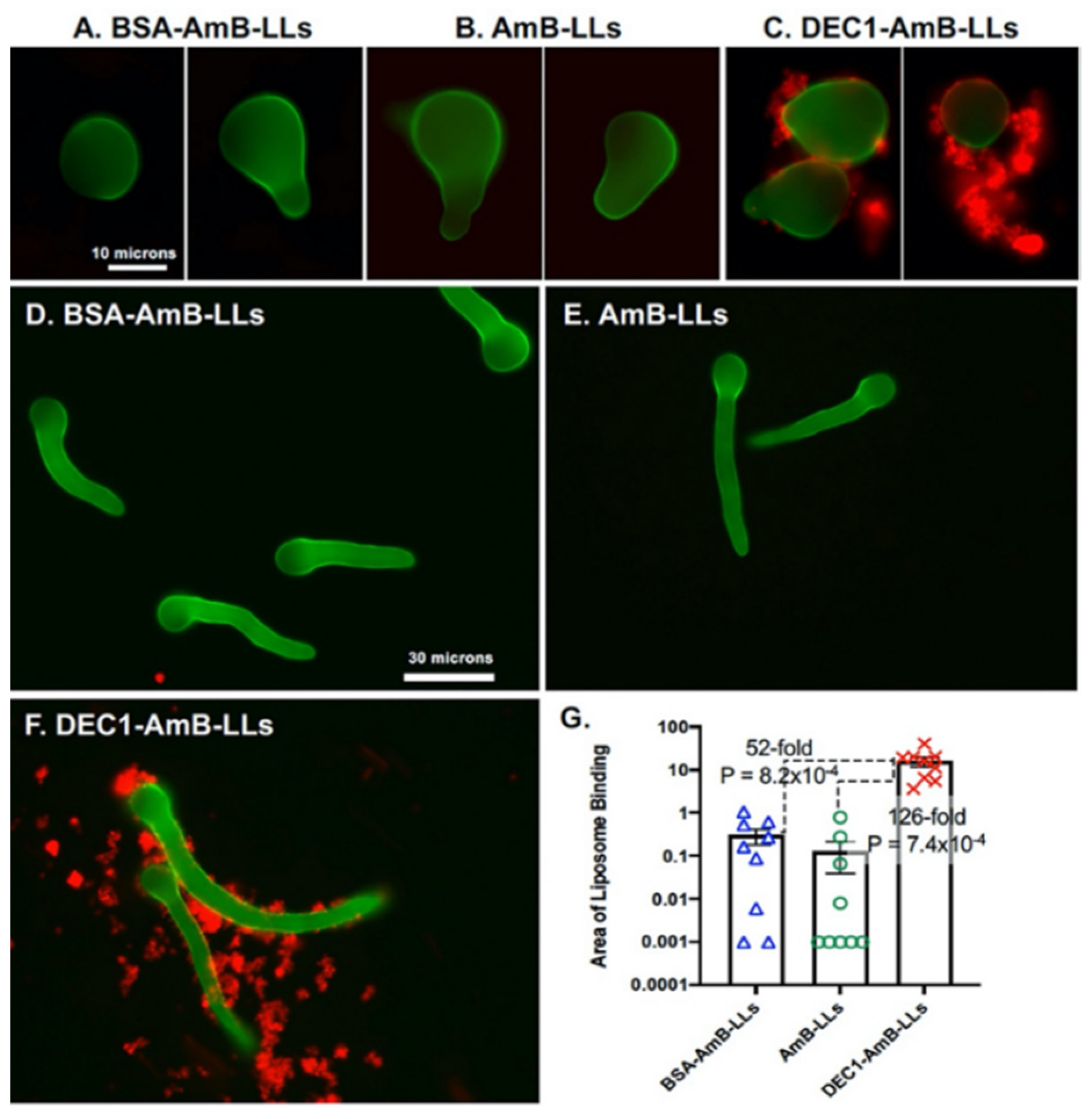
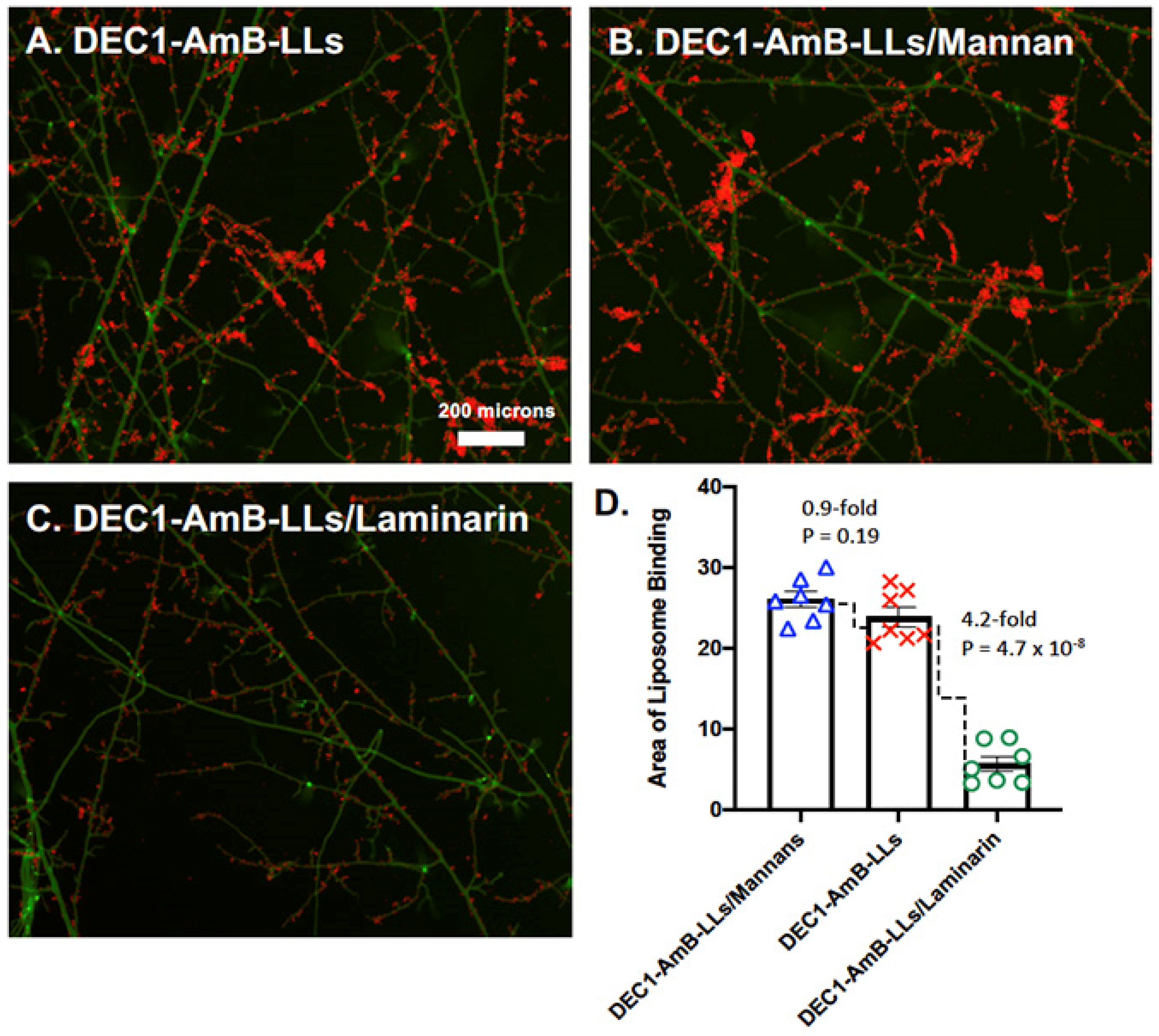
3.1. Dectin-1 Targeted DEC1-AmB-LLs Bind to R. delemar
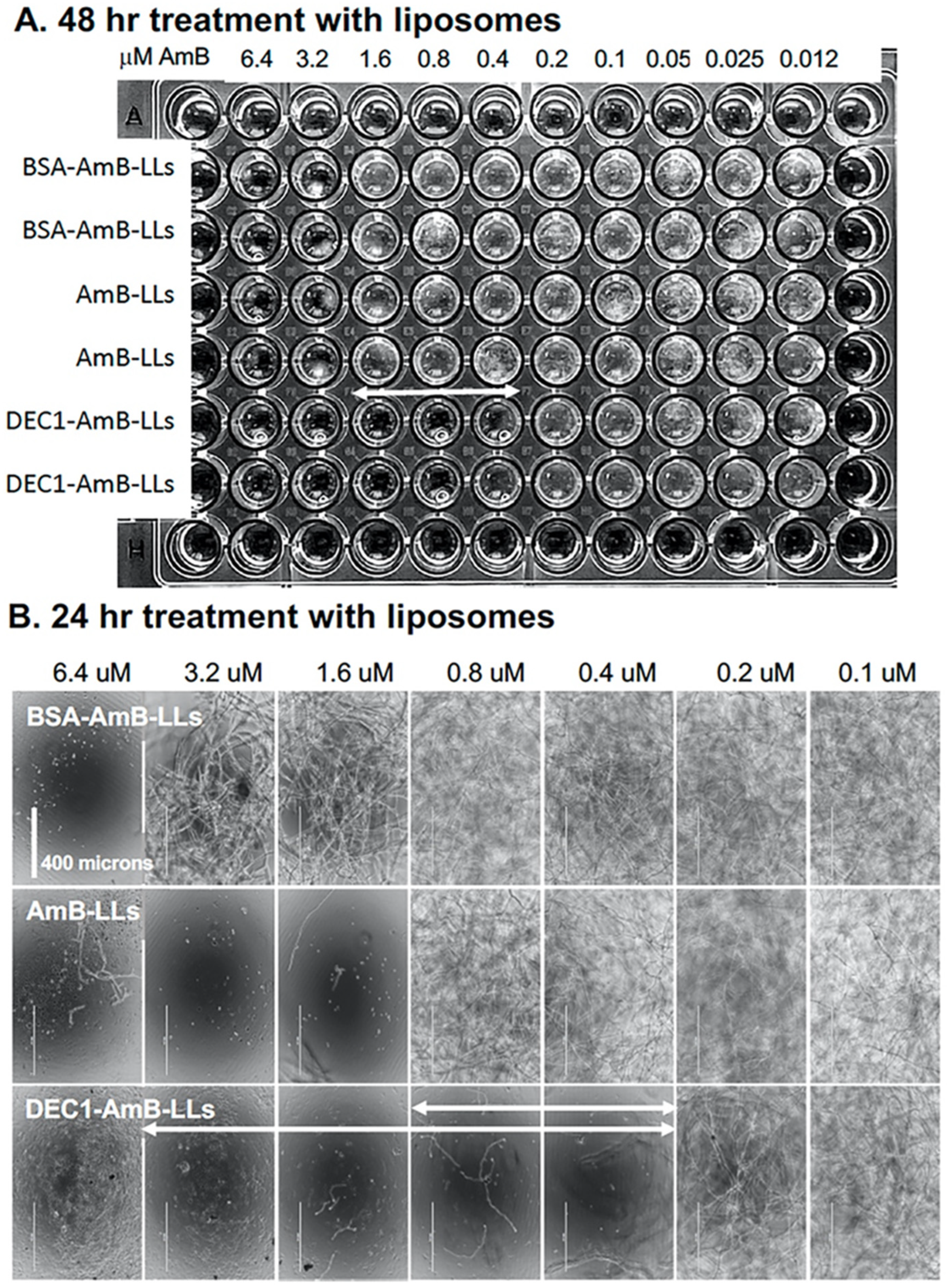
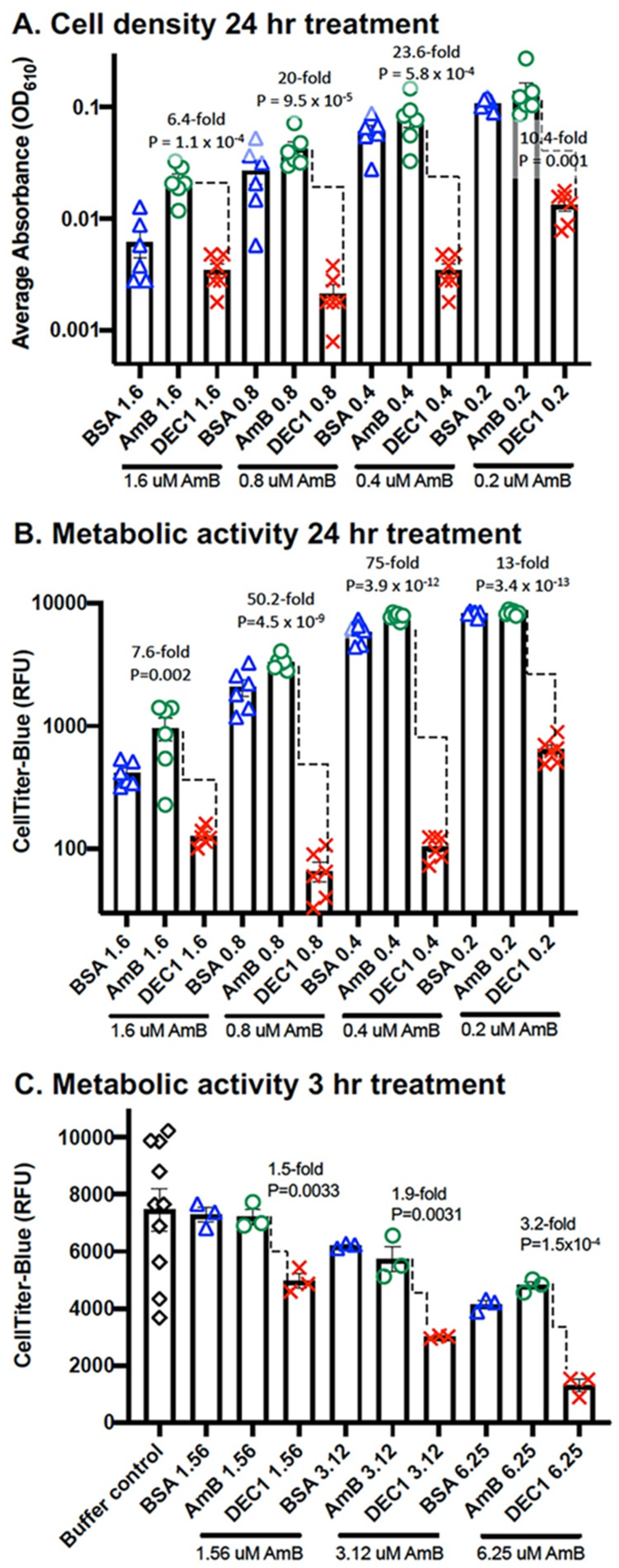
3.2. DEC1-AmB-LLs Efficiently Inhibit and/or Kill R. delemar
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prakash, H.; Chakrabarti, A. Global Epidemiology of Mucormycosis. J. Fungi 2019, 5, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Lewis, R. Invasive Zygomycosis: Update on Pathogenesis, Clinical Manifestations, and Management. Infect. Dis. Clin. N. Am. 2006, 20, 581–607. [Google Scholar] [CrossRef] [PubMed]
- Pyrgos, V.; Shoham, S.; Walsh, T. Pulmonary Zygomycosis. Semin. Respir. Crit. Care Med. 2008, 29, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Voigt, K. Pathogenicity patterns of mucormycosis: Epidemiology, interaction with immune cells and virulence factors. Med. Mycol. 2019, 57, S245–S256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Kong, D.C.M.; Chen, S.C.-A. The epidemiology and clinical manifestations of mucormycosis: A systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019, 25, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Qiao, J.; Giovanni, G.; Liu, G.; Yang, H.; Wu, J.; Chen, J. Mucormycosis in renal transplant recipients: Review of 174 reported cases. BMC Infect. Dis. 2017, 17, 283. [Google Scholar] [CrossRef]
- Banerjee, I.; Robinson, J.; Asim, M.; Sathian, B.; Banerjee, I. Mucormycosis and COVID-19 an epidemic in a pandemic? Nepal J. Epidemiol. 2021, 11, 1034–1039. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Spellberg, B.; Walsh, T.J.; Kontoyiannis, D.P. Pathogenesis of Mucormycosis. Clin. Infect. Dis. 2012, 54, S16–S22. [Google Scholar] [CrossRef]
- Prakash, H.; Skiada, A.; Paul, R.; Chakrabarti, A.; Rudramurthy, S. Connecting the Dots: Interplay of Pathogenic Mechanisms between COVID-19 Disease and Mucormycosis. J. Fungi 2021, 7, 616. [Google Scholar] [CrossRef]
- Garre, V. Recent Advances and Future Directions in the Understanding of Mucormycosis. Front. Cell. Infect. Microbiol. 2022, 12, 850581. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Castrillón-Betancur, J.C.; Klaile, E.; Slevogt, H. The Interaction of Human Pathogenic Fungi with C-Type Lectin Receptors. Front. Immunol. 2018, 9, 1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, D. (Ed.) Fungi Pathogenic for Humans and Animals; Dekker Incorporated, Marcel: New York, NY, USA, 1983; p. 652. [Google Scholar]
- Klimko, N.; Khostelidi, S.; Shadrivova, O.; Volkova, A.; Popova, M.; Uspenskaya, O.; Shneyder, T.; Bogomolova, T.; Ignatyeva, S.; Zubarovskaya, L.; et al. Contrasts between mucormycosis and aspergillosis in oncohematological patients. Med. Mycol. 2019, 57, S138–S144. [Google Scholar] [CrossRef]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef]
- Patel, A.; Kaur, H.; Xess, I.; Michael, J.; Savio, J.; Rudramurthy, S.; Singh, R.; Shastri, P.; Umabala, P.; Sardana, R.; et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin. Microbiol. Infect. 2019, 26, 944.e9–944.e15. [Google Scholar] [CrossRef]
- Hamilos, G.; Samonis, G.; Kontoyiannis, D.P. Pulmonary Mucormycosis. Semin. Respir. Crit. Care Med. 2011, 32, 693–702. [Google Scholar] [CrossRef]
- Hong, H.-L.; Lee, Y.-M.; Kim, T.; Lee, J.-Y.; Chung, Y.-S.; Kim, M.-N.; Kim, S.-H.; Choi, S.-H.; Kim, Y.S.; Woo, J.H.; et al. Risk Factors for Mortality in Patients with Invasive Mucormycosis. Infect. Chemother. 2013, 45, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Chamilos, G.; Ganguly, D.; Lande, R.; Gregorio, J.; Meller, S.; Goldman, W.E.; Gilliet, M.; Kontoyiannis, D.P. Generation of IL-23 Producing Dendritic Cells (DCs) by Airborne Fungi Regulates Fungal Pathogenicity via the Induction of TH-17 Responses. PLoS ONE 2010, 5, e12955. [Google Scholar] [CrossRef] [Green Version]
- Camargo, J.F.; Bhimji, A.; Kumar, D.; Kaul, R.; Pavan, R.; Schuh, A.; Seftel, M.; Lipton, J.H.; Gupta, V.; Humar, A.; et al. Impaired T Cell Responsiveness to Interleukin-6 in Hematological Patients with Invasive Aspergillosis. PLoS ONE 2015, 10, e0123171. [Google Scholar] [CrossRef]
- Brown, J.; O’Callaghan, C.A.; Marshall, A.; Gilbert, R.; Siebold, C.; Gordon, S.; Brown, G.; Jones, E.Y. Structure of the fungal β-glucan-binding immune receptor dectin-1: Implications for function. Protein Sci. 2007, 16, 1042–1052. [Google Scholar] [CrossRef]
- Ambati, S.; Pham, T.; Lewis, Z.A.; Lin, X.; Meagher, R.B. DC-SIGN targets amphotericin B-loaded liposomes to diverse pathogenic fungi. Fungal Biol. Biotechnol. 2021, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Ambati, S.; Pham, T.; Lewis, Z.A.; Lin, X.; Meagher, R.B. DectiSomes: Glycan Targeting of Liposomal Drugs Improves the Treatment of Disseminated Candidiasis. Antimicrob. Agents Chemother. 2022, 66, e01467-21. [Google Scholar] [CrossRef]
- Meagher, R.B.; Lewis, Z.A.; Ambati, S.; Lin, X. Aiming for a bull’s-eye: Targeting antifungals to fungi with dectin-decorated liposomes. PLoS Pathog. 2021, 17, e1009699. [Google Scholar] [CrossRef] [PubMed]
- Ambati, S.; Ferarro, A.R.; Khang, S.E.; Lin, J.; Lin, X.; Momany, M.; Lewis, Z.A.; Meagher, R.B. Dectin-1-Targeted Antifungal Liposomes Exhibit Enhanced Efficacy. mSphere 2019, 4, e00025-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramana, L.N.; Sharma, S.; Sethuraman, S.; Ranga, U.; Krishnan, U.M. Stealth anti-CD4 conjugated immunoliposomes with dual antiretroviral drugs—Modern Trojan horses to combat HIV. Eur. J. Pharm. Biopharm. 2015, 89, 300–311. [Google Scholar] [CrossRef]
- Tenchov, R. Understanding the Nanotechnology in COVID-19 Vaccines. CAS. 18 February 2021, pp. 1–15. Available online: https://www.cas.org/resource/blog/understanding-nanotechnology-covid-19-vaccines (accessed on 18 February 2021).
- Ambati, S.; Ellis, E.C.; Lin, J.; Lin, X.; Lewis, Z.A.; Meagher, R.B. Dectin-2-Targeted Antifungal Liposomes Exhibit Enhanced Efficacy. mSphere 2019, 4, e00715-19. [Google Scholar] [CrossRef] [Green Version]
- Andrianaki, A.M.; Kyrmizi, I.; Thanopoulou, K.; Baldin, C.; Drakos, E.; Soliman, S.S.M.; Shetty, A.C.; McCracken, C.; Akoumianaki, T.; Stylianou, K.; et al. Iron restriction inside macrophages regulates pulmonary host defense against Rhizopus species. Nat. Commun. 2018, 9, 3333. [Google Scholar] [CrossRef] [Green Version]
- Hamidi, M.; Okoro, O.V.; Milan, P.B.; Khalili, M.R.; Samadian, H.; Nie, L.; Shavandi, A. Fungal exopolysaccharides: Properties, sources, modifications, and biomedical applications. Carbohydr. Polym. 2022, 284, 119152. [Google Scholar] [CrossRef]
- Adams, E.L.; Rice, P.J.; Graves, B.; Ensley, H.E.; Yu, H.; Brown, G.D.; Gordon, S.; Monteiro, M.A.; Papp-Szabo, E.; Lowman, D.W.; et al. Differential High-Affinity Interaction of Dectin-1 with Natural or Synthetic Glucans Is Dependent upon Primary Structure and Is Influenced by Polymer Chain Length and Side-Chain Branching. J. Pharmacol. Exp. Ther. 2008, 325, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Anderson, T.M.; Clay, M.C.; Cioffi, A.G.; Diaz, K.A.; Hisao, G.S.; Tuttle, M.D.; Nieuwkoop, A.; Comellas, G.; Maryum, N.; Wang, S.; et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 2014, 10, 400–406. [Google Scholar] [CrossRef]
- Ribes, J.A.; Vanover-Sams, C.L.; Baker, D.J. Zygomycetes in Human Disease. Clin. Microbiol. Rev. 2000, 13, 236–301. [Google Scholar] [CrossRef] [PubMed]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [Green Version]
- Spatafora, J.W.; Aime, M.C.; Grigoriev, I.V.; Martin, F.; Stajich, J.E.; Blackwell, M. The Fungal Tree of Life: From Molecular Systematics to Genome-Scale Phylogenies. Microbiol. Spectr. 2017, 5, 3–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padovan, A.C.B.; Sanson, G.F.; Brunstein, A.; Briones, M.R. Fungi Evolution Revisited: Application of the Penalized Likelihood Method to a Bayesian Fungal Phylogeny Provides a New Perspective on Phylogenetic Relationships and Divergence Dates of Ascomycota Groups. J. Mol. Evol. 2005, 60, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Bartnicki-Garcia, S.; Reyes, E. Chemistry of spore wall differentiation in Mucor rouxii. Arch. Biochem. Biophys. 1964, 108, 125–133. [Google Scholar] [CrossRef]
- Lecointe, K.; Cornu, M.; Leroy, J.; Coulon, P.; Sendid, B. Polysaccharides Cell Wall Architecture of Mucorales. Front. Microbiol. 2019, 10, 469. [Google Scholar] [CrossRef]
- Yu, W.; Chen, G.; Zhang, P.; Chen, K. Purification, partial characterization and antitumor effect of an exopolysaccharide from Rhizopus nigricans. Int. J. Biol. Macromol. 2016, 82, 299–307. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Latge, J.-P.; Munro, C.A. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5, 28513415. [Google Scholar] [CrossRef] [Green Version]
- Reichhardt, C.; A Stevens, D.; Cegelski, L. Fungal biofilm composition and opportunities in drug discovery. Future Med. Chem. 2016, 8, 1455–1468. [Google Scholar] [CrossRef] [Green Version]
- Lagree, K.; Mitchell, A.P. Fungal Biofilms: Inside Out. Microbiol. Spectr. 2017, 5, 873–886. [Google Scholar] [CrossRef]
- Hamill, R.J. Amphotericin B Formulations: A Comparative Review of Efficacy and Toxicity. Drugs 2013, 73, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Deray, G. Amphotericin B nephrotoxicity. J. Antimicrob. Chemother. 2002, 49, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Stanzani, M.; Vianelli, N.; Cavo, M.; Maritati, A.; Morotti, M.; Lewis, R.E. Retrospective Cohort Analysis of Liposomal Amphotericin B Nephrotoxicity in Patients with Hematological Malignancies. Antimicrob. Agents Chemother. 2017, 61, e02651-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Chen, S.C.-A.; Kong, D.C. Contemporary management and clinical outcomes of mucormycosis: A systematic review and meta-analysis of case reports. Int. J. Antimicrob. Agents 2019, 53, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Floros, L.; Kuessner, D.; Posthumus, J.; Bagshaw, E.; Sjölin, J. Cost-effectiveness analysis of isavuconazole versus voriconazole for the treatment of patients with possible invasive aspergillosis in Sweden. BMC Infect. Dis. 2019, 19, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skiada, A.; Lass-Floerl, C.; Klimko, N.; Ibrahim, A.; Roilides, E.; Petrikkos, G. Challenges in the diagnosis and treatment of mucormycosis. Med. Mycol. 2018, 56, S93–S101. [Google Scholar] [CrossRef] [Green Version]
- Dannaoui, E. Antifungal resistance in mucorales. Int. J. Antimicrob. Agents 2017, 50, 617–621. [Google Scholar] [CrossRef]
- Riley, T.T.; Muzny, C.; Swiatlo, E.; Legendre, D.P. Breaking the Mold: A Review of Mucormycosis and Current Pharmacological Treatment Options. Ann. Pharmacother. 2016, 50, 747–757. [Google Scholar] [CrossRef]
- Merino, M.; Zalba, S.; Garrido, M.J. Immunoliposomes in clinical oncology: State of the art and future perspectives. J. Control. Release 2018, 275, 162–176. [Google Scholar] [CrossRef]
- Eloy, J.O.; Petrilli, R.; Trevizan, L.N.F.; Chorilli, M. Immunoliposomes: A review on functionalization strategies and targets for drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 454–467. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Sun, Y.; Liu, Y.; Meng, F.; Lee, R.J. Clinical translation of immunoliposomes for cancer therapy: Recent perspectives. Expert Opin. Drug Deliv. 2018, 15, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Suvvari, T.K.; Arigapudi, N.; Kandi, V.R.; Kutikuppala, L.S. Mucormycosis: A killer in the shadow of COVID-19. J. Mycol. Med. 2021, 31, 101161. [Google Scholar] [CrossRef] [PubMed]
- Selarka, L.; Sharma, S.; Saini, D.; Sharma, S.; Batra, A.; Waghmare, V.T.; Dileep, P.; Patel, S.; Shah, M.; Parikh, T.; et al. Mucormycosis and COVID-19: An epidemic within a pandemic in India. Mycoses 2021, 64, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Singh, B.; Bhadada, S.K.; Banerjee, M.; Bhogal, R.S.; Hage, N.; Kumar, A. COVID-19-associated mucormycosis: An updated systematic review of literature. Mycoses 2021, 64, 1452–1459. [Google Scholar] [CrossRef]
- Bhanuprasad, K.; Manesh, A.; Devasagayam, E.; Varghese, L.; Cherian, L.M.; Kurien, R.; Karthik, R.; Deodhar, D.; Vanjare, H.; Peter, J.; et al. Risk factors associated with the mucormycosis epidemic during the COVID-19 pandemic. Int. J. Infect. Dis. 2021, 111, 267–270. [Google Scholar] [CrossRef]
- Walther, G.; Wagner, L.; Kurzai, O. Updates on the Taxonomy of Mucorales with an Emphasis on Clinically Important Taxa. J. Fungi 2019, 5, 106. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhury, Q.J.; Ambati, S.; Lewis, Z.A.; Meagher, R.B. Targeted Delivery of Antifungal Liposomes to Rhizopus delemar. J. Fungi 2022, 8, 352. https://doi.org/10.3390/jof8040352
Choudhury QJ, Ambati S, Lewis ZA, Meagher RB. Targeted Delivery of Antifungal Liposomes to Rhizopus delemar. Journal of Fungi. 2022; 8(4):352. https://doi.org/10.3390/jof8040352
Chicago/Turabian StyleChoudhury, Quanita J., Suresh Ambati, Zachary A. Lewis, and Richard B. Meagher. 2022. "Targeted Delivery of Antifungal Liposomes to Rhizopus delemar" Journal of Fungi 8, no. 4: 352. https://doi.org/10.3390/jof8040352
APA StyleChoudhury, Q. J., Ambati, S., Lewis, Z. A., & Meagher, R. B. (2022). Targeted Delivery of Antifungal Liposomes to Rhizopus delemar. Journal of Fungi, 8(4), 352. https://doi.org/10.3390/jof8040352





