Identification of Mucormycosis by Fluorescence In Situ Hybridization Targeting Ribosomal RNA in Tissue Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of FISH Probes Targeting the Ribosomal RNA of Agents of Mucormycosis
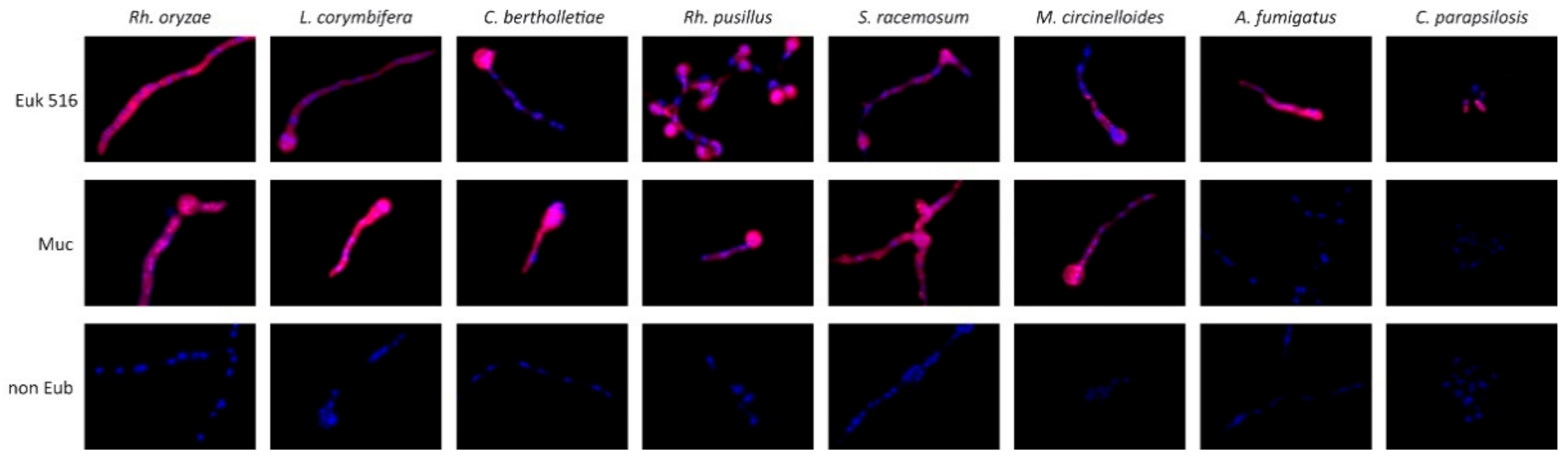
2.2. Evaluation of FISH Probes with Cultivated Fungi
2.3. Mucormycosis Mouse Model
2.4. Clinical Samples of Patients with Invasive Fungal Infections
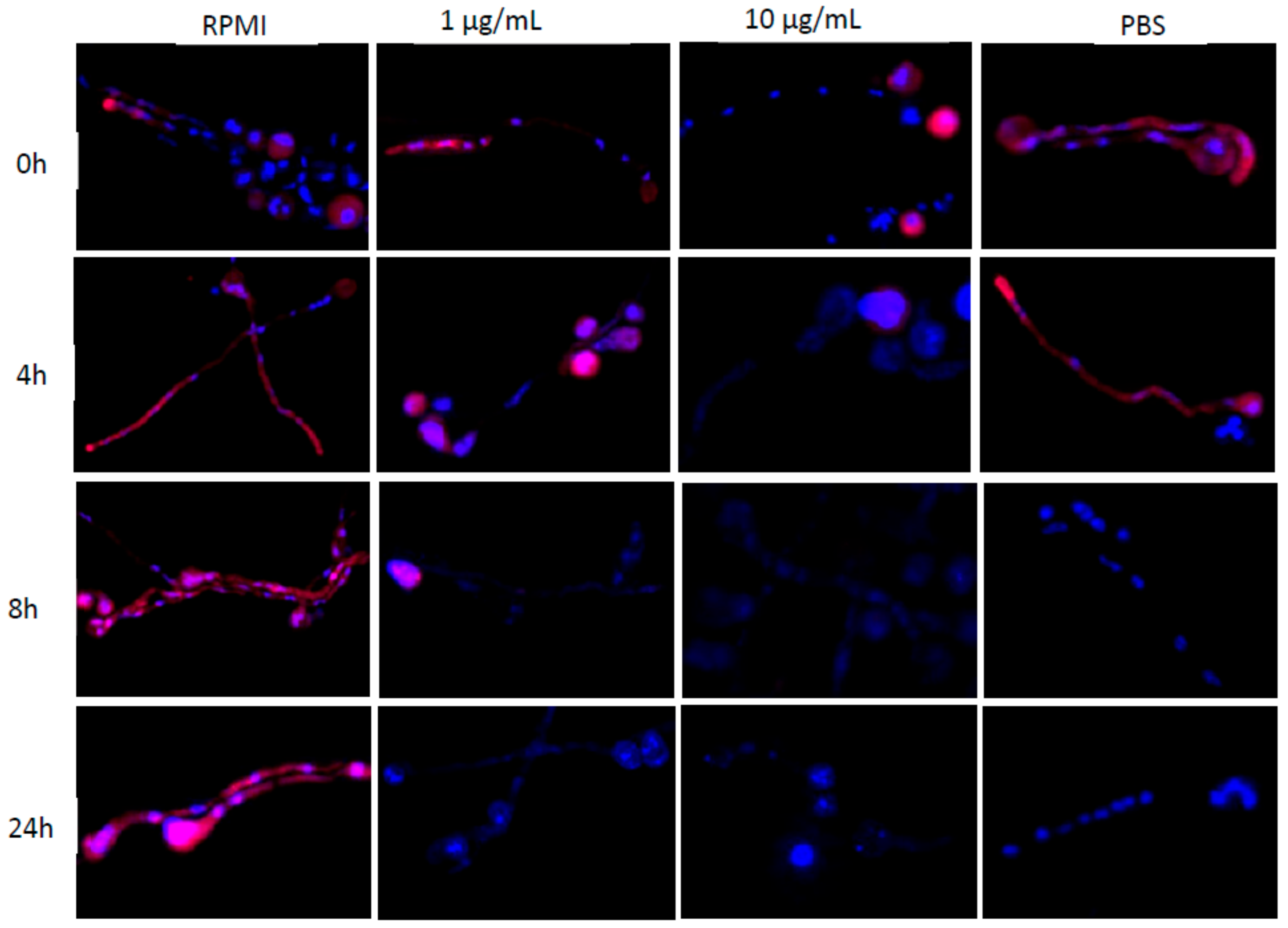
2.5. Assessing rRNA FISH Signal Intensity in Alternative Growth Conditions
3. Results
3.1. Generation of a Probe Combination Targeting Agents of Mucormycosis
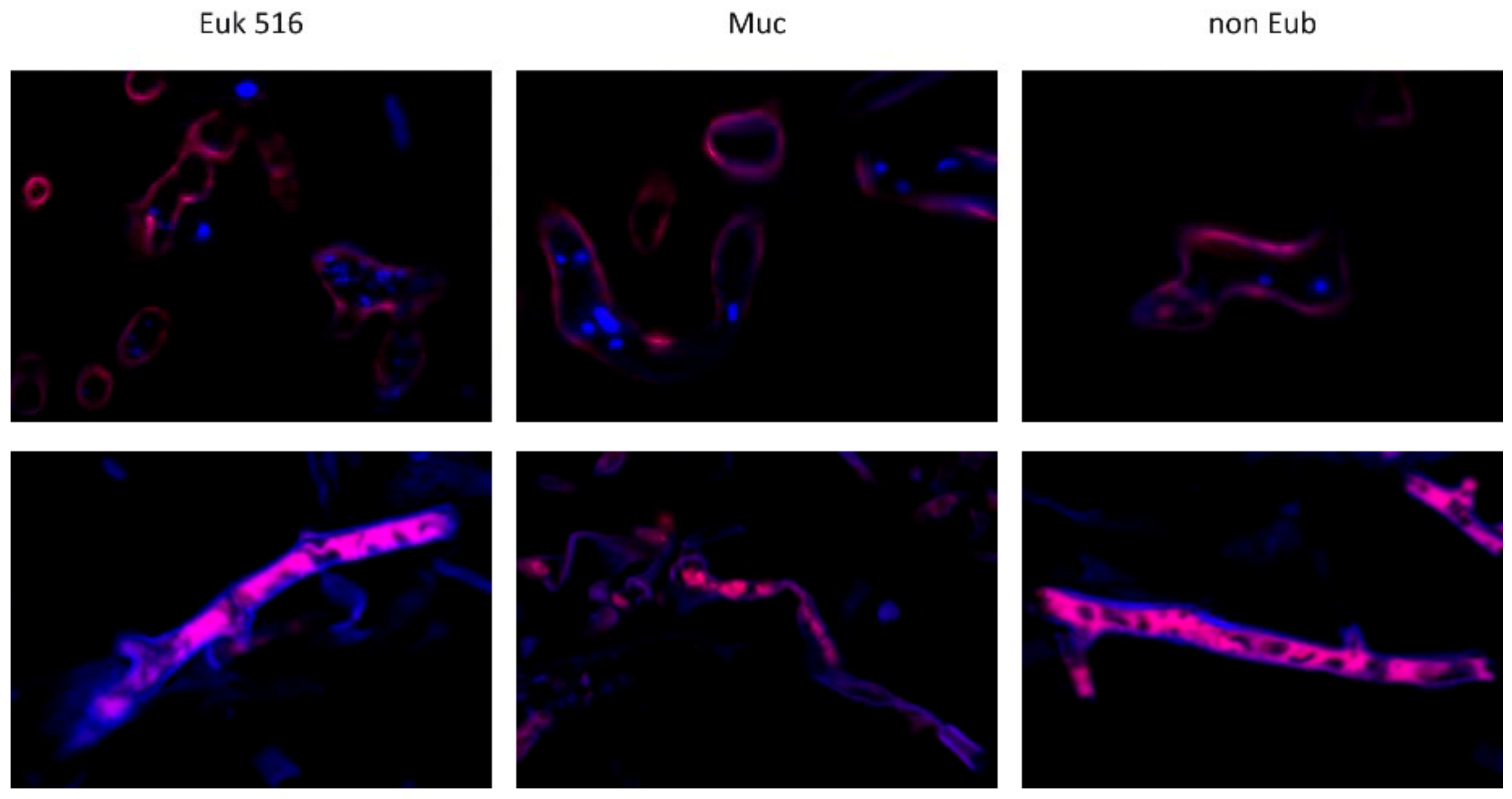
3.2. Evaluation of the Mucorales Probe Combination with Mouse Tissue
3.3. Evaluation of Mucorales Probe with Clinical Samples
3.4. Assessing rRNA FISH Signal under Alternative Growth Conditions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hassan, M.I.A.; Voigt, K. Pathogenicity patterns of mucormycosis: Epidemiology, interaction with immune cells and virulence factors. Med. Mycol. 2019, 57, S245–S256. [Google Scholar] [CrossRef] [Green Version]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef]
- Fredricks, D.N.; Relman, D.A. Sequence-based identification of microbial pathogens: A reconsideration of Koch′s postulates. Clin. Microbiol. Rev. 1996, 9, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Hayden, R.T.; Qian, X.; Procop, G.W.; Roberts, G.D.; Lloyd, R.V. In situ hybridization for the identification of filamentous fungi in tissue section. Diagn. Mol. Pathol. 2002, 11, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Rickerts, V.; Khot, P.D.; Myerson, D.; Ko, D.L.; Lambrecht, E.; Fredricks, D.N. Comparison of quantitative real time PCR with Sequencing and ribosomal RNA-FISH for the identification of fungi in formalin fixed, paraffin-embedded tissue specimens. BMC Infect. Dis. 2011, 11, 202. [Google Scholar] [CrossRef] [Green Version]
- Amann, R.; Fuchs, B.M. Single-cell identification in microbial communities by improved fluorescence in situ hybridization techniques. Nat. Rev. Microbiol. 2008, 6, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Behrens, S.; Fuchs, B.M.; Mueller, F.; Amann, R. Is the in situ accessibility of the 16S rRNA of Escherichia coli for Cy3-labeled oligonucleotide probes predicted by a three-dimensional structure model of the 30S ribosomal subunit? Appl. Environ. Microbiol. 2003, 69, 4935–4941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rooms, I.; Mugisha, P.; Gambichler, T.; Hadaschik, E.; Esser, S.; Rath, P.M.; Haase, G.; Wilmes, D.; McCormick-Smith, I.; Rickerts, V. Disseminated Emergomycosis in a Person with HIV Infection, Uganda. Emerg. Infect. Dis. 2019, 25, 1750–1751. [Google Scholar] [CrossRef] [PubMed]
- Neofytos, D.; Horn, D.; Anaissie, E.; Steinbach, W.; Olyaei, A.; Fishman, J.; Pfaller, M.; Chang, C.; Webster, K.; Marr, K. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: Analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin. Infect. Dis. 2009, 48, 265–273. [Google Scholar] [CrossRef]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef] [Green Version]
- Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin. Infect. Dis. 2008, 47, 503–509. [Google Scholar] [CrossRef]
- Zhang, S.X.; Babady, N.E.; Hanson, K.E.; Harrington, A.T.; Larkin, P.M.K.; Leal, S.M., Jr.; Luethy, P.M.; Martin, I.W.; Pancholi, P.; Procop, G.W.; et al. Recognition of Diagnostic Gaps for Laboratory Diagnosis of Fungal Diseases: Expert Opinion from the Fungal Diagnostics Laboratories Consortium (FDLC). J. Clin. Microbiol. 2021, 59, e0178420. [Google Scholar] [CrossRef] [PubMed]

| Agents of Mucormycosis | Strain Number |
| Lichtheimia corymbifera | 11–0171 |
| Cunninghamella bertholletiae | 09–0536 |
| Rhizopus oryzae | 10–077 |
| Mucor circinelloides | 13–1227 |
| Syncephalastrum racemosum | 13–1228 |
| Non-Mucorales Species | |
| Aspergillus fumigatus | 11–0169 |
| Candida glabrata | ATCC 64677 |
| Candida parapsilosis | DSM 11225 |
| Scedosporium prolificans | 04–0286 |
| Sample No. | Euk | Non Eub | Mucorales Probes | No Probe | Fungal PCR and Sequencing (Histopathology) |
| 1 | + | - | + | - | Rhizopus sp.(M) |
| 2 | + | - | + | nt | Rhizomucor sp. (M) |
| 3 | + | - | + | nt | Rhizopus sp. (M) |
| 4 | + | - | + | - | Rhizopus sp. (M) |
| 5 | + | + | + | + | Rhizopus sp. (M) |
| 6 | + | + | + | + | mixed sequence (M) |
| 7 | + | + | + | + | Rhizopus sp. (M) |
| 8 | - | - | - | - | Lichtheimia sp. (M) |
| 9 | - | - | - | - | negative (M) |
| 10 | - | - | - | - | mixed sequence (M) |
| 11 | - | - | - | - | Rhizopus sp. (M) |
| 12 | - | - | - | - | negative (M) |
| 13 | - | - | - | - | A. fumigatus (H) |
| 14 | + | - | - | - | C. glabrata (C) |
| 15 | - | - | - | - | negative (H) |
| 16 | - | - | - | - | A. fumigatus (A) |
| 17 | - | - | - | - | S. boydii (H) |
| 18 | + | - | - | nt | A. flavus (H) |
| 19 | - | - | - | - | negative (H) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalimot, J.J.; Smith, I.M.C.; Gerkrath, J.; Hartmann, S.; Cornely, O.A.; Lee, S.C.; Heitman, J.; Rickerts, V. Identification of Mucormycosis by Fluorescence In Situ Hybridization Targeting Ribosomal RNA in Tissue Samples. J. Fungi 2022, 8, 289. https://doi.org/10.3390/jof8030289
Dalimot JJ, Smith IMC, Gerkrath J, Hartmann S, Cornely OA, Lee SC, Heitman J, Rickerts V. Identification of Mucormycosis by Fluorescence In Situ Hybridization Targeting Ribosomal RNA in Tissue Samples. Journal of Fungi. 2022; 8(3):289. https://doi.org/10.3390/jof8030289
Chicago/Turabian StyleDalimot, Jill Jasmine, Ilka Mc Cormick Smith, Jasmin Gerkrath, Sylvia Hartmann, Oliver A. Cornely, Soo Chan Lee, Joseph Heitman, and Volker Rickerts. 2022. "Identification of Mucormycosis by Fluorescence In Situ Hybridization Targeting Ribosomal RNA in Tissue Samples" Journal of Fungi 8, no. 3: 289. https://doi.org/10.3390/jof8030289
APA StyleDalimot, J. J., Smith, I. M. C., Gerkrath, J., Hartmann, S., Cornely, O. A., Lee, S. C., Heitman, J., & Rickerts, V. (2022). Identification of Mucormycosis by Fluorescence In Situ Hybridization Targeting Ribosomal RNA in Tissue Samples. Journal of Fungi, 8(3), 289. https://doi.org/10.3390/jof8030289







