Comparison Approach for Identifying Missed Invasive Fungal Infections in Formalin-Fixed, Paraffin-Embedded Autopsy Specimens
Abstract
1. Introduction
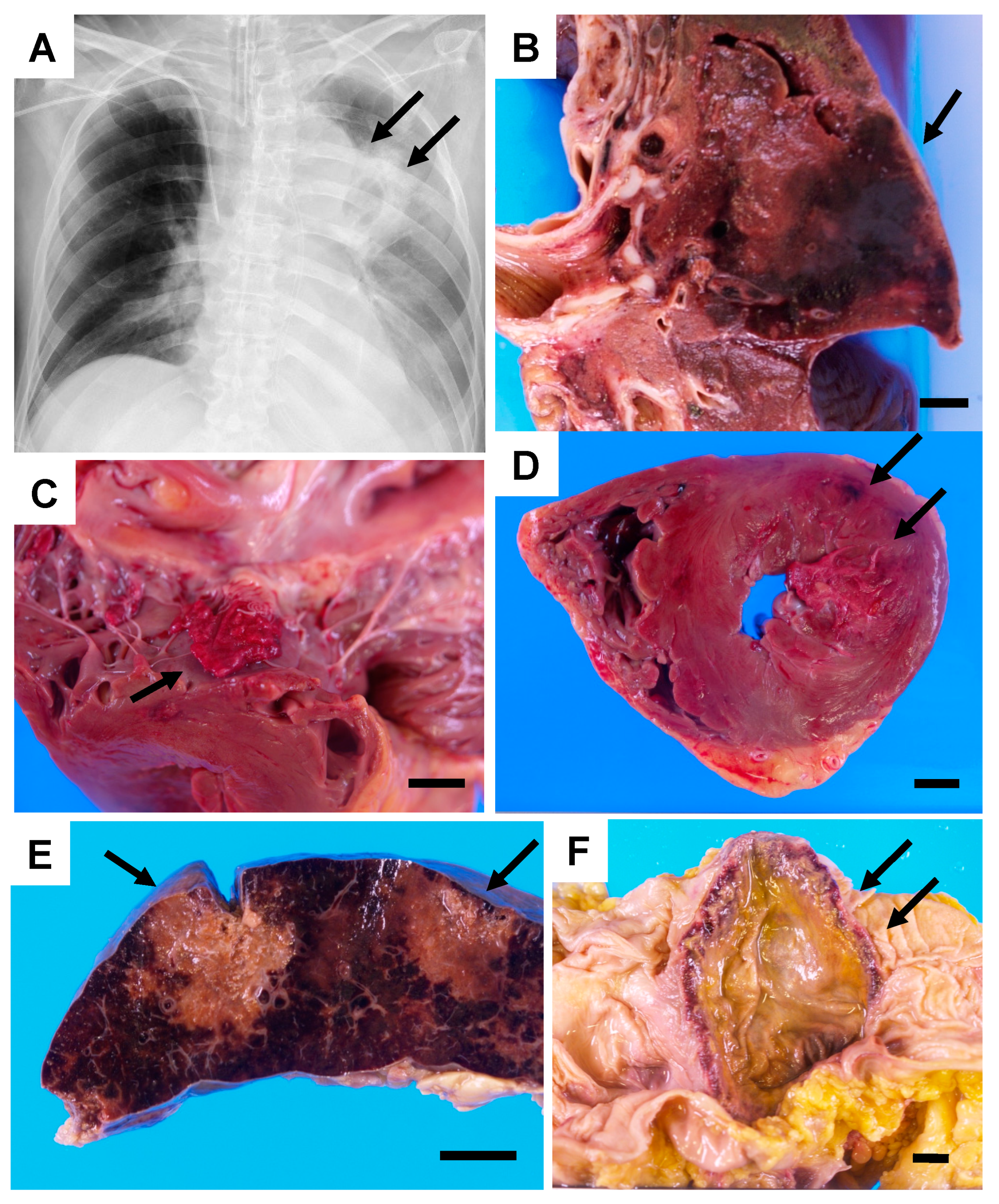
2. Case Description
3. Materials and Methods
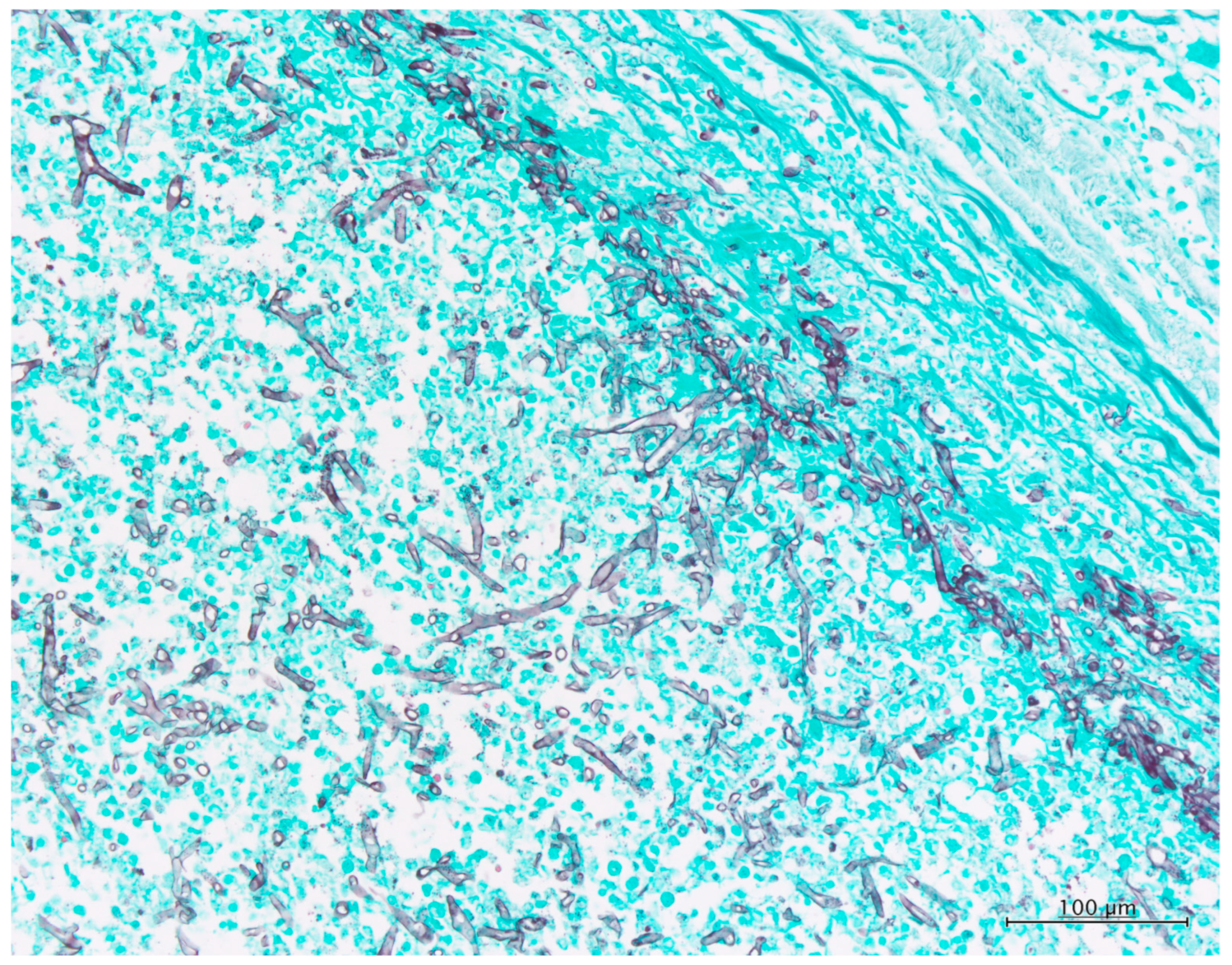
3.1. Histopathology
3.2. DNA Extraction, PCR, and Sequencing
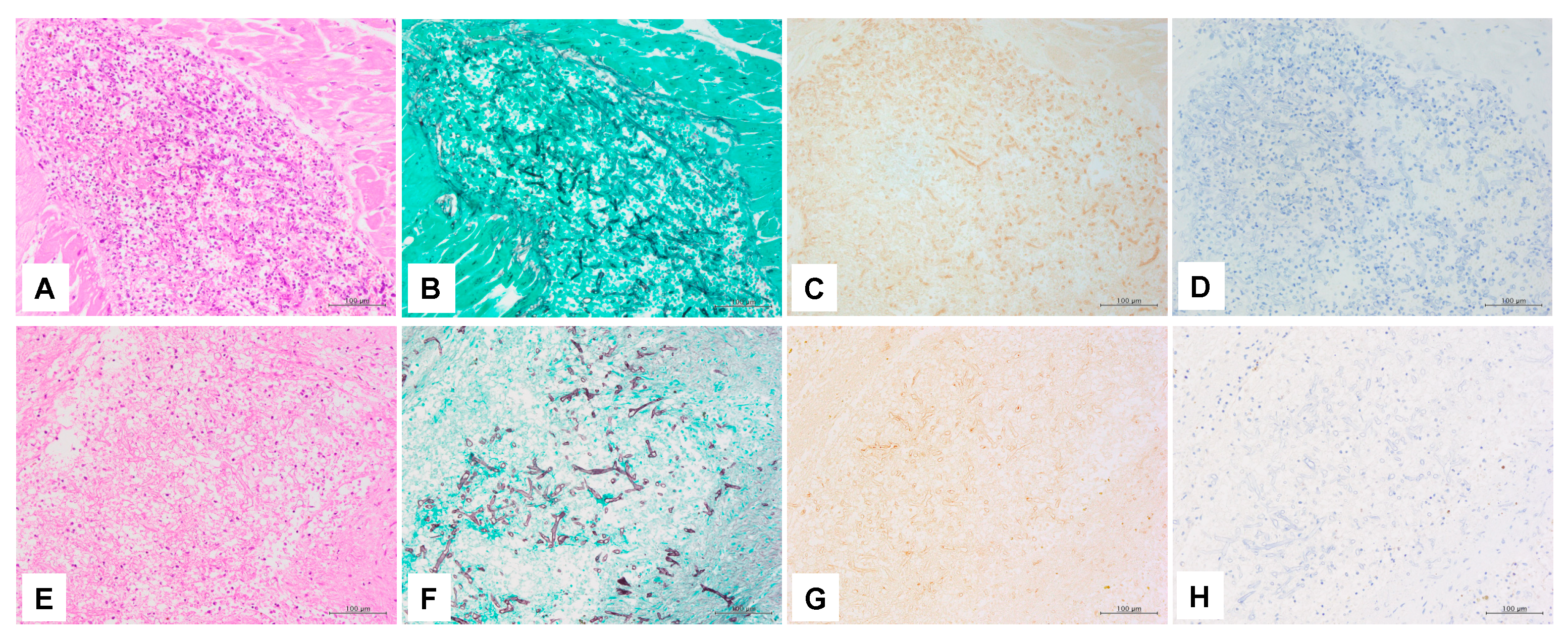
3.3. IHC
3.4. ISH
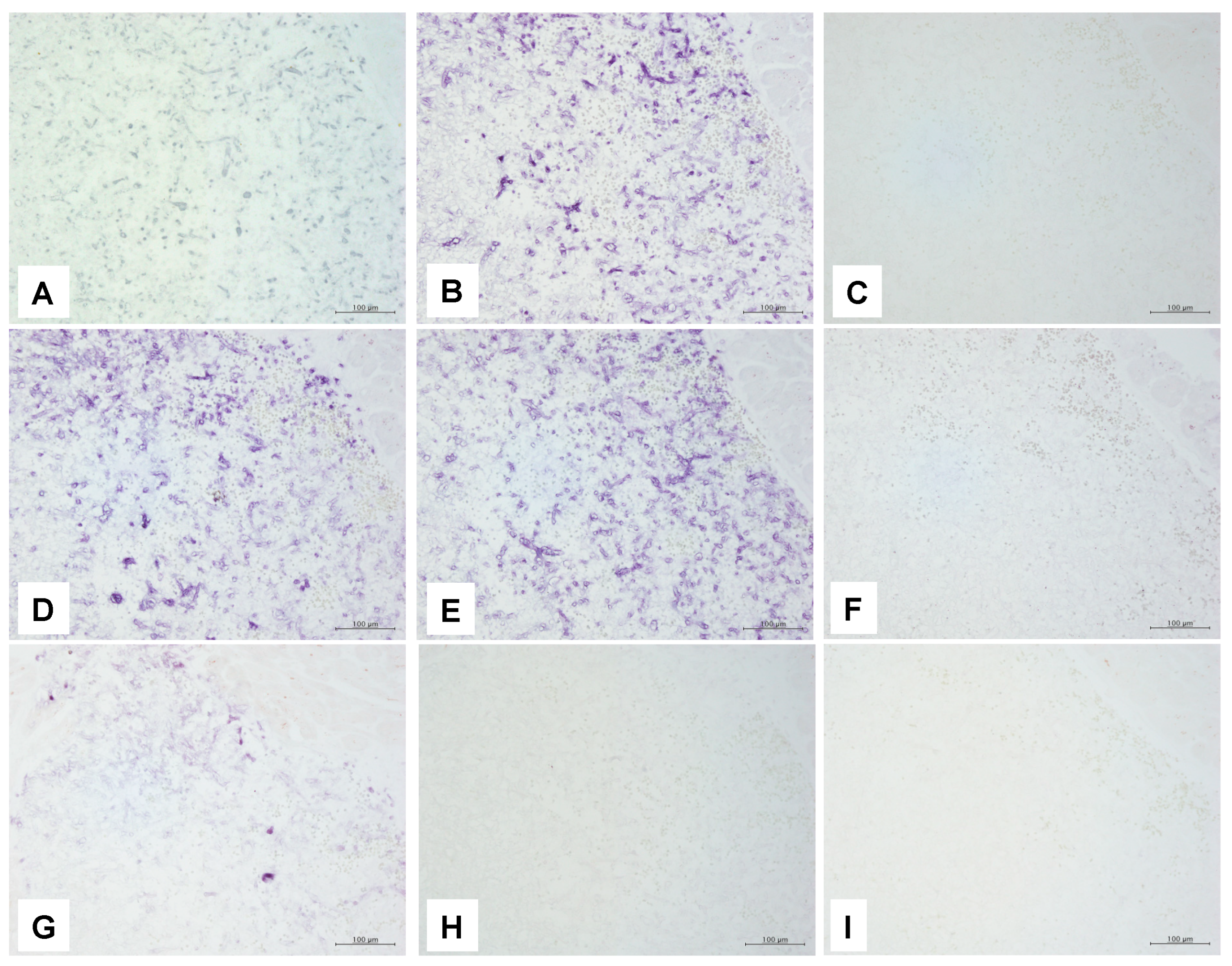
3.4.1. ISH Probes Used in This Study
3.4.2. ISH Using the PNA Probe
3.4.3. ISH Using dsDNA and Oligonucleotide DNA Probes
4. Results
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guarner, J.; Brandt, M.E. Histopathologic diagnosis of fungal infections in the 21st century. Clin. Microbiol. Rev. 2011, 24, 247–280. [Google Scholar] [PubMed]
- Sangoi, A.R.; Rogers, W.M.; Longacre, T.A.; Montoya, J.G.; Baron, E.J.; Banaei, N. Challenges and Pitfalls of Morphologic Identification of Fungal Infections in Histologic and Cytologic Specimens. Am. J. Clin. Pathol. 2009, 131, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Bialek, R.; Kibbler, C.C.; Cuenca-Estrella, M.; Jensen, H.E.; Kontoyiannis, D.P. Molecular Techniques for Genus and Species Determination of Fungi from Fresh and Paraffin-Embedded Formalin-Fixed Tissue in the Revised EORTC/MSGERC Definitions of Invasive Fungal Infection. Clin. Infect. Dis. 2021, 72, S109–S113. [Google Scholar] [CrossRef]
- Sengüven, B.; Baris, E.; Oygur, T.; Berktas, M. Comparison of methods for the extraction of DNA from formalin-fixed, paraffin-embedded archival tissues. Int. J. Med. Sci. 2014, 11, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Simbolo, M.; Gottardi, M.; Corbo, V.; Fassan, M.; Mafficini, A.; Malpeli, G.; Lawlor, R.T.; Scarpa, A. DNA Qualification Workflow for Next Generation Sequencing of Histopathological Samples. PLoS ONE 2013, 8, e62692. [Google Scholar] [CrossRef]
- Sato, M.; Kojima, M.; Nagatsuma, A.K.; Nakamura, Y.; Saito, N.; Ochiai, A. Optimal fixation for total preanalytic phase evaluation in pathology laboratories. A comprehensive study including immunohistochemistry, DNA, and mRNA assays. Pathol. Int. 2014, 64, 209–216. [Google Scholar] [CrossRef]
- Zhi, Y.; Sasai, D.; Okubo, Y.; Shinozaki, M.; Nakayama, H.; Murayama, S.Y.; Wakayama, M.; Ide, T.; Zhang, Z.; Shibuya, K. Comparison between the effectiveness of polymerase chain reaction and In situ hybridization in detecting the presence of pathogenic fungi by using the preserved DNA in formalin-fixed and paraffin-embedded tissues. Jpn. J. Infect. Dis. 2013, 66, 173–179. [Google Scholar] [CrossRef]
- Shinozaki, M.; Tochigi, N.; Sadamoto, S.; Yamagata Murayama, S.; Wakayama, M.; Nemoto, T.; Shibuya, K. Technical Aspects and Applications for Developing in situ Hybridization Procedures for Formalin-Fixed and Paraffin-Embedded (FFPE) Tissues for Diagnosis of Fungal Infections. Med. Mycol. J. 2017, 58, E33–E37. [Google Scholar] [CrossRef]
- Fukumoto, H.; Sato, Y.; Hasegawa, H.; Saeki, H.; Katano, H. Development of a new real-time PCR system for simultaneous detection of bacteria and fungi in pathological samples. Int. J. Clin. Exp. Pathol. 2015, 8, 15479–15488. [Google Scholar]
- The Japanese Society of Pathology [Guidelines on the Handling of Pathological Tissue Samples for Genomic Medicine] Genomu Shinryo-you Biyouri Soshikikentaitoriatukaikitei. Available online: http://pathology.or.jp/genome_med/pdf/textbook.pdf (accessed on 18 January 2022). (In Japanese).
- Multani, A.; Allard, L.S.; Wangjam, T.; Sica, R.A.; Epstein, D.J.; Rezvani, A.R.; Ho, D.Y. Missed diagnosis and misdiagnosis of infectious diseases in hematopoietic cell transplant recipients: An autopsy study. Blood Adv. 2019, 3, 3602–3612. [Google Scholar] [CrossRef]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kume, H.; Togano, T.; Ohto, H. Epidemiology of zygomycosis: Analysis of national data from pathological autopsy cases in japan: Data from 1989 to 2009. Med. Mycol. J. 2017, 58, E89–E95. [Google Scholar] [CrossRef] [PubMed]
- Montone, K.T. Differentiation of Fusarium From Aspergillus Species by Colorimetric In Situ Hybridization in Formalin-Fixed, Paraffin-Embedded Tissue Sections Using Dual Fluorogenic-Labeled LNA Probes. Am. J. Clin. Pathol. 2009, 132, 866–870. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal Rna Genes for Phylogenetics. In PCR Protocols; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Robnett, C.J. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5’ end of the large-subunit (26S) ribosomal DNA gene. J. Clin. Microbiol. 1997, 35, 1216–1223. [Google Scholar] [CrossRef]
- Rickerts, V.; Just-Nübling, G.; Konrad, F.; Kern, J.; Lambrecht, E.; Böhme, A.; Jacobi, V.; Bialek, R. Diagnosis of invasive aspergillosis and mucormycosis in immunocompromised patients by seminested PCR assay of tissue samples. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 8–13. [Google Scholar] [CrossRef]
- Sadamoto, S.; Shinozaki, M.; Nagi, M.; Nihonyanagi, Y.; Ejima, K.; Mitsuda, A.; Wakayama, M.; Tochigi, N.; Murakami, Y.; Hishima, T.; et al. Histopathological study on the prevalence of trichosporonosis in formalin-fixed and paraffin-embedded tissue autopsy sections by in situ hybridization with peptide nucleic acid probe. Med. Mycol. 2020, 58, 460–468. [Google Scholar] [CrossRef]
- Shinozaki, M.; Okubo, Y.; Sasai, D.; Nakayama, H.; Murayama, S.Y.; Ide, T.; Wakayama, M.; Hiruta, N.; Shibuya, K. Identification of Fusarium Species in Formalin-Fixed and Paraffin-Embedded Sections by In Situ Hybridization Using Peptide Nucleic Acid Probes. J. Clin. Microbiol. 2011, 49, 808–813. [Google Scholar] [CrossRef]
- Hanazawa, R.; Murayama, S.Y.; Yamaguchi, H. In-situ detection of Aspergillus fumigatus. J. Med. Microbiol. 2000, 49, 285–290. [Google Scholar] [CrossRef][Green Version]
- Makimura, K.; Murayama, S.Y.; Yamaguchi, H. Detection of a wide range of medically important fungi by the polymerase chain reaction. J. Med. Microbiol. 1994, 40, 358–364. [Google Scholar] [CrossRef]
- Eriko, F.; Mikawa, T.; Nihei, H.; Endo, S.; Suzuki, M.; Hasegawa, M.; Yaguchi, T.; Kimura, M. Specific Detection Method of 18S rRNA for Conidiobolomycosis-, Mucormycosis- and Aspergillosis-causing Fungi by in situ Hybridization Method Using MicroProbe System on Paraffin-embedded Tissue Sections. Jpn. J. Med. Mycol. 2019, 60, 77–84. (In Japanese) [Google Scholar]
- Hayden, R.T.; Qian, X.; Procop, G.W.; Roberts, G.D.; Lloyd, R.V. In situ hybridization for the identification of filamentous fungi in tissue section. Diagn. Mol. Pathol. 2002, 11, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Lass-Florl, C.; Bouza, E.; Klimko, N.; Gaustad, P.; et al. Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011, 17, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.E. Histopathology in the Diagnosis of Invasive Fungal Diseases. Curr. Fungal Infect. Rep. 2021, 15, 23–31. [Google Scholar]
- Dannaoui, E.; Schwarz, P.; Slany, M.; Loeffler, J.; Jorde, A.T.; Cuenca-Estrella, M.; Hauser, P.M.; Shrief, R.; Huerre, M.; Freiberger, T.; et al. Molecular Detection and Identification of Zygomycetes Species from Paraffin-Embedded Tissues in a Murine Model of Disseminated Zygomycosis: A Collaborative European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Fungal Infection Study G. J. Clin. Microbiol. 2010, 48, 2043–2046. [Google Scholar] [CrossRef]
- Ruangritchankul, K.; Chindamporn, A.; Worasilchai, N.; Poumsuk, U.; Keelawat, S.; Bychkov, A. Invasive fungal disease in university hospital: A PCR-based study of autopsy cases. Int. J. Clin. Exp. Pathol. 2015, 8, 14840–14852. [Google Scholar]
- Rickerts, V. Identification of fungal pathogens in Formalin-fixed, Paraffin-embedded tissue samples by molecular methods. Fungal Biol. 2016, 120, 279–287. [Google Scholar] [CrossRef]
- Vallor, A.C.; Kirkpatrick, W.R.; Najvar, L.K.; Bocanegra, R.; Kinney, M.C.; Fothergill, A.W.; Herrera, M.L.; Wickes, B.L.; Graybill, J.R.; Patterson, T.F. Assessment of Aspergillus fumigatus burden in pulmonary tissue of guinea pigs by quantitative PCR, galactomannan enzyme immunoassay, and quantitative culture. Antimicrob. Agents Chemother. 2008, 52, 2593–2598. [Google Scholar] [CrossRef]
- Springer, J.; McCormick Smith, I.; Hartmann, S.; Winkelmann, R.; Wilmes, D.; Cornely, O.; Kessel, J.; Löffler, J.; Rickerts, V. Identification of Aspergillus and Mucorales in formalin-fixed, paraffin-embedded tissue samples: Comparison of specific and broad-range fungal qPCR assays. Med. Mycol. 2019, 57, 308–313. [Google Scholar] [CrossRef]
- Jung, J.; Park, Y.S.; Sung, H.; Song, J.S.; Lee, S.-O.; Choi, S.-H.; Kim, Y.S.; Woo, J.H.; Kim, S.-H. Using Immunohistochemistry to Assess the Accuracy of Histomorphologic Diagnosis of Aspergillosis and Mucormycosis. Clin. Infect. Dis. 2015, 61, 1664–1670. [Google Scholar] [CrossRef]
- Abdo, W.; Kakizoe, Y.; Ryono, M.; Dover, S.R.; Fukushi, H.; Okuda, H.; Kano, R.; Shibahara, T.; Okada, E.; Sakai, H.; et al. Pulmonary Zygomycosis with Cunninghamella bertholletiae in a Killer Whale (Orcinus orca). J. Comp. Pathol. 2012, 147, 94–99. [Google Scholar] [CrossRef]
- Challa, S.; Uppin, S.G.; Uppin, M.S.; Pamidimukkala, U.; Vemu, L. Diagnosis of filamentous fungi on tissue sections by immunohistochemistry using anti-aspergillus antibody. Med. Mycol. 2015, 53, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Rickerts, V.; Khot, P.D.; Myerson, D.; Ko, D.L.; Lambrecht, E.; Fredricks, D.N.; Bialek, R.; Konrad, F.; Kern, J.; Aepinus, C.; et al. Comparison of quantitative real time PCR with Sequencing and ribosomal RNA-FISH for the identification of fungi in Formalin fixed, paraffin-embedded tissue specimens. BMC Infect. Dis. 2011, 11, 202. [Google Scholar] [CrossRef]
- Hirayama, Y.; Yajima, N.; Kaimori, M.; Akagi, T.; Kubo, K.; Saito, D.; Hizawa, Y.; Wada, R.; Yagihashi, S. Disseminated infection and pulmonary embolization of Cunninghamella bertholletiae complicated with hemophagocytic lymphohistiocytosis. Intern. Med. 2013, 52, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Song, Y.; Li, R. The use of combined PCR, fluorescence in situ hybridisation and immunohistochemical staining to diagnose mucormycosis from formalin-fixed paraffin-embedded tissues. Mycoses 2021, 64, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
| Tissue Organ | Heart | Lung | Liver | Spleen | Kidney | Colon | ||
|---|---|---|---|---|---|---|---|---|
| Histopathology | Area of Fungal element/Total area of FFPE | 70% | 60% | 50% | 80% | 50% | 40% | |
| Density of fungal components | High | Medium | Low | Medium | Low | Low | ||
| Necrosis background | − | − | + | + | − | − | ||
| PCR | Panfungal | ITS1-ITS2 | − | − | − | − | − | − |
| ITS3-ITS4 | − | − | − | − | − | − | ||
| ITS1-ITS4 | − | − | − | − | − | − | ||
| NL1-NL4 | + | − | − | − | − | − | ||
| Mucorales | ZM1-ZM2 | − | − | − | − | − | − | |
| ZM1-ZM3 | + | + | + | − | + | + | ||
| IHC | Anti-Aspergillus antibody | ab20419 | − | − | − | − | − | − |
| Anti-Rhizopus arrhizus antibody | WSSA-RA-1 | + | + | + | + | + | + | |
| ISH | Panfungal | PNA probe | + | + | +(focal) | − | +(focal) | +(focal) |
| 18S rRNA probe | + | + | + | +(focal) | + | + | ||
| Oligo nucleotide DNA probe | − | − | − | − | − | − | ||
| Mucormycosis | 28S rRNA probe | + | + | + | + | + | + | |
| 18S rRNA probe | + | + | + | + | + | + | ||
| Aspergillosis | A. fumigatus alkaline proteinase gene | - | NT | NT | NT | NT | NT | |
| Mucor, Rhizomucor, Rhizopus, Saksenaea | Oligonucleotide DNA probe | − | NT | NT | NT | NT | NT | |
| Absida | − | NT | NT | NT | NT | NT | ||
| Cunninghamella | + | − | + | − | + | + | ||
| Method | Accessibility | Standardization | Time for Detection | Cost | Automation Technique | Advantage | Disadvantage |
|---|---|---|---|---|---|---|---|
| Histopathology | Easy | Yes | Minutes | Low | Yes | Low cost, Fast, High sensitivity. | Difficult for identifying up to species level. |
| IHC | Easy-Limited | No | Hours | Intermediate | Yes | It can be fully automated. Good sensitivity. | Commercially available antibody is few. Specificity depends on the quality of the antibody. |
| ISH | Limited | No | Hours-days | Intermediate | Partial or no | Good sensitivity with good specify. | Takes time. Specific probes needed for detecting different species. |
| Broad range PCR with sequencing | Easy-Limited | Yes | Hours-day | Intermediate | Partial or no | Can be identify up to the species level. | Contamination of ubiquitous fungi can happen. |
| Real-time PCR | Limited | Yes | Hours | High | Partial | The result can be shown by real-time with quantification. | Detectible species depend on the primer use. |
| NGS | Limited | No | Hours | High | Partial | Can potentially detect any fungal pathogen. | Limited evidence. High cost and special equipment should be needed. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadamoto, S.; Mitsui, Y.; Nihonyanagi, Y.; Amemiya, K.; Shinozaki, M.; Murayama, S.Y.; Abe, M.; Umeyama, T.; Tochigi, N.; Miyazaki, Y.; et al. Comparison Approach for Identifying Missed Invasive Fungal Infections in Formalin-Fixed, Paraffin-Embedded Autopsy Specimens. J. Fungi 2022, 8, 337. https://doi.org/10.3390/jof8040337
Sadamoto S, Mitsui Y, Nihonyanagi Y, Amemiya K, Shinozaki M, Murayama SY, Abe M, Umeyama T, Tochigi N, Miyazaki Y, et al. Comparison Approach for Identifying Missed Invasive Fungal Infections in Formalin-Fixed, Paraffin-Embedded Autopsy Specimens. Journal of Fungi. 2022; 8(4):337. https://doi.org/10.3390/jof8040337
Chicago/Turabian StyleSadamoto, Sota, Yurika Mitsui, Yasuhiro Nihonyanagi, Kazuki Amemiya, Minoru Shinozaki, Somay Yamagata Murayama, Masahiro Abe, Takashi Umeyama, Naobumi Tochigi, Yoshitsugu Miyazaki, and et al. 2022. "Comparison Approach for Identifying Missed Invasive Fungal Infections in Formalin-Fixed, Paraffin-Embedded Autopsy Specimens" Journal of Fungi 8, no. 4: 337. https://doi.org/10.3390/jof8040337
APA StyleSadamoto, S., Mitsui, Y., Nihonyanagi, Y., Amemiya, K., Shinozaki, M., Murayama, S. Y., Abe, M., Umeyama, T., Tochigi, N., Miyazaki, Y., & Shibuya, K. (2022). Comparison Approach for Identifying Missed Invasive Fungal Infections in Formalin-Fixed, Paraffin-Embedded Autopsy Specimens. Journal of Fungi, 8(4), 337. https://doi.org/10.3390/jof8040337







