Isolation, Identification and Insecticidal Activity of the Secondary Metabolites of Talaromyces purpureogenus BS5
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolated Strains
2.2. Production of Culture Filtrates and Extracts
2.3. Bioassays
2.3.1. Bioassays with Culture Filtrates
2.3.2. Bioassays with Extracts
2.3.3. Bioassays with Fractions
2.4. Statistical Analysis
2.5. UPLC-Q-Exactive Orbitrap/MS Analysis
2.6. Fungal Identification
2.6.1. Morphological Analysis
2.6.2. Molecular Identification and Phylogenetic Analysis
3. Results
3.1. Determination of Insecticidal Activity of Culture Filtrates
3.2. Determination of Insecticidal Activity of Extracts and Fractions of Fungus BS5
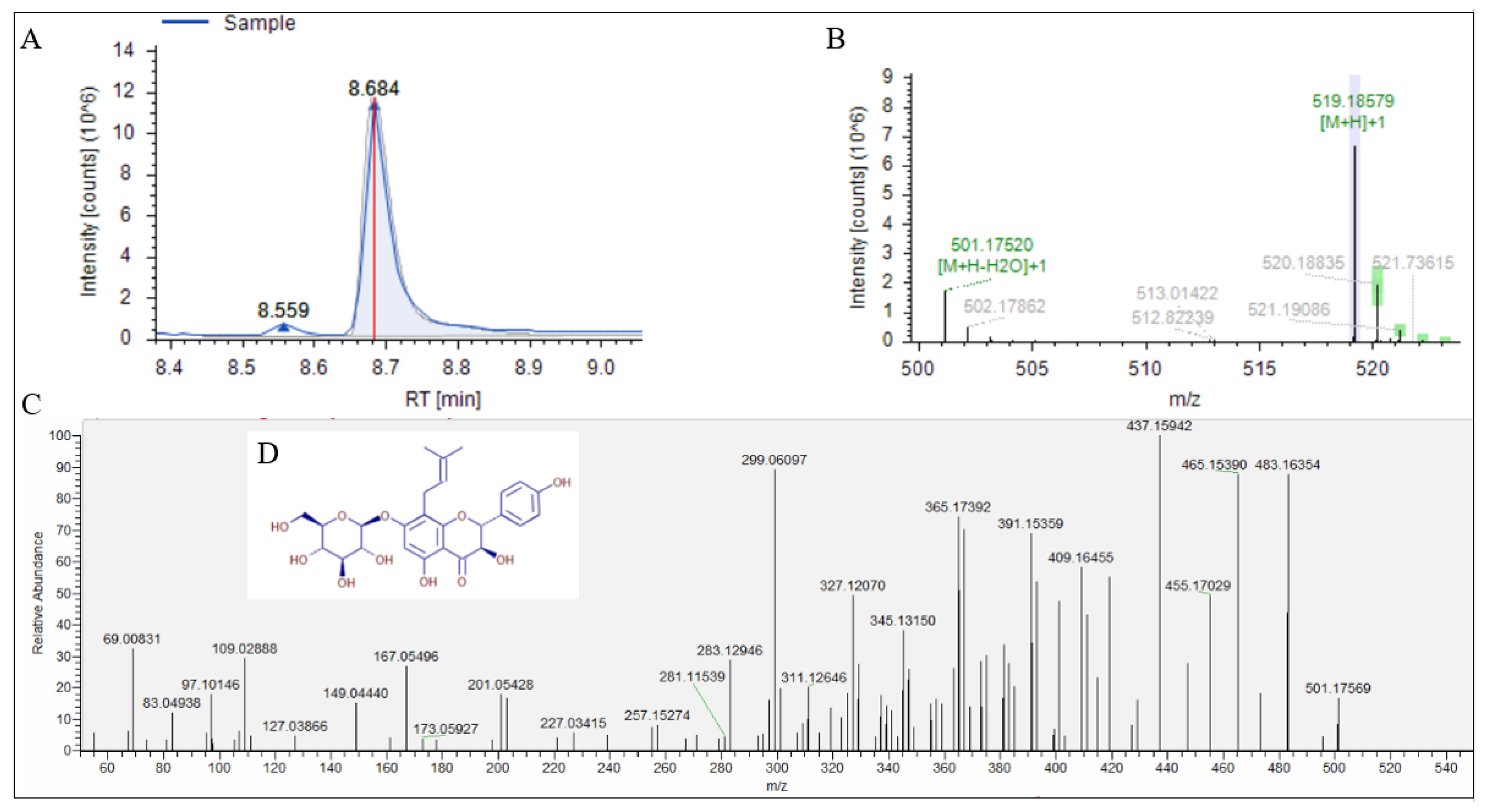
3.3. UPLC-Q-Exactive Orbitrap/MS Analysis of Fr.2.2.2
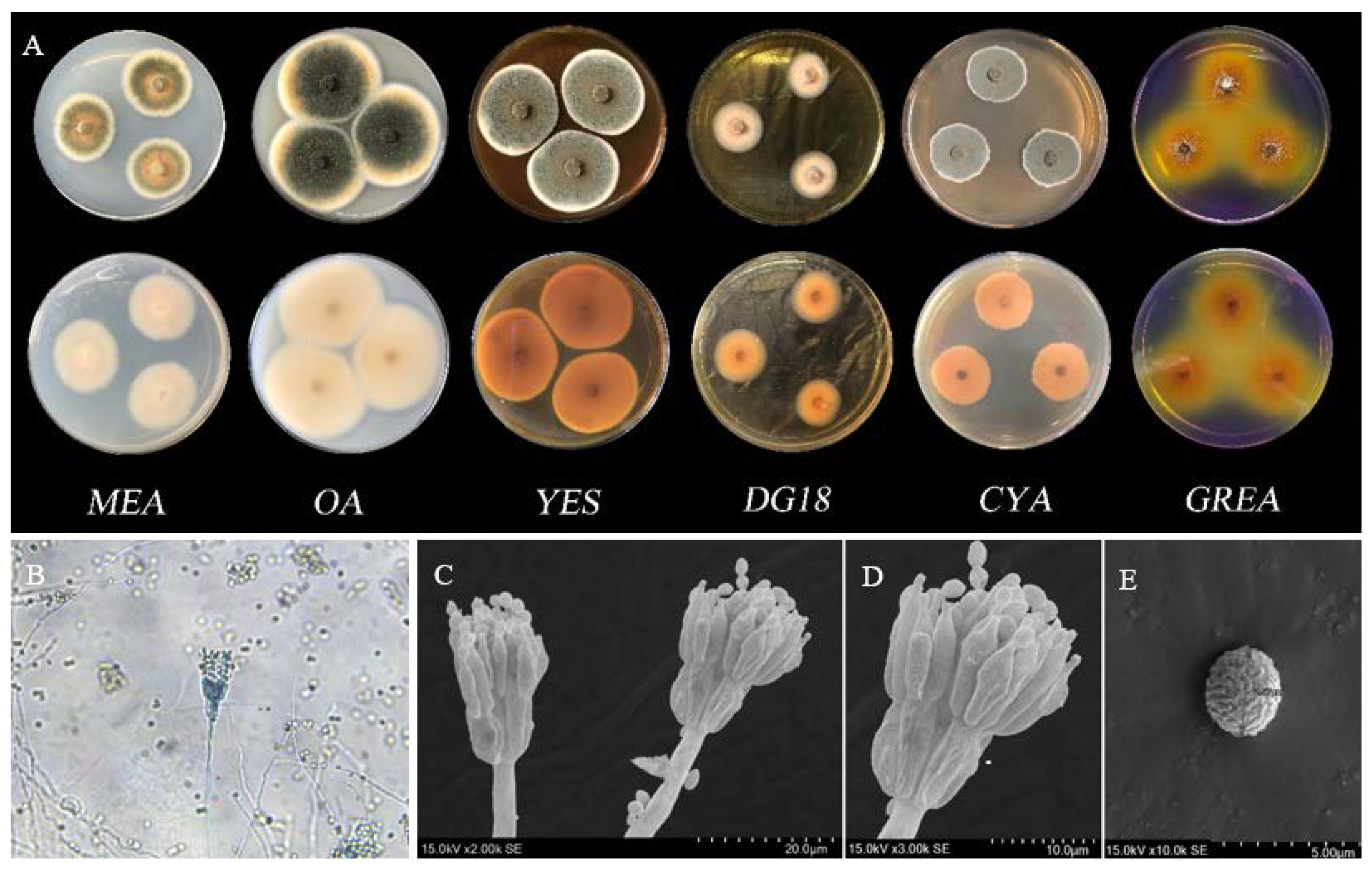
3.4. Fungal Identification of BS5
3.4.1. Morphological Analysis
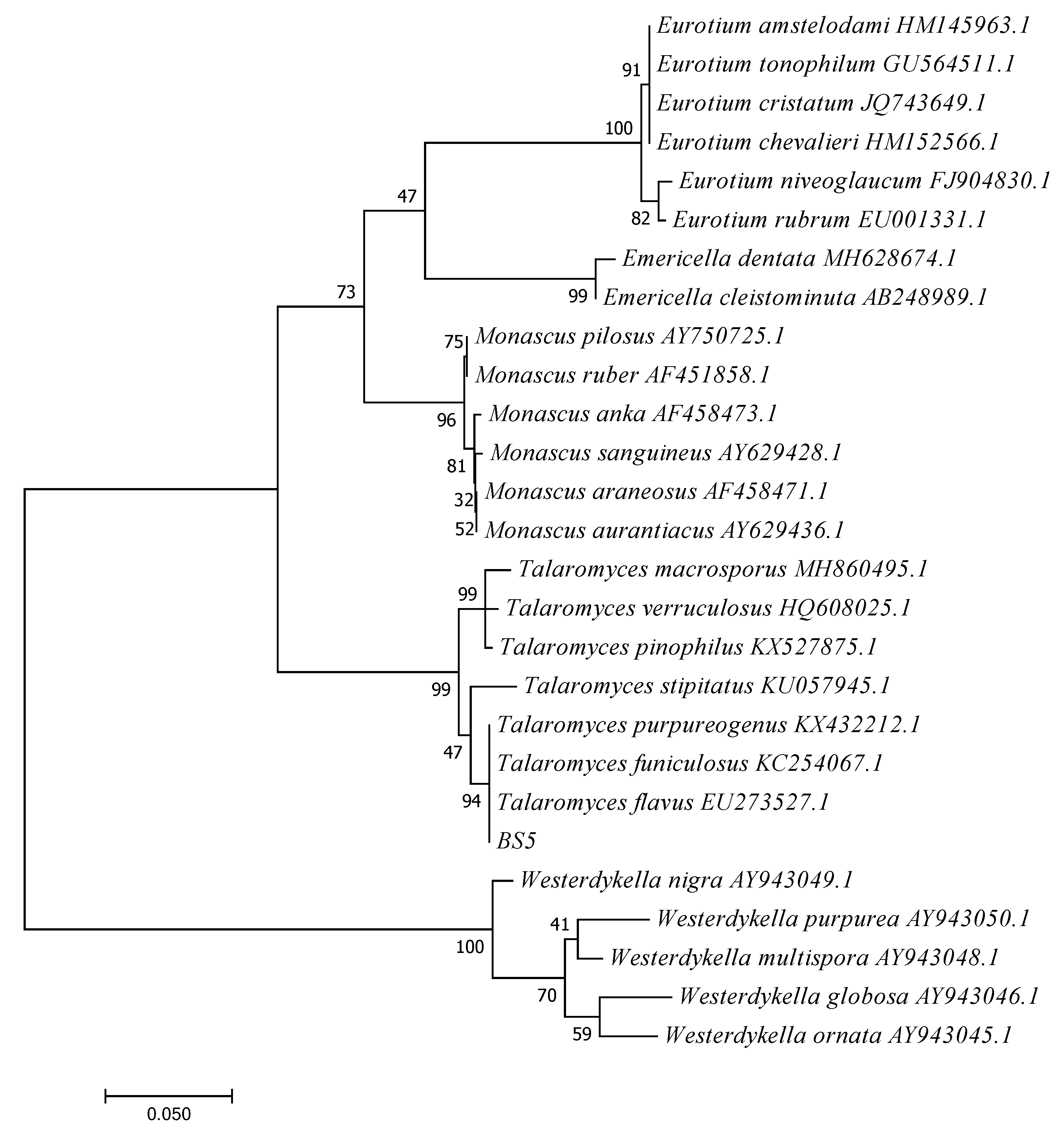
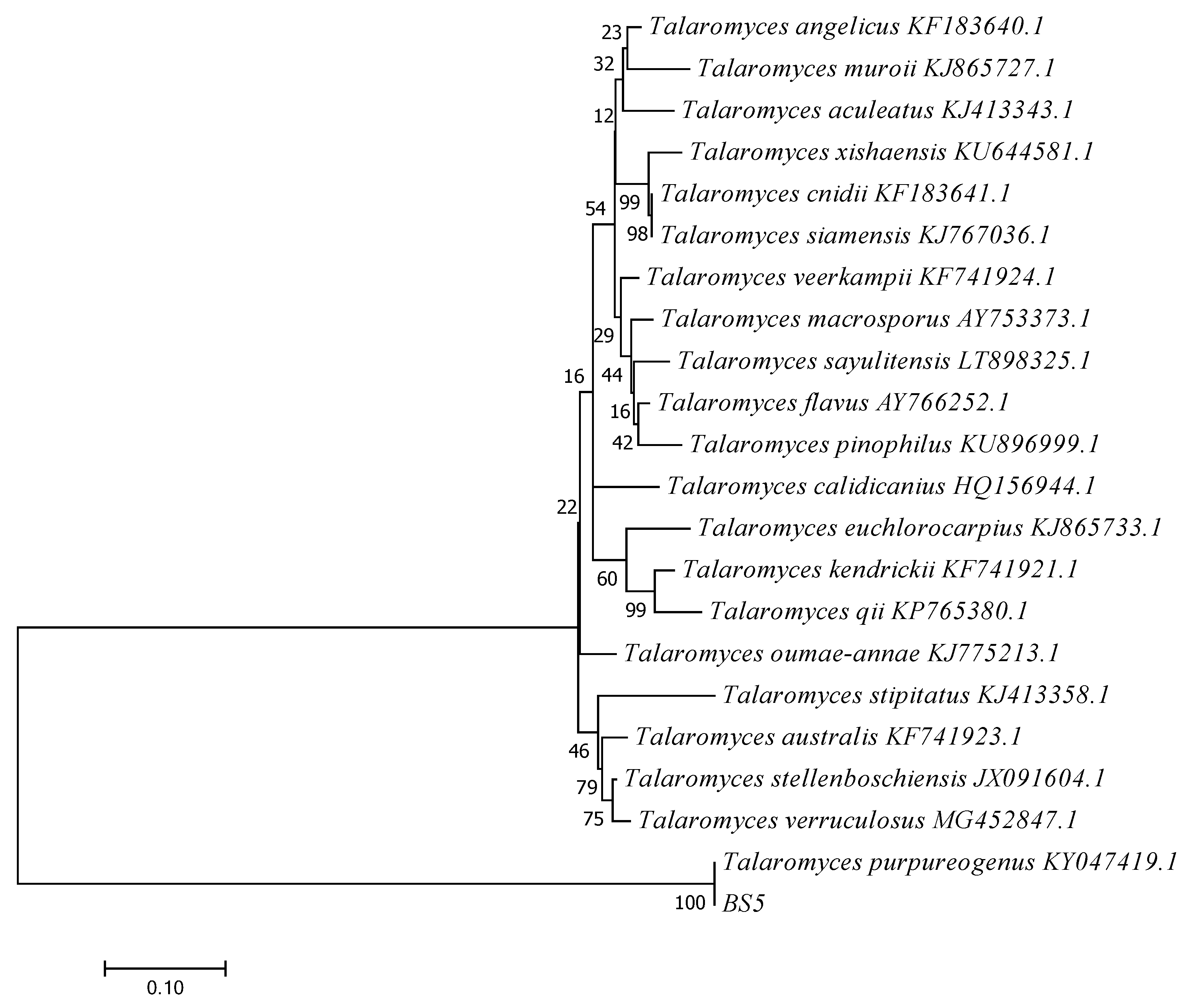
3.4.2. Molecular Identification and Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhang, L.; Lecoq, M.; Latchininsky, A.; Hunter, D. Locust and Grasshopper Management. Annu. Rev. Entomol. 2019, 64, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Cullen, D.A.; Cease, A.J.; Latchininsky, A.V.; Ayali, A.; Berry, K.; Buhl, J.; Keyser, R.D.; Foquet, B.; Hadrich, J.C.; Matheson, T.; et al. From Molecules to Management: Mechanisms and Consequences of Locust Phase Polyphenism. Adv. Insect Phys. 2017, 53, 165–285. [Google Scholar]
- Ariel, G.; Ayali, A. Locust Collective Motion and Its Modeling. PLoS Comput. Biol. 2015, 11, e1004522. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, D.; Ye, S.; Yao, X.; Li, J.; Zhang, N.; Han, Y.; Zhang, L. Design and implementation of geographic information systems, remote sensing, and global positioning system-based information platform for locust control. J. Appl. Remote Sens. 2014, 8, 084899. [Google Scholar] [CrossRef][Green Version]
- Aw, K.M.S.; Hue, S.M. Mode of Infection of Metarhizium spp. Fungus and Their Potential as Biological Control Agents. J. Fungi 2017, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Djinni, I.; Defant, A.; Kecha, M.; Mancini, I. Metabolite profile of marine-derived endophytic Streptomyces sundarbansensis WR1L1S8 by liquid chromatography-mass spectrometry and evaluation of culture conditions on antibacterial activity and mycelia growth. J. Appl. Microbiol. 2014, 116, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Slama, N.; Mankai, H.; Ayed, A.; Mezhoud, K.; Rauch, C.; Lazim, H.; Barkallah, I.; Gtari, M.; Limam, F. Streptomyces tunisiensis sp. nov., a novel Streptomyces species with antibacterial activity. Anton. Leeuw. Int. J. 2014, 105, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.Z.; D’Andrea, W.J.; Liu, Z. The influence of 14C reservoir age on interpretation of paleolimnological records from the Tibetan Plateau. Quat. Sci. Rev. 2012, 48, 67–79. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Chung, H.; Yu, L.; Mi, Z.; Geng, Y.; Jing, X.; Wang, S.; Zeng, H.; Cao, G.; et al. Non-growing-season soil respiration is controlled by freezing and thawing processes in the summer monsoon-dominated Tibetan alpine grassland. Global Biogeochem. Cycles 2014, 28, 1081–1095. [Google Scholar] [CrossRef]
- Quade, J.; Breecker, D.O.; Daëron, M.; Eiler, J. The paleoaltimetry of Tibet: An isotopic perspective. Am. J. Sci. 2011, 311, 77–115. [Google Scholar] [CrossRef]
- Zhang, Z. Determination of Virulence of Insecticides: Principles, Methods, Applications; Science Press: Beijing, China, 1988; pp. 85–104. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Duncan, D.B. A significance test for differences between ranked treatments in an analysis of variance. Vac. J. Sci. 1951, 2, 171–189. [Google Scholar]
- Norušis, M.J. IBM SPSS Statistics 19 Statistical Procedures Companion; Prentice Hall: New York, NY, USA, 2012; pp. 396–398. [Google Scholar]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: London, UK, 1971; p. 333. [Google Scholar]
- Tang, Q. Data Processing System; Science Press: Beijing, China, 2001; pp. 188–195. [Google Scholar]
- Accelerated Unknown Compound Annotation with Confidence: From Spectra to Structure in Untargeted Metabolomics Experiments. Available online: https://assets.thermofisher.cn/TFS-Assets/CMD/Application-Notes/an-65362-ms-compound-annotation-an65362-en.pdf (accessed on 3 May 2021).
- Lee, S.B.; Taylor, J.W. Isolation of DNA from fungal mycelia and single spores. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: San Diego, CA, USA, 1990; pp. 282–314. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Sang, H.; An, T.J.; Kim, C.S.; Shin, G.S.; Sung, H.Y. Two novel Talaromyces species isolated from medicinal crops in Korea. J. Microbiol. 2013, 51, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi; CBS Laboratory Manual Series 2; CBS-KNAW Fungal Biodiversity Centre: Utrecht, NL, USA, 2010; pp. 322–368. [Google Scholar]
- Yilmaz, N.; Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of the genus Talaromyces. Stud. Mycol. 2014, 78, 175–341. [Google Scholar] [CrossRef]
- Bara, R.; Zerfass, I.; Aly, A.H.; Goldbach-Gecke, H.; Raghavan, V.; Sass, P.; Lin, W. Atropisomeric dihydroanthracenones as inhibitors of multiresistant Staphylococcus aureus. J. Med. Chem. 2013, 56, 3257–3272. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.G.; Puttananjaih, M.H.; Harohally, N.V.; Dhale, M.A. Functional attributes of a new molecule-2-hydroxymethyl-benzoic acid 2′-hydroxy-tetradecyl ester isolated from Talaromyces purpureogenus. Food Chem. 2018, 255, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Anand, B.G.; Thomas, C.N.; Prakash, S. In Vitro cytotoxicity and antimicrobial activity of Talaromyces flavus SP5 inhabited in the marine sediment of Southern Coast of India. Chin. J. Nat. Med. 2016, 14, 913–921. [Google Scholar] [CrossRef]
- Kumar, M.; Qadri, M.; Sharma, P.R.; Kumar, A.; Andotra, S.S.; Kaur, T.; Johri, S. Tubulin inhibitors from an endophytic fungus isolated from Cedrus deodara. J. Nat. Prod. 2013, 76, 194–199. [Google Scholar] [CrossRef]
- Ngokpol, S.; Suwakulsiri, W.; Sureram, S.; Lirdprapamongkol, K.; Aree, T.; Wiyakrutta, S.; Kittakoop, P. Drimane sesquiterpene-conjugated amino acids from a marine isolate of the fungus Talaromyces minioluteus (Penicillium minioluteum). Mar. Drugs 2015, 13, 3567–3580. [Google Scholar] [CrossRef]
- Kumari, M.; Taritla, S.; Sharma, A.; Jayabaskaran, C. Antiproliferative and Antioxidative Bioactive Compounds in Extracts of Marine-Derived Endophytic Fungus Talaromyces purpureogenus. Front. Microbiol. 2018, 9, 1777. [Google Scholar] [CrossRef]
- Matsunaga, H.; Kamisuki, S.; Kaneko, M.; Yamaguchi, Y.; Takeuchi, T.; Watashi, K.; Sugawara, F. Isolation and structure of vanitaracin A, a novel anti-hepatitis B virus compound from Talaromyces sp. Bioorg. Med. Chem. Lett. 2015, 47, 4325–4328. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, K.; Chiba, T.; Suga, T.; Asami, Y.; Iwatsuki, M.; Masuma, R.; Shiomi, K. Coculnol, a new penicillic acid produced by a coculture of Fusarium solani FKI-6853 and Talaromyces sp. FKA-65. J. Antibiot. 2015, 68, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Ohlendorf, B.; Oesker, V.; Wiese, J.; Malien, S.; Schmaljohann, R.; Imhoff, J.F. Acetylcholinesterase inhibitors from a marine fungus Talaromyces sp. strain LF458. Mar. Biotechnol. 2015, 17, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Y.; Liu, Z.; Cai, R.; Lu, Y.; Huang, X.; She, Z. Isocoumarins and benzofurans from the mangrove endophytic fungus Talaromyces amestolkiae possess α-glucosidase inhibitory and antibacterial activities. RSC Adv. 2016, 6, 26412–26420. [Google Scholar] [CrossRef]
- Hayashi, H.; Oka, Y.; Kai, K.; Akiyama, K. A new meroterpenoid, chrodrimanin C, from YO-2 of Talaromyces sp. Biosci. Biotechnol. Biochem. 2012, 76, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Oka, Y.; Kai, K.; Akiyama, K. New Chrodrimanin Congeners, Chrodrimanins D–H, from YO-2 of Talaromyces sp. Biosci. Biotechnol. Biochem. 2012, 76, 1765–1768. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.P.; Zhu, C.Y.; Zhang, C.P. Thermolides, potent nematocidal PKS-NRPS hybrid metabolites from thermophilic fungus Talaromyces thermophilus. J. Am. Chem. Soc. 2012, 134, 20306–20309. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Ômura, H.; Chachin, M. Synergistic or Antagonistic Modulation of Oviposition Response of Two Swallowtail Butterflies, Papilio maackii and P. protenor, to Phellodendron amurense by Its Constitutive Prenylated Flavonoid, Phellamurin. J. Chem. Ecol. 2011, 37, 575–581. [Google Scholar] [CrossRef]
- Bai, J.; Yan, D.; Zhang, T.; Guo, Y.; Liu, Y.; Zou, Y.; Tang, M. A Cascade of Redox Reactions Generates Complexity in the Biosynthesis of the Protein Phosphatase-2 Inhibitor Rubratoxin A. Angew. Chem. Int. Edit. 2017, 56, 4782–4786. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, N.; Houbraken, J.; Hoekstra, E.S.; Frisvad, J.C.; Visagie, C.M.; Samson, R.A. Delimitation and characterisation of Talaromyces purpurogenus and related species. Persoonia 2012, 29, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Frisvad, J.C.; Kirk, P.M.; Lim, H.J.; Lee, H.B. Discovery and Extrolite Production of Three New Species of Talaromyces Belonging to Sections Helici and Purpurei from Freshwater in Korea. J. Fungi 2021, 7, 722. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Yilmaz, N.; Houbraken, J. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud. Mycol. 2011, 70, 159–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, Y.; Wang, Y. Anti-tumor effects of Rubratoxin B on cell toxicity, inhibition of cell proliferation, cytotoxic activity and matrix metalloproteinase-2,9. Toxicol. Vitro 2007, 21, 646–650. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Set Value | |
|---|---|---|
| Source of ion | Spray voltage | 3.5 kV (+)/3.2 kV (−) |
| Capillary temperature | 320 °C | |
| Sheath gas | 35 arb | |
| AUX gas | 10 arb | |
| Sweep gas | 0 arb | |
| Probe heater temperature | 350 °C | |
| S-lens | 60 | |
| Mass spectrometry scanning | Scan mode | Full MS-ddms2 |
| Full MS scan range | 100 to 1500 m/z | |
| Spectrum data type | Profile | |
| Resolution | Full MS: 70,000 | |
| MS/MS: 17,500 | ||
| AGC target | Full MS: 1e6 | |
| MS/MS: 2e5 | ||
| Maximum IT | Full MS: 100 ms | |
| MS/MS: 50 ms | ||
| Loop count | 3 | |
| MSX count | 1 | |
| Isolation width | 1.5 m/z | |
| NCE (stepped NCE) | 20, 40, 60 | |
| Minimum AGC target | 8e3 | |
| Intensity threshold | 1.6e5 | |
| Dynamic exclution | 5 s |
| Extracts | Concentration | Mortality (%; means ± SE) | |||
|---|---|---|---|---|---|
| (µg/mL) | 1st Day | 2nd Day | 3rd Day | 4th Day | |
| PEE | 625 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 2.27 | 0.00 ± 4.65h |
| 1250 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 2.27 | 0.00 ± 4.65h | |
| 2500 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 2.27 | 0.00 ± 4.65h | |
| 5000 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 2.27 | 2.33 ± 4.03h | |
| 10,000 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 2.27 | 6.98 ± 2.33gh | |
| DE | 625 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 2.27 | 0.00 ± 4.65h |
| 1250 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 2.27 | 2.33 ± 4.03h | |
| 2500 | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.28 ± 2.27 | 4.66 ± 4.65h | |
| 5000 | 0.00 ± 0.00 | 0.00 ± 0.00 | 4.55 ± 3.94 | 11.63 ± 2.32gh | |
| 10,000 | 0.00 ± 0.00 | 6.67 ± 3.85 | 13.64 ± 2.27 | 27.91 ± 6.15ef | |
| EAE | 625 | 0.00 ± 0.00 | 8.89 ± 2.22 | 13.64 ± 2.27 | 37.21 ± 4.03e |
| 1250 | 0.00 ± 0.00 | 11.11 ± 2.22 | 25.00 ± 3.94 | 55.82 ± 4.65cd | |
| 2500 | 0.00 ± 0.00 | 20.0 ± 3.85 | 38.64 ± 3.94 | 67.44 ± 4.65c | |
| 5000 | 4.45 ± 2.22 | 24.4 ± 5.88 | 52.27 ± 3.93 | 81.40 ± 4.65b | |
| 10,000 | 17.78 ± 2.22 | 53.3 ± 3.85 | 77.27 ± 6.01 | 93.02 ± 0.00a | |
| NE | 625 | 0.00 ± 0.00 | 4.45 ± 2.22 | 6.82 ± 2.27 | 11.63 ± 2.32gh |
| 1250 | 0.00 ± 0.00 | 6.67 ± 3.85 | 11.37 ± 3.93 | 18.61 ± 2.33fg | |
| 2500 | 0.00 ± 0.00 | 13.33 ± 3.85 | 25.00 ± 3.94 | 34.89 ± 2.32e | |
| 5000 | 2.22 ± 2.22 | 15.55 ± 2.22 | 31.82 ± 3.94 | 53.49 ± 2.32d | |
| 10,000 | 6.67 ± 3.85 | 26.67 ± 6.67 | 47.73 ± 6.01 | 60.47 ± 6.15cd | |
| Extract/Factions | TRE * | LC50 (µg/mL) (95% CL) † | r # | p Value |
|---|---|---|---|---|
| EAE | y = 0.6140 + 1.4463x | 1077.94 (927.46–1252.83) | 0.9948 | 0.0005 |
| Fr.1 | y = 1.2406 + 0.9305x | 10,968.79 (9340.81–12,880.50) | 0.9968 | 0.0002 |
| Fr.2 | y = 0.6119 + 1.4862x | 896.66 (576.55–1394.51) | 0.9642 | 0.0081 |
| Fr.3 | y = 0.4328 + 1.6338x | 2114.72 (1745.95–2561.37) | 0.9858 | 0.0020 |
| Fr.4 | y = 1.8511 + 1.6674x | 12,845.69 (6790.08–24,301.87) | 0.9591 | 0.0099 |
| Fr.2.1 | y = 0.2814 + 1.3418x | 8630.19 (6948.45–10,718.98) | 0.9927 | 0.0007 |
| Fr.2.2 | y = 2.5809 + 0.9076x | 462.89 (389.67–549.86) | 0.9970 | 0.0002 |
| Fr.2.3 | y = 2.7254 + 0.6768x | 2294.07 (1742.27–3020.64) | 0.9708 | 0.0060 |
| Fr.2.4 | y = 1.4135 + 0.8991x | 9746.56 (7192.64–13,207.32) | 0.9874 | 0.0017 |
| Fr.2.2.1 | y = 2.9517 + 0.6593x | 1278.95 (1026.05–1594.17) | 0.9868 | 0.0018 |
| Fr.2.2.2 | y = 3.3508 + 0.5829x | 674.87 (494.19–921.62) | 0.9861 | 0.0020 |
| No. | RT (min) | Deduced Compound | Formula | Molecular Weight | Fragment Ions (m/z) |
|---|---|---|---|---|---|
| 1 | 8.684 | Phellamurin | C26H30O11 | 518.17839 | 365.27392, 437.15942 465.15390, 483.16354 |
| 2 | 10.421 | Rubratoxin B | C26H30O11 | 518.17843 | 365.17410, 437.15942, 465.15399, 483.16473 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, Y.; Jiang, M.; Hu, H.; Wu, J.; Sun, H.; Jin, H.; Hou, T.; Tao, K. Isolation, Identification and Insecticidal Activity of the Secondary Metabolites of Talaromyces purpureogenus BS5. J. Fungi 2022, 8, 288. https://doi.org/10.3390/jof8030288
Yue Y, Jiang M, Hu H, Wu J, Sun H, Jin H, Hou T, Tao K. Isolation, Identification and Insecticidal Activity of the Secondary Metabolites of Talaromyces purpureogenus BS5. Journal of Fungi. 2022; 8(3):288. https://doi.org/10.3390/jof8030288
Chicago/Turabian StyleYue, Ying, Mingfang Jiang, Hanying Hu, Jinghui Wu, Haoran Sun, Hong Jin, Taiping Hou, and Ke Tao. 2022. "Isolation, Identification and Insecticidal Activity of the Secondary Metabolites of Talaromyces purpureogenus BS5" Journal of Fungi 8, no. 3: 288. https://doi.org/10.3390/jof8030288
APA StyleYue, Y., Jiang, M., Hu, H., Wu, J., Sun, H., Jin, H., Hou, T., & Tao, K. (2022). Isolation, Identification and Insecticidal Activity of the Secondary Metabolites of Talaromyces purpureogenus BS5. Journal of Fungi, 8(3), 288. https://doi.org/10.3390/jof8030288






