Anti-Adipogenic Lanostane-Type Triterpenoids from the Edible and Medicinal Mushroom Ganoderma applanatum
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Fungal Materials
2.3. Extraction and Isolation
2.4. Mosher’s Method
2.5. Inhibition of Lipogenesis Assay
2.5.1. Cell Culture and Adipocyte Differentiation
2.5.2. Cell Viability Assay
2.5.3. Lipid Content Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sudheer, S.; Bai, R.G.; Muthoosamy, K.; Tuvikene, R.; Gupta, V.K.; Manickam, S. Biosustainable production of nanoparticles via mycogenesis for biotechnological applications: A critical review. Environ. Res. 2022, 204, 111963–111979. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lv, R.; Xu, X.; Ge, Q.; Lin, S. Tricholoma matsutake-Derived Peptides Show Gastroprotective Effects against Ethanol-Induced Acute Gastric Injury. J. Agric. Food Chem. 2021, 69, 14985–14994. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ge, Q.; Du, H.; Lin, S. Tricholoma matsutake-derived peptides ameliorate inflammation and mitochondrial dysfunction in RAW264.7 macrophages by modulating the NF-κB/COX-2 pathway. Foods 2021, 10, 2680. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shen, X.; Chen, H.; Dong, H.; Zhang, L.; Yuan, T.; Zhang, D.; Shang, X.; Tan, Q.; Liu, J.; et al. Analysis of heavy metal content in Lentinula edodes and the main influencing factors. Food Control 2021, 130, 108198–108205. [Google Scholar] [CrossRef]
- Shi, D.; Yin, C.; Fan, X.; Yao, F.; Qiao, Y.; Xue, S.; Lu, Q.; Feng, C.; Meng, J.; Gao, H. Effects of ultrasound and gamma irradiation on quality maintenance of fresh Lentinula edodes during cold storage. Food Chem. 2022, 373, 131478. [Google Scholar] [CrossRef]
- Chen, Y.-h.; Chen, J. Optimization of extraction conditions for polysaccharides from Collybia albuminosa via response surface methodology. Mod. Food Sci. Technol. 2012, 28, 541. [Google Scholar]
- Peng, X.R.; Su, H.G.; Liu, J.H.; Huang, Y.J.; Yang, X.Z.; Li, Z.R.; Zhou, L.; Qiu, M.H. C30 and C31 triterpenoids and triterpene sugar esters with cytotoxic activities from edible mushroom Fomitopsis pinicola (Sw. Ex Fr.) Krast. J. Agric. Food. Chem. 2019, 67, 10330–10341. [Google Scholar] [CrossRef]
- Ying, Y.M.; Yu, H.F.; Tong, C.P.; Shan, W.G.; Zhan, Z.J. Spiroinonotsuoxotriols A and B, two highly rearranged triterpenoids from Inonotus obliquus. Org. Lett. 2020, 22, 3377–3380. [Google Scholar] [CrossRef]
- Yu, J.; Xiang, J.Y.; Xiang, H.; Xie, Q. Cecal butyrate (Not Propionate) was connected with metabolism-related chemicals of mice, based on the different effects of the two Inonotus obliquus extracts on obesity and their mechanisms. ACS Omega 2020, 5, 16690–16700. [Google Scholar] [CrossRef]
- Li, Y.-T.; Zhang, Z.; Feng, Y.; Cheng, Y.; Li, S.; Li, C.; Tian, L.-W. Cardioprotective 22-hydroxylanostane triterpenoids from the fruiting bodies of Phellinus igniarius. Phytochemistry 2021, 191, 112907–112914. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Y.; Wang, G.; Wang, W.; Li, Y.; Xuan, L. Styryl pyranones with apoptosis activities from the sporocarps of Phellinus igniarius. Phytochem. Lett. 2021, 44, 154–159. [Google Scholar] [CrossRef]
- Shao, W.; Xiao, C.; Yong, T.; Zhang, Y.; Hu, H.; Xie, T.; Liu, R.; Huang, L.; Li, X.; Xie, Y.; et al. A polysaccharide isolated from Ganoderma lucidum ameliorates hyperglycemia through modulating gut microbiota in type 2 diabetic mice. Int. J. Biol. Macromol. 2022, 197, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Sheng, Z.; Wang, J.; Jiang, Y.; Yang, B. Structure of water-soluble polysaccharides in spore of Ganoderma lucidum and their anti-inflammatory activity. Food Chem. 2022, 373, 131374. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Chen, H.; Zhou, L.; Zou, H.; Luo, X.; Sun, B.; Zhuang, X. Polysaccharides from Ganoderma Sinense—rice bran fermentation products and their anti-tumor activities on non-small-cell lung cancer. BMC Complementary Med. Ther. 2021, 21, 169–179. [Google Scholar] [CrossRef]
- Teseo, S.; Houot, B.; Yang, K.; Monnier, V.; Liu, G.; Tricoire, H. G. sinense and P. notoginseng extracts improve healthspan of aging flies and provide protection in a huntington disease model. Aging Dis. 2021, 12, 425–440. [Google Scholar] [CrossRef]
- Ahmad, R.; Riaz, M.; Khan, A.; Aljamea, A.; Algheryafi, M.; Sewaket, D.; Alqathama, A. Ganoderma lucidum (Reishi) an edible mushroom; a comprehensive and critical review of its nutritional, cosmeceutical, mycochemical, pharmacological, clinical, and toxicological properties. Phytother. Res. 2021, 35, 6030–6062. [Google Scholar] [CrossRef]
- Zeng, M.; Qi, L.; Guo, Y.; Zhu, X.; Tang, X.; Yong, T.; Xie, Y.; Wu, Q.; Zhang, M.; Chen, D. Long-term administration of triterpenoids From Ganoderma lucidum mitigates age-associated brain physiological decline via regulating sphingolipid metabolism and enhancing autophagy in Mice. Front. Aging Neurosci. 2021, 13, 628860–628881. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xiao, C.; Xu, H.; Yang, S.; Chen, Z.; Wang, H.; Zheng, B.; Mao, B.; Wu, X. Anti-inflammatory effects of Ganoderma lucidum sterols via attenuation of the p38 MAPK and NF-κB pathways in LPS-induced RAW 264.7 macrophages. Food Chem. Toxicol. 2021, 150, 112073–112082. [Google Scholar] [CrossRef]
- Shao, C.S.; Feng, N.; Zhou, S.; Zheng, X.X.; Wang, P.; Zhang, J.S.; Huang, Q. Ganoderic acid T improves the radiosensitivity of HeLa cells via converting apoptosis to necroptosis. Toxicol. Res. 2021, 10, 531–541. [Google Scholar] [CrossRef]
- Kou, R.W.; Gao, Y.Q.; Xia, B.; Wang, J.Y.; Liu, X.N.; Tang, J.J.; Yin, X.; Gao, J.M. Ganoderterpene A, a New Triterpenoid from Ganoderma lucidum, Attenuates LPS-Induced Inflammation and Apoptosis via Suppressing MAPK and TLR-4/NF-kappaB Pathways in BV-2 Cells. J. Agric. Food. Chem. 2021, 69, 12730–12740. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Wang, N.-X.; Luo, Y.; Yu, C.-Y.; Xiao, J.-H. Ganoderal A effectively induces osteogenic differentiation of human amniotic mesenchymal stem cells via cross-talk between Wnt/β-catenin and BMP/SMAD signaling pathways. Biomed. Pharmacother. 2020, 123, 109807–109817. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, J.; Chen, Q.; Liu, W.; Qi, Y.; Aisa, H.A.; Yuan, T. Applanaic acids A-C, three new highly oxygenated lanostane triterpenoids from the fruiting bodies of Ganoderma applanatum. Nat. Prod. Res. 2020, 35, 3918–3924. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Cui, W.; Gao, Z.; Zhang, J.; Jia, L. Structural characterization and amelioration of sulfated polysaccharides from Ganoderma applanatum residue against CCl4-induced hepatotoxicity. Int. Immunopharmacol. 2021, 96, 107554–107563. [Google Scholar] [CrossRef]
- Mfopa, A.; Mediesse, F.K.; Mvongo, C.; Nkoubatchoundjwen, S.; Lum, A.A.; Sobngwi, E.; Kamgang, R.; Boudjeko, T. Antidyslipidemic potential of water-soluble polysaccharides of Ganoderma applanatum in MACAPOS-2-induced obese rats. Evid. Based Complement. Alternat. Med. 2021, 2021, 2452057. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.M.M.; Islam, M.J.; Hossain, M.R.; Barua, A.; Uddin, M.G.; Emon, N.U. CNS anti-depressant, anxiolytic and analgesic effects of Ganoderma applanatum (mushroom) along with ligand-receptor binding screening provide new insights: Multi-disciplinary approaches. Biochem. Biophys. Rep. 2021, 27, 101062. [Google Scholar] [CrossRef]
- Hossain, M.S.; Barua, A.; Tanim, M.A.H.; Hasan, M.S.; Islam, M.J.; Hossain, R.M.; Emon, N.U.; Hossen, S.M.M. Ganoderma applanatum mushroom provides new insights into the management of diabetes mellitus, hyperlipidemia, and hepatic degeneration: A comprehensive analysis. Food Sci. Nutr. 2021, 9, 4364–4374. [Google Scholar] [CrossRef] [PubMed]
- Su, H.G.; Wang, Q.; Zhou, L.; Peng, X.R.; Xiong, W.Y.; Qiu, M.H. Functional triterpenoids from medicinal fungi Ganoderma applanatum: A continuous search for antiadipogenic agents. Bioorg. Chem. 2021, 112, 104977–104986. [Google Scholar] [CrossRef]
- Su, H.G.; Wang, Q.; Zhou, L.; Peng, X.R.; Xiong, W.Y.; Qiu, M.H. Highly oxygenated lanostane triterpenoids from Ganoderma applanatum as a class of agents for inhibiting lipid accumulation in adipocytes. Bioorg. Chem. 2020, 104, 104263–104276. [Google Scholar] [CrossRef]
- Peng, X.R.; Wang, Q.; Wang, H.R.; Hu, K.; Xiong, W.Y.; Qiu, M.H. FPR2-based anti-inflammatory and anti-lipogenesis activities of novel meroterpenoid dimers from Ganoderma. Bioorg. Chem. 2021, 116, 105338–105348. [Google Scholar] [CrossRef]
- Peng, X.; Su, H.; Wang, H.; Hu, G.; Hu, K.; Zhou, L.; Qiu, M. Applanmerotic acids A and B, two meroterpenoid dimers with an unprecedented polycyclic skeleton from Ganoderma applanatum that inhibit formyl peptide receptor 2. Org. Chem. Front. 2021, 8, 3381–3389. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, J.S.; Lee, S.; Kang, S.S. Polyoxygenated ergostane-type sterols from the liquid culture of Ganoderma applanatum. Nat. Prod. Res. 2011, 25, 1304–1311. [Google Scholar] [CrossRef]
- Peng, X.; Liu, J.; Xia, J.; Wang, C.; Li, X.; Deng, Y.; Bao, N.; Zhang, Z.; Qiu, M. Lanostane triterpenoids from Ganoderma hainanense J. D. Zhao. Phytochemistry 2015, 114, 137–145. [Google Scholar] [CrossRef]
- Wang, Q.; Mu, R.F.; Liu, X.; Zhou, H.M.; Xu, Y.H.; Qin, W.Y.; Yang, C.R.; Wang, L.B.; Li, H.Z.; Xiong, W.Y. Steaming changes the composition of saponins of Panax notoginseng (Burk.) F.H. Chen that function in treatment of hyperlipidemia and obesity. J. Agric. Food. Chem. 2020, 68, 4865–4875. [Google Scholar] [CrossRef] [PubMed]
- Pu, D.; Li, X.; Lin, J.; Zhang, R.; Luo, T.; Wang, Y.; Gao, J.; Zeb, M.A.; Zhang, X.; Li, X.; et al. Triterpenoids from Ganoderma gibbosum: A class of sensitizers of FLC-Resistant candida albicans to fluconazole. J. Nat. Prod. 2019, 82, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Pu, D.-B.; Zheng, X.; Gao, J.-B.; Zhang, X.-J.; Qi, Y.; Li, X.-S.; Wang, Y.-M.; Li, X.-N.; Li, X.-L.; Wan, C.-P.; et al. Highly oxygenated lanostane-type triterpenoids and their bioactivity from the fruiting body of Ganoderma gibbosum. Fitoterapia 2017, 119, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, L.; Dong, J.; Lu, S.; Lu, J.; Li, X.; Zhou, L.; Qiu, M. Lanostane-type triterpenoids from the fruiting bodies of Ganoderma applanatum. Phytochemistry 2019, 157, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Peng, X.-R.; Dong, J.-R.; Lu, S.-Y.; Li, X.-N.; Zhou, L.; Qiu, M.-H. Rearranged lanostane-type triterpenoids with anti-hepatic fibrosis activities from Ganoderma applanatum. RSC Adv. 2018, 8, 31287–31295. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, K.; Nishimura, N.; Bando, S.; Arihara, S.; Matsumura, E.; Katayama, S. New lanostanoids, elfvingic acids A-H, from the fruit body of Elfvingia applanata. J. Nat. Prod. 2002, 65, 548–552. [Google Scholar] [CrossRef]
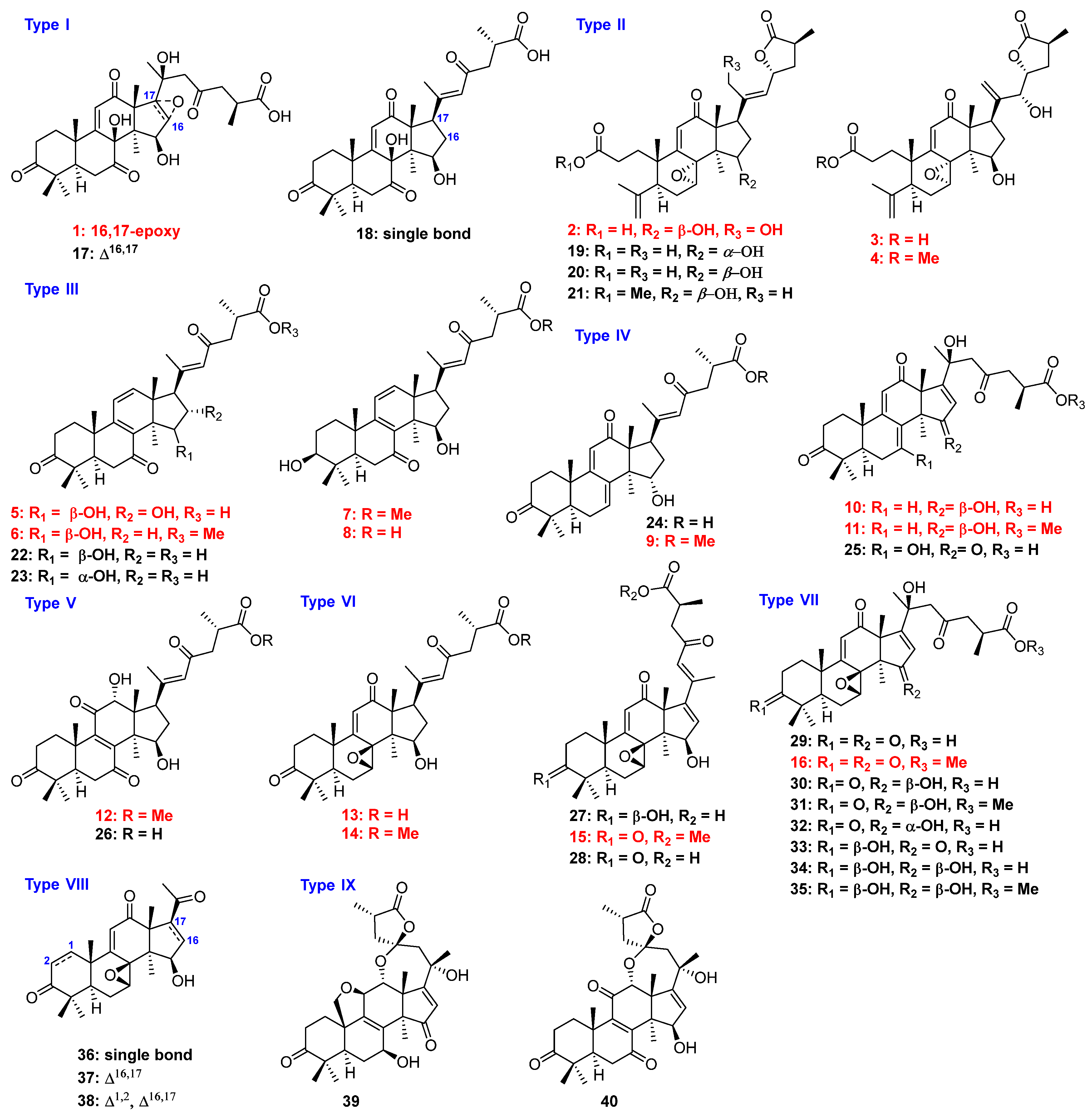
 H) correlations of compounds 1–3, 5, 10, 13, and 15. (B) Selected ROESY correlations of compounds 1, 2, 5, and 9.
H) correlations of compounds 1–3, 5, 10, 13, and 15. (B) Selected ROESY correlations of compounds 1, 2, 5, and 9.
 H) correlations of compounds 1–3, 5, 10, 13, and 15. (B) Selected ROESY correlations of compounds 1, 2, 5, and 9.
H) correlations of compounds 1–3, 5, 10, 13, and 15. (B) Selected ROESY correlations of compounds 1, 2, 5, and 9.



| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.87 dt (13.7 4.5) 2.21 dd (15.4 2.8) | 1.86 m 2.20 m | 1.82 m 2.16 m | 1.85 m 2.17 m | 1.91 m 2.29 m | 1.87 m 2.16 m | 2.02 m 1.51 m | 1.50 m 2.02 m |
| 2 | 2.40 m 2.95 m | 2.19 m 2.36 m | 2.17 m 2.34 m | 2.25 m 2.40 m | 2.55 m 2.74 m | 2.51 m 2.71 m | 1.78 m | 1.77 m |
| 3 | 3.24 t (8.4) | 3.23 t (8.4) | ||||||
| 5 | 1.74 dd (14.8 2.8) | 2.97 t (8.3) | 2.94 t (8.4) | 2.95 dd (9.3 7.5) | 2.28 m | 2.19 m | 1.66 dd (14.4 3.6) | 1.66 dd (14.4 3.6) |
| 6 | 2.30 dd (15.4 2.8) 3.30 m | 2.03 m | 1.99 m | 2.03 m | 2.44 dd (16.3 3.3) | 2.52 m 2.66 m | 2.62 m 2.48 m | 2.48 m 2.59 m |
| 7 | 4.93 s | 4.95 s | 4.98 s | |||||
| 11 | 5.98 s | 6.04 s | 6.02 s | 6.04 s | 6.28 d (9.9) | 6.14 d (10.2) | 6.31 d (10.2) | 6.31 d (10.2) |
| 12 | 6.59 d (9.9) | 6.59 d (10.2) | 6.64 d (10.2) | 6.65 d (10.2) | ||||
| 15 | 4.83 s | 3.95 d (6.5) | 3.91 d (6.5) | 3.94 d (6.4) | 4.20 s | 4.44 d (7.8) | 4.39 d (7.2) | 4.39 d (7.2) |
| 16 | 3.36 s | 1.80 m 4.65 m | 1.73 m 2.64 m | 1.76 m 2.67 m | 4.57 d (7.8) | 2.20 m 2.42 m | 2.16 m 2.51 m | 2.16 m 2.51 m |
| 17 | 3.33 m | 3.21 dd (10.7 8.7) | 3.23 m | 2.73 m | 2.73 m | 2.87 m | 2.83 m | |
| 18 | 1.97 s | 1.30 s | 1.37 s | 1.39 s | 0.99 s | 1.01 s | 1.00 s | 0.99 s |
| 19 | 1.65 s | 1.01 s | 1.04 s | 1.05 s | 1.14 s | 1.27 s | 1.16 s | 1.16 s |
| 21 | 1.34 s | 4.37 s | 5.33 s 5.39 s | 5.36 s 5.43 s | 1.21 s | 2.23 s | 2.19 s | 2.19 s |
| 22 | 2.66 d (14.9) 3.00 d (14.9) | 5.78 d (8.6) | 4.47 d (5.6) | 4.50 d (5.6) | 6.36 s | 6.22 s | 6.40 s | 6.32 s |
| 23 | 5.59 dd (8.6 6.6) | 4.72 m | 4.73 ddd (8.6 5.6 3.2) | |||||
| 24 | 2.62 dd (18.3 5.6) 3.07 dd (18.3 7.7) | 2.19 m | 1.97 m 2.16 m | 2.00 m 2.56 m | 2.61 m 2.90 m | 2.54 m 2.94 m | 2.64 m 2.90 m | 2.53 m 2.55 m |
| 25 | 1.74 dd (14.8 2.8) | 2.82 q (7.7) | 2.84 m | 2.86 m | 2.74 m | 2.97 m | 2.85 m | 2.84 m |
| 26 | 1.16 d (6.6) | 1.29 d (7.3) | 1.22 d (6.5) | 1.25 d (6.5) | 1.18 d (7.0) | 1.19 d (7.2) | 1.17 d (7.2) | 1.17 d (7.2) |
| 28 | 1.14 s | 4.80 s 4.98 s | 4.77 s 4.95 s | 4.77 s 4.95 s | 1.12 s | 1.13 s | 1.00 s | 1.00 s |
| 29 | 1.07 s | 1.80 s | 1.78 s | 1.80 s | 1.10 s | 1.14 s | 0.90 s | 0.90 s |
| 30 | 0.74 s | 1.06 s | 1.01 s | 1.04 s | 1.10 s | 0.94 s | 0.95 s | 0.95 s |
| OMe | 3.65 s | 3.68 s | 3.65 s |
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1 | 37.6 CH2 | 37.7 CH2 | 37.7 CH2 | 37.5 CH2 | 36.5 CH2 | 35.6 CH2 | 36.3 CH2 | 36.3 CH2 |
| 2 | 35.1 CH2 | 30.4 CH2 | 30.5 CH2 | 30.5 CH2 | 35.0 CH2 | 34.0 CH2 | 28.1 CH2 | 28.1 CH2 |
| 3 | 215.8 C | 177.4 C | 177.4 C | 175.6 C | 217.1 C | 214.3 C | 78.3 CH | 78.3 CH |
| 4 | 48.7 C | 146.5 C | 146.5 C | 146.3 C | 51.9 C | 46.9 C | 39.7 C | 39.8 C |
| 5 | 48.5 CH | 45.0 CH | 45.3 CH | 45.3 CH | 50.7 CH | 49.3 CH | 50.8 CH | 50.8 CH |
| 6 | 35.8 CH2 | 28.2 CH2 | 28.1 CH2 | 28.1 CH2 | 37.5 CH2 | 36.8 CH2 | 37.0 CH2 | 37.0 CH2 |
| 7 | 205.9 C | 64.7 CH | 64.8 CH | 65.0 CH | 203.0 C | 210.4 C | 204.3 C | 204.3 C |
| 8 | 85.5 C | 67.4 C | 67.5 C | 67.5 C | 135.8 C | 134.9 C | 135.7 C | 135.7 C |
| 9 | 165.0 C | 167.2 C | 167.8 C | 167.3 C | 161.4 C | 160.6 C | 164.5 C | 164.5 C |
| 10 | 41.0 C | 45.0 C | 45.2 C | 45.3 C | 39.2 C | 37.9 C | 39.9 C | 39.7 C |
| 11 | 127.3 CH | 130.7 CH | 130.6 CH | 130.6 CH | 123.2 CH | 122.0 CH | 123.3 CH | 123.3 CH |
| 12 | 204.0 C | 206.5 C | 207.3 C | 207.0 C | 147.4 CH | 147.5 CH | 148.3 CH | 148.3 C |
| 13 | 62.3 C | 60.8 C | 60.4 C | 60.4 C | 52.2 C | 50.2 C | 51.4 C | 51.4 C |
| 14 | 47.0 C | 54.4 C | 54.4 C | 54.4 C | 48.0 C | 52.5 C | 53.9 C | 53.8 C |
| 15 | 80.0 CH | 77.4 CH | 77.2 CH | 77.2 CH | 84.9 CH | 75.0 CH | 76.5 CH | 76.6 CH |
| 16 | 61.2 CH | 41.3 CH2 | 43.7 CH2 | 43.5 CH2 | 83.6 CH | 36.2 CH2 | 37.2 CH2 | 37.2 CH2 |
| 17 | 71.4 C | 43.3 CH | 41.6 CH | 41.6 CH | 58.7 CH | 48.5 CH | 49.7 CH | 49.7 C |
| 18 | 24.6 CH3 | 20.2 CH3 | 20.2 CH3 | 20.2 CH3 | 19.2 CH3 | 17.1 CH3 | 20.2 CH3 | 21.2 CH3 |
| 19 | 19.9 CH3 | 24.2 CH3 | 23.4 CH3 | 23.4 CH3 | 21.1 CH3 | 19.5 CH3 | 18.0 CH3 | 18.0 CH3 |
| 20 | 73.4 C | 144.1 C | 148.7 C | 148.7 C | 156.5 C | 157.3 C | 158.8 C | 158.4 C |
| 21 | 27.8 CH3 | 61.8 CH2 | 117.6 CH2 | 117.5 CH2 | 19.7 CH3 | 21.4 CH3 | 21.4 CH3 | 21.3 CH3 |
| 22 | 53.9 CH2 | 130.8 CH | 76.5 CH | 76.5 CH | 126.9 CH | 124.8 CH | 126.0 CH | 126.4 CH |
| 23 | 209.9 C | 76.8 CH | 80.9 CH | 80.9 CH | 200.8 C | 198.3 C | 200.6 C | 201.3 C |
| 24 | 48.7 CH2 | 38.3 CH2 | 32.2 CH2 | 32.2 CH2 | 48.7 CH2 | 47.7 CH2 | 48.8 CH2 | 49.0 CH2 |
| 25 | 35.8 CH | 35.7 CH | 35.3 CH | 35.3 CH | 36.2 CH | 34.8 CH | 36.6 CH | 36.6 CH |
| 26 | 17.4 CH3 | 15.9 CH3 | 16.3 CH3 | 16.0 CH3 | 17.5 CH3 | 17.1 CH3 | 17.4 CH3 | 17.7 CH3 |
| 27 | 179.7 C | 182.7 C | 183.1 C | 183.2 C | 179.7 C | 176.4 C | 178.1 C | 180.8 C |
| 28 | 22.3 CH3 | 115.9 CH2 | 115.9 CH2 | 115.9 CH2 | 21.0 CH3 | 25.6 CH3 | 27.8 CH3 | 27.8 CH3 |
| 29 | 24.9 CH3 | 23.5 CH3 | 23.4 CH3 | 23.4 CH3 | 25.9 CH3 | 20.4 CH3 | 15.7 CH3 | 15.7 CH3 |
| 30 | 30.7 CH3 | 21.3 CH3 | 21.4 CH3 | 21.4 CH3 | 19.7 CH3 | 20.6 CH3 | 21.2 CH3 | 20.2 CH3 |
| OMe | 52.3 CH3 | 51.8 CH3 | 52.3 CH3 |
| Position | 9 a | 10 b | 11 b | 12 b | 13 b | 14 b | 15 b | 16 a |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.27 m 1.86 m | 1.83 m 2.39 m | 1.83 m 2.38 m | 2.80 m 2.95 m | 1.82 m 2.26 m | 1.79 m 2.23 m | 1.81 m 2.23 m | 1.80 m 1.80 m |
| 2 | 2.42 m 2.78 m | 2.37 m 2.93 m | 2.37 m 2.94 m | 2.53 m 2.59 m | 2.31 m 2.95 m | 2.27 m 2.94 m | 2.28 m 2.93 m | 2.36 m 2.89 m |
| 5 | 1.65 m | 1.78 dd (12.8 3.1) | 1.76 dd (11.4 3.60 | 2.36 dd (15.0 3.0) | 1.29 dd (12.4 5.9) | 1.67 dd (12.6 6.0) | 1.66 m | 1.56 dd (12.66.0) |
| 6 | 1.11 m 2.28 m | 2.33 m 2.49 m | 2.34 m 2.53 m | 2.54 m 2.68 m | 2.25 m | 2.25 m | 2.30 m | 2.16 m |
| 7 | 6.50 br s | 6.62 m | 6.63 d (7.2) | 3.96 d (5.7) | 3.93 d (5.4) | 3.90 d (3.6) | 4.45 d (5.4) | |
| 11 | 5.65 s | 5.74 s | 5.74 s | 6.04 s | 6.01 s | 5.98 s | 6.03 s | |
| 12 | 3.70 s | |||||||
| 15 | 4.58 t (8.3) | 4.47 d (3.0) | 4.47 d (3.0) | 4.40 d (7.8) | 4.08 d (6.0) | 4.05 d (6.0) | 4.35 d (3.0) | |
| 16 | 1.80 m 2.48 m | 5.75 d (3.0) | 5.74 overlapped | 2.07 m 2.43 m | 1.94 m 2.43 m | 1.91 m 2.42 m | 6.20 d (3.0) | 5.66 s |
| 17 | 3.26 m | 3.28 t (9.6) | 3.24 dd (10.7 7.3) | 3.21 dd (10.8 7.8) | ||||
| 18 | 0.80 s | 1.54 s | 1.54 s | −0.096 s | 1.46 s | 1.43 s | 1.90 s | 1.76 s |
| 19 | 1.28 s | 1.39 s | 1.40 s | 1.31 s | 1.47 s | 1.45 s | 1.47 s | 1.44 s |
| 20 | ||||||||
| 21 | 2.22 s | 1.42 s | 1.40 s | 2.24 s | 2.28 s | 2.25 s | 2.30 s | 1.49 s |
| 22 | 6.37 s | 2.82 d (14.2) 3.01 d (14.2) | 2.79 d (14.4) 2.79 dd (14.4) | 6.31 s | 6.56 s | 6.52 s | 6.50 s | 2.92 d (15.0) 2.98 d (15.0) |
| 24 | 2.54 m 2.94 m | 2.66 dd (18.4 5.2) 3.02 m | 2.79 dd (18.6 5.4) 3.02 dd (18.6 8.4) | 2.54 m 2.94 m | 2.62 m 2.92 m | 2.62 m 2.88 m | 2.58 m 2.93 m | 3.04 m 2.59 dd (18.0 5.4) |
| 25 | 2.93 m | 2.80 m | 2.81 m | 1.19 m | 2.89 m | 2.89 m | 2.89 m | 2.89 m |
| 26 | 1.17 d (6.7) | 1.15 d (7.2) | 113 d (7.2) | 1.19 d (7.2) | 1.20 d (7.0) | 1.16 d (7.2) | 1.20 d (7.0) | 1.15 d (7.2) |
| 28 | 1.11 s | 1.20 s | 1.20 s | 1.15 s | 1.09 s | 1.09 s | 1.10 s | 1.11 s |
| 29 | 1.15 s | 1.11 s | 1.06 s | 1.13 s | 1.13 s | 1.13 s | 1.38 s | 1.12 s |
| 30 | 1.08 s | 1.06 s | 3.63 s | 1.24 s | 0.96 s | 0.96 s | 1.00 s | 1.28 s |
| OMe | 3.68 s | 3.56 s | 3.69 s | 3.65 s | 3.63 s | 3.65 s |
| Position | 9 a | 10 b | 11 b | 12 b | 13 b | 14 b | 15 b | 16 a |
|---|---|---|---|---|---|---|---|---|
| 1 | 35.6 CH2 | 36.7 CH2 | 36.8 CH2 | 35.1 CH2 | 38.4 CH2 | 38.4 CH2 | 38.4 CH2 | 37.2 CH2 |
| 2 | 34.2 CH2 | 35.4 CH2 | 35.4 CH2 | 34.3 CH2 | 34.9 CH2 | 34.9 CH2 | 34.8 CH2 | 33.8 CH2 |
| 3 | 214.8 C | 217.2 C | 217.2 C | 214.9 C | 216.4 C | 216.4 C | 216.5 C | 213.6 C |
| 4 | 47.0 C | 48.4 C | 48.8 C | 47.1 C | 48.7 C | 48.5 C | 48.8 C | 47.8 C |
| 5 | 49.5 CH | 51.1 CH | 51.2 CH | 49.8 CH | 50.7 CH | 50.7 CH | 51.2 CH | 49.9 CH |
| 6 | 23.9 CH2 | 25.2 CH2 | 25.2 CH2 | 37.6 CH2 | 22.6 CH2 | 22.6 CH2 | 22.4 CH2 | 21.6 CH2 |
| 7 | 130.9 CH | 135.5 CH | 135.5 CH | 203.4 C | 59.7 CH | 59.7 CH | 58.8 CH | 57.1 CH |
| 8 | 139.8 C | 137.3 C | 137.3 C | 150.2 C | 65.7 C | 65.8 C | 64.4 C | 59.0 C |
| 9 | 161.0 C | 165.5 C | 165.6 C | 151.9 C | 162.2 C | 162.3 C | 163.0 C | 165.0 C |
| 10 | 38.0 C | 39.6 C | 39.7 C | 39.3 C | 39.0 C | 39.0 C | 38.6 C | 38.2 C |
| 11 | 118.2 CH | 118.3 CH | 118.3 CH | 203.9 C | 127.6 CH | 127.6 CH | 127.2 CH | 125.0 CH |
| 12 | 202.5 C | 206.5 C | 206.5 C | 79.6 CH | 204.6 C | 204.6 C | 201.9 C | 200.6 C |
| 13 | 52.3 C | 64.0 C | 64.0 C | 52.9 C | 59.4 C | 59.4 C | 63.1 C | 61.8 C |
| 14 | 57.8 C | 54.3 C | 54.3 C | 50.0 C | 51.3 C | 51.3 C | 50.0 C | 54.4 C |
| 15 | 72.9 CH | 79.3 CH | 79.4 CH | 77.5 CH | 79.0 CH | 79.0 CH | 79.9 CH | 202.7 C |
| 16 | 36.5 CH2 | 127.5 CH | 127.6 CH | 34.2 CH2 | 38.2 CH2 | 38.2 CH2 | 134.9 CH | 124.4 CH |
| 17 | 45.7 CH | 158.9 C | 159.0 C | 46.9 CH | 49.6 CH | 49.6 CH | 148.8 C | 181.9 C |
| 18 | 17.8 CH3 | 29.0 CH3 | 29.1 CH3 | 18.6 CH3 | 20.3 CH3 | 20.3 CH3 | 27.0 CH3 | 30.9 CH3 |
| 19 | 21.3 CH3 | 21.5 CH3 | 21.5 CH3 | 19.0 CH3 | 21.0 CH3 | 21.0 CH3 | 21.2 CH3 | 20.8 CH3 |
| 20 | 157.6 C | 72.6 C | 72.6 C | 157.5 C | 159.0 C | 159.2 C | 156.0 C | 72.6 C |
| 21 | 20.4 CH3 | 29.0 CH3 | 29.1 CH3 | 20.3 CH3 | 21.5 CH3 | 21.5 CH3 | 17.5 CH3 | 29.1 CH3 |
| 22 | 125.7 CH | 54.6 CH2 | 54.7 CH2 | 125.1 CH | 127.5 CH | 127.5 CH | 127.1 CH | 52.7 CH2 |
| 23 | 198.3 C | 209.8 C | 209.7 C | 198.6 C | 201.0 C | 201.0 C | 201.7 C | 206.3 C |
| 24 | 47.7 CH2 | 48.8 CH2 | 48.7 CH2 | 47.9 CH2 | 48.7 CH2 | 48.7 CH2 | 48.9 CH2 | 47.8 CH2 |
| 25 | 34.7 CH | 35.8 CH | 35.9 CH | 35.0 CH | 36.3 CH | 36.3 CH | 36.3 CH | 34.5 CH |
| 26 | 17.0 CH3 | 17.4 CH3 | 17.3 CH3 | 16.9 CH3 | 17.4 CH3 | 17.4 CH3 | 17.5 CH3 | 17.0 CH3 |
| 27 | 176.4 C | 179.7 C | 178.2 C | 176.7 C | 179.7 C | 178.2 C | 180.1 C | 176.2 C |
| 28 | 25.2 CH3 | 22.9 CH3 | 25.5 CH3 | 26.9 CH3 | 24.9 CH3 | 24.9 CH3 | 24.9 CH3 | 24.5 CH3 |
| 29 | 22.3 CH3 | 25.2 CH3 | 22.9 CH3 | 20.4 CH3 | 22.3 CH3 | 22.3 CH3 | 22.5 CH3 | 22.1 CH3 |
| 30 | 18.1 CH3 | 29.0 CH3 | 29.1 CH3 | 27.4 CH3 | 22.5 CH3 | 22.5 CH3 | 24.9 CH3 | 25.9 CH3 |
| OMe | 51.8 CH3 | 52.2 CH3 | 52.0 CH3 | 52.3 CH3 | 52.0 CH3 | 51.9 CH3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.-R.; Wang, Q.; Su, H.-G.; Zhou, L.; Xiong, W.-Y.; Qiu, M.-H. Anti-Adipogenic Lanostane-Type Triterpenoids from the Edible and Medicinal Mushroom Ganoderma applanatum. J. Fungi 2022, 8, 331. https://doi.org/10.3390/jof8040331
Peng X-R, Wang Q, Su H-G, Zhou L, Xiong W-Y, Qiu M-H. Anti-Adipogenic Lanostane-Type Triterpenoids from the Edible and Medicinal Mushroom Ganoderma applanatum. Journal of Fungi. 2022; 8(4):331. https://doi.org/10.3390/jof8040331
Chicago/Turabian StylePeng, Xing-Rong, Qian Wang, Hai-Guo Su, Lin Zhou, Wen-Yong Xiong, and Ming-Hua Qiu. 2022. "Anti-Adipogenic Lanostane-Type Triterpenoids from the Edible and Medicinal Mushroom Ganoderma applanatum" Journal of Fungi 8, no. 4: 331. https://doi.org/10.3390/jof8040331
APA StylePeng, X.-R., Wang, Q., Su, H.-G., Zhou, L., Xiong, W.-Y., & Qiu, M.-H. (2022). Anti-Adipogenic Lanostane-Type Triterpenoids from the Edible and Medicinal Mushroom Ganoderma applanatum. Journal of Fungi, 8(4), 331. https://doi.org/10.3390/jof8040331







