Ascophyllum nodosum Extract and Mycorrhizal Colonization Synergistically Trigger Immune Responses in Pea Plants against Rhizoctonia Root Rot, and Enhance Plant Growth and Productivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pea Cultivar, Seaweed, and Fungal Inocula
2.2. Screening for Antifungal Activity of the Seaweed Extract
2.3. Greenhouse Experiment
2.3.1. Expression Profiles of Defense-Related Genes
2.3.2. Growth and Yield Parameters
2.3.3. Disease Assessment
2.3.4. Phenolic Content and Activity of Antioxidant Enzymes
2.3.5. Total Photosynthetic Pigments and Biochemical Analyses
2.3.6. Estimation of Mycorrhizal Colonization
2.3.7. Ultrastructural Investigation
2.4. Statistical Analyses
3. Results
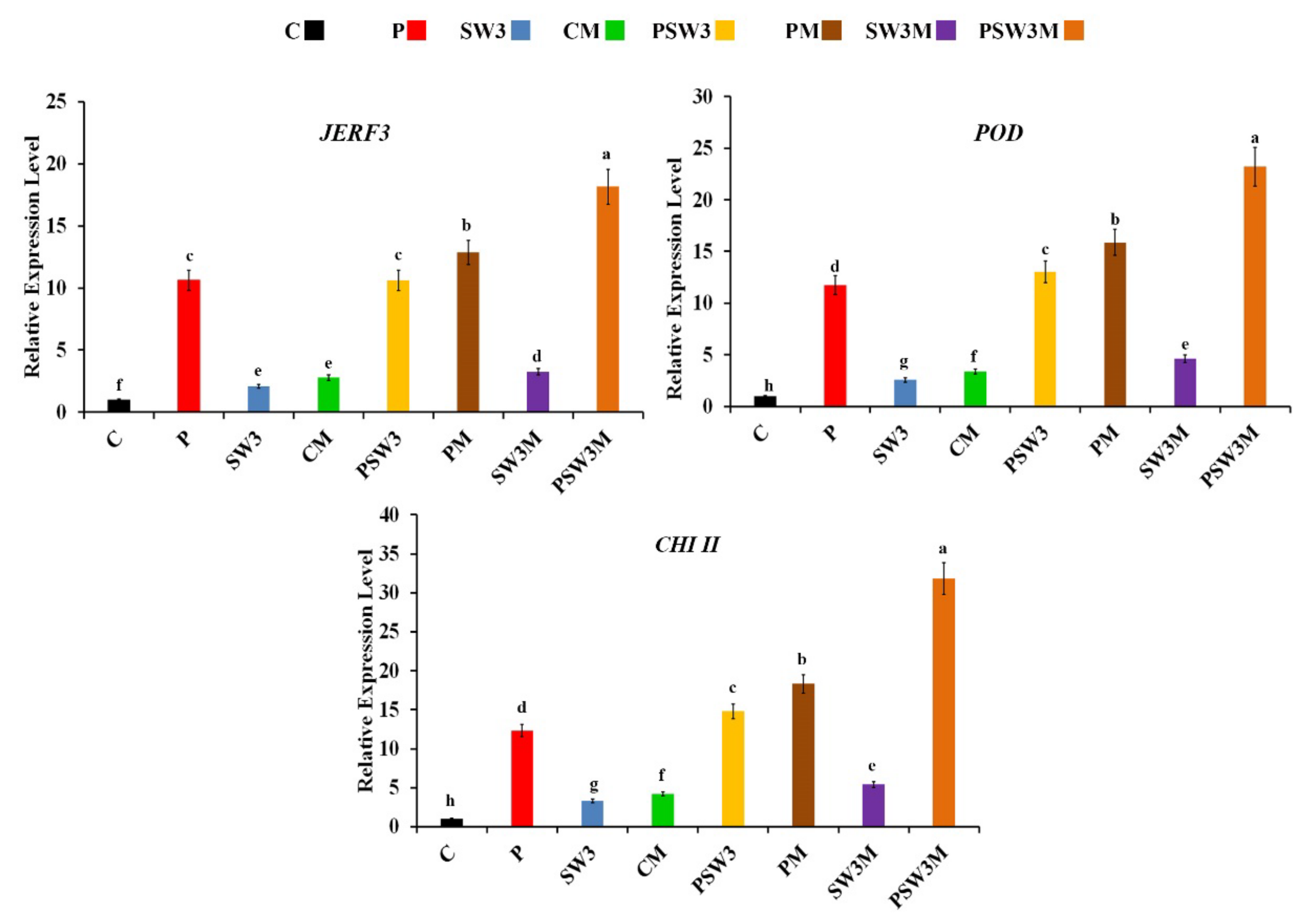
3.1. Expression Profiles of Defense-Related Genes
3.2. Effect on the Growth and Yield Parameters
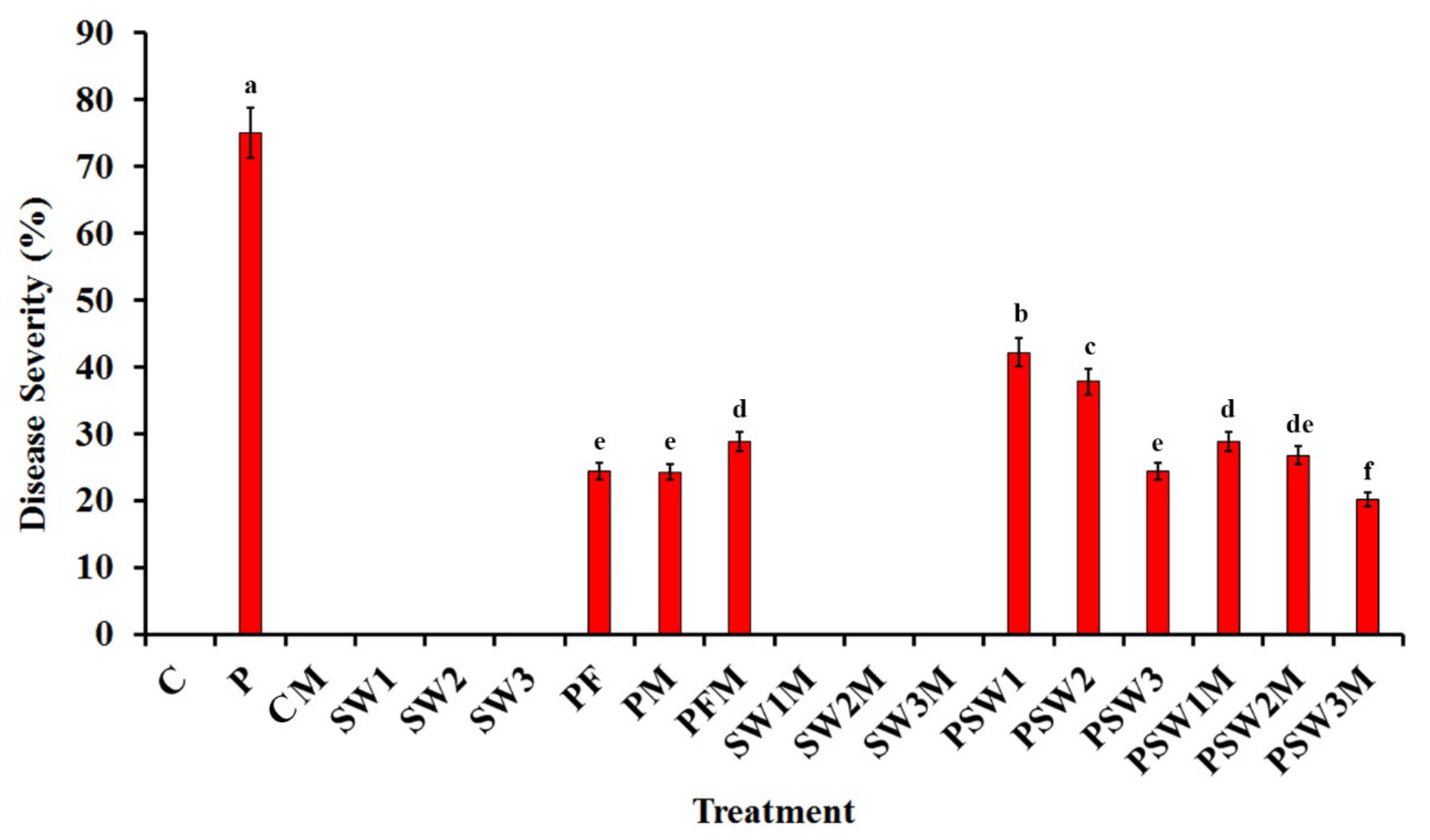
3.3. Effect on Disease Severity
3.4. Effect on Phenolic Content, Activity of POD and PPO Enzymes, Electrolyte Leakage, and TSS
3.5. Effect on Total Photosynthetic Pigments
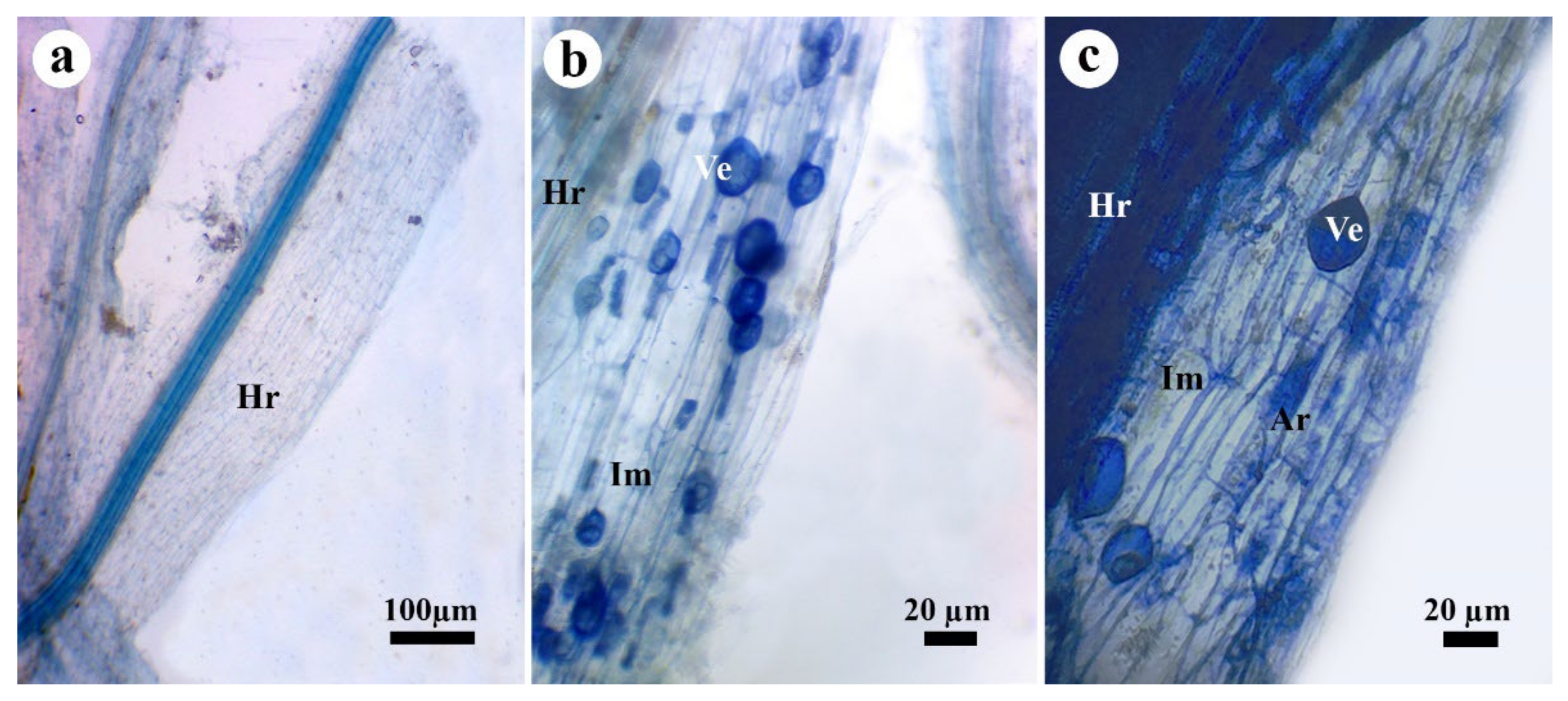
3.6. Effect on Mycorrhizal Colonization
3.7. TEM Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Wan, S.; Hao, J.; Hu, J.; Yang, T.; Zong, X. Large-scale evaluation of pea (Pisum sativum L.) germplasm for cold tolerance in the field during winter in Qingdao. Crop J. 2016, 4, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the health benefits of peas (Pisum sativum L.). Br. J. Nutr. 2012, 108, S3–S10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAOSTAT. Statistics Division. 2022. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 19 January 2022).
- Fang, X.; Finnegan, P.M.; Barbetti, M.J. Wide variation in virulence and genetic diversity of binucleate Rhizoctonia isolates associated with root rot of strawberry in Western Australia. PLoS ONE 2013, 8, e55877. [Google Scholar] [CrossRef]
- Dubey, S.C.; Tripathi, A.; Upadhyay, B.K.; Deka, U.K. Diversity of Rhizoctonia solani associated with pulse crops in different agro-ecological regions of India. World J. Microbiol. Biotechnol. 2014, 30, 1699–1715. [Google Scholar] [CrossRef]
- Grünwald, N.J.; Chen, W.; Larsen, R.C. Pea Diseases and their Management. In Diseases of Fruits and Vegetables: Volume II; Naqvi, S.A.M.H., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2004; pp. 301–331. ISBN 978-1-4020-2607-2. [Google Scholar]
- Sharma-Poudyal, D.; Paulitz, T.C.; Porter, L.D.; Sharma-Poudyal, D. Characterization and pathogenicity of Rhizoctonia and Rhizoctonia-like spp. from pea crops in the Columbia Basin of Oregon and Washington. Plant Dis. 2015, 99, 604–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, F.M.; Lamppa, R.S.; Chittem, K.; Chang, Y.W.; Botschner, M.; Kinzer, K.; Goswami, R.S.; Markell, S.G. Characterization and Pathogenicity of Rhizoctonia solani Isolates Affecting Pisum sativum in North Dakota. Plant Dis. 2011, 96, 666–672. [Google Scholar] [CrossRef] [Green Version]
- Bartholomäus, A.; Mittler, S.; Märländer, B.; Varrelmann, M. Control of Rhizoctonia solani in Sugar Beet and Effect of Fungicide Application and Plant Cultivar on Inoculum Potential in the Soil. Plant Dis. 2017, 101, 941–947. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Gao, X.; Nan, J.; Zhang, T.; Xie, X.; Cai, Q. Fungicides alter the distribution and diversity of bacterial and fungal communities in ginseng fields. Bioengineered 2021, 12, 8043–8056. [Google Scholar] [CrossRef] [PubMed]
- Rashad, Y.M.; Moussa, T.A.A. Biocontrol Agents for Fungal Plant Diseases Management. In Cottage Industry of Biocontrol Agents and Their Applications: Practical Aspects to Deal Biologically with Pests and Stresses Facing Strategic Crops; El-Wakeil, N., Saleh, M., Abu-hashim, M., Eds.; Springer International Publishing: Cham, Germany, 2020; pp. 337–363. ISBN 978-3-030-33161-0. [Google Scholar]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [Green Version]
- Rashad, Y.M.; Abbas, M.A.; Soliman, H.M.; Abdel-Fattah, G.; Abdel-Fattah, G. Synergy between endophytic Bacillus amyloliquefaciens GGA and arbuscular mycorrhizal fungi induces plant defense responses against white rot of garlic and improves host plant growth. Phytopathol. Mediterr. 2020, 59, 169–186. [Google Scholar] [CrossRef]
- Rashad, Y.M.; Fekry, W.M.E.; Sleem, M.M.; Elazab, N.T. Effects of Mycorrhizal Colonization on Transcriptional Expression of the Responsive Factor JERF3 and Stress-Responsive Genes in Banana Plantlets in Response to Combined Biotic and Abiotic Stresses. Front. Plant Sci. 2021, 12, 742628. [Google Scholar] [CrossRef] [PubMed]
- Yousef, S.; Sharkawy, H.; Metwaly, H. Use of Beneficial Microorganisms to Minimize the Recommended Rates of Macronutrients to Control Cucumber Damping off. Egypt. J. Phytopathol. 2016, 44, 17–34. [Google Scholar] [CrossRef]
- El-Sharkawy, H.H.A.; Rashad, Y.M.; Ibrahim, S.A. Biocontrol of stem rust disease of wheat using arbuscular mycorrhizal fungi and Trichoderma spp. Physiol. Mol. Plant Pathol. 2018, 103, 84–91. [Google Scholar] [CrossRef]
- Rashad, Y.; Aseel, D.; Hammad, S.; Elkelish, A. Rhizophagus irregularis and Rhizoctonia solani differentially elicit systemic transcriptional expression of polyphenol biosynthetic pathways genes in sunflower. Biomolecules 2020, 10, 379. [Google Scholar] [CrossRef] [Green Version]
- Aseel, D.G.; Rashad, Y.M.; Hammad, S.M. Arbuscular Mycorrhizal Fungi Trigger Transcriptional Expression of Flavonoid and Chlorogenic Acid Biosynthetic Pathways Genes in Tomato against Tomato Mosaic Virus. Sci. Rep. 2019, 9, 9692. [Google Scholar] [CrossRef] [Green Version]
- Goicoechea, N. Mycorrhizal Fungi as Bioprotectors of Crops Against Verticillium Wilt—A Hypothetical Scenario Under Changing Environmental Conditions. Plants 2020, 9, 1468. [Google Scholar] [CrossRef]
- Abdel-Fattah, G.M.; El-Haddad, S.A.; Hafez, E.E.; Rashad, Y.M. Induction of defense responses in common bean plants by arbuscular mycorrhizal fungi. Microbiol. Res. 2011, 166, 268–281. [Google Scholar] [CrossRef]
- Kratzer, R.; Murkovic, M. Food Ingredients and Nutraceuticals from Microalgae: Main Product Classes and Biotechnological Production. Foods 2021, 10, 1626. [Google Scholar] [CrossRef]
- Fertah, M.; Belfkira, A.; Dahmane, E.M.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-J.; Shim, C.-K.; Kim, Y.-K.; Ko, B.-G.; Park, J.-H.; Hwang, S.-G.; Kim, B.-H. Effect of Biostimulator Chlorella fusca on Improving Growth and Qualities of Chinese Chives and Spinach in Organic Farm. Plant Pathol. J. 2018, 34, 567–574. [Google Scholar] [CrossRef]
- De Corato, U.; Salimbeni, R.; De Pretis, A.; Avella, N.; Patruno, G. Antifungal activity of crude extracts from brown and red seaweeds by a supercritical carbon dioxide technique against fruit postharvest fungal diseases. Postharvest Biol. Technol. 2017, 131, 16–30. [Google Scholar] [CrossRef]
- Graff, K.H.; Raj, T.S. Effect of Sargassum tenerrimum on controlling sheath blight of rice caused by Rhizoctonia solani Kuhn. Plant Arch. 2019, 19, 1132–1135. [Google Scholar]
- Pegoraro, C.; da Farias, D.R.; Mertz, L.M.; Santos, R.S.; da Maia, L.C.; Rombaldi, C.V.; de Oliveira, A.C. Ethylene response factors gene regulation and expression profiles under different stresses in rice. Theor. Exp. Plant Physiol. 2013, 25, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Zhang, K.; Sadeghnezhad, E.; Jiu, S.; Zhu, X.; Dong, T.; Liu, Z.; Guan, L.; Jia, H.; Fang, J. Chitinase family genes in grape differentially expressed in a manner specific to fruit species in response to Botrytis cinerea. Mol. Biol. Rep. 2020, 47, 7349–7363. [Google Scholar] [CrossRef] [PubMed]
- Shigeto, J.; Tsutsumi, Y. Diverse functions and reactions of class III peroxidases. New Phytol. 2016, 209, 1395–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentes, A.; Ortiz, J.; Saavedra, N.; Salazar, L.A.; Meneses, C.; Arriagada, C. Reference gene selection for quantitative real-time PCR in Solanum lycopersicum L. inoculated with the mycorrhizal fungus Rhizophagus irregularis. Plant Physiol. Biochem. 2016, 101, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Carling, D.E.; Pope, E.J.; Brainard, K.A.; Carter, D.A. Characterization of mycorrhizal isolates of Rhizoctonia solani from an orchid, including AG-12, a new anastomosis group. Phytopathology 1999, 89, 942–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malick, C.P.; Singh, M.B. Estimation of Total Phenols, Plant Enzymology and Histo Enzymology; Kalyani Publishers: New Delhi, India, 1980; p. 286. [Google Scholar]
- Maxwell, D.P.; Bateman, D.F. Changes in the activities of some oxidases in extracts of Rhizoctonia-infected bean hypocotyls in relation to lesion maturation. Phytopathology 1967, 57, 132–136. [Google Scholar]
- Galeazzi, M.A.M.; Sgarbieri, V.C.; Constantinides, S.M. Isolation, Purification and Physicochemical Characterization of Polyphenoloxidases (PPO) from a Dwarf Variety of Banana (Musa cavendishii, L). J. Food Sci. 1981, 46, 150–155. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; Chapman and Hall: London, UK, 1984. [Google Scholar]
- Shi, Q.; Bao, Z.; Zhu, Z.; Ying, Q.; Qian, Q. Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence, and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regul. 2006, 48, 127–135. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158-IN18. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.; Gianinazzi, V. Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche de methodes d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; INRA Press: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [Green Version]
- Abkhoo, J.; Sabbagh, S.K. Control of Phytophthora melonis damping-off, induction of defense responses, and gene expression of cucumber treated with commercial extract from Ascophyllum nodosum. J. Appl. Phycol. 2016, 28, 1333–1342. [Google Scholar] [CrossRef]
- Almagro, L.; Gómez Ros, L.V.; Belchi-Navarro, S.; Bru, R.; Ros Barceló, A.; Pedreño, M.A. Class III peroxidases in plant defence reactions. J. Exp. Bot. 2009, 60, 377–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poria, V.; Rana, A.; Kumari, A.; Grewal, J.; Pranaw, K.; Singh, S. Current Perspectives on Chitinolytic Enzymes and Their Agro-Industrial Applications. Biology 2021, 10, 1319. [Google Scholar] [CrossRef]
- Rashad, Y.M.; Al-Askar, A.A.; Ghoneem, K.M.; Saber, W.I.A.; Hafez, E.E. Chitinolytic Streptomyces griseorubens E44G enhances the biocontrol efficacy against Fusarium wilt disease of tomato. Phytoparasitica 2017, 45, 227–237. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulatory activities of Ascophyllum nodosum extract in tomato and sweet pepper crops in a tropical environment. PLoS ONE 2019, 14, e0216710. [Google Scholar] [CrossRef] [Green Version]
- Ng, L.M.; Melcher, K.; Teh, B.T.; Xu, H.E. Abscisic acid perception and signaling: Structural mechanisms and applications. Acta Pharmacol. Sin. 2014, 35, 567–584. [Google Scholar] [CrossRef] [Green Version]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.Y.; Wang, Y.; Tao, X.; Fan, Y.F.; Dai, Y.; Yang, H.; Ma, X.R. Genomic profiling of exogenous abscisic acid-responsive microRNAs in tomato (Solanum lycopersicum). BMC Genom. 2016, 17, 423. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Chen, L.; Yang, Y.; Guo, X.; Chen, G.; Xiong, X.; Dong, D.; Li, G. Transcriptome analysis reveals that exogenous ethylene activates immune and defense responses in a high late blight resistant potato genotype. Sci. Rep. 2020, 10, 21294. [Google Scholar] [CrossRef] [PubMed]
- Vangelisti, A.; Natali, L.; Bernardi, R.; Sbrana, C.; Turrini, A.; Hassani-Pak, K.; Hughes, D.; Cavallini, A.; Giovannetti, M.; Giordani, T. Transcriptome changes induced by arbuscular mycorrhizal fungi in sunflower (Helianthus annuus L.) roots. Sci. Rep. 2018, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-Induced Resistance and Priming of Plant Defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Sehrawat, K.D.; Mundlia, P.; Sehrawat, A.R.; Choudhary, R.; Rajput, V.D.; Minkina, T.; van Hullebusch, E.D.; Siddiqui, M.H.; Alamri, S. Potential use of ascophyllum nodosum as a biostimulant for improving the growth performance of vigna aconitifolia (Jacq.) marechal. Plants 2021, 10, 2361. [Google Scholar] [CrossRef] [PubMed]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Lee, Z.H.; Hirakawa, T.; Yamaguchi, N.; Ito, T. The roles of plant hormones and their interactions with regulatory genes in determining meristem activity. Int. J. Mol. Sci. 2019, 20, 4065. [Google Scholar] [CrossRef] [Green Version]
- Goñi, O.; Fort, A.; Quille, P.; McKeown, P.C.; Spillane, C.; O’Connell, S. Comparative Transcriptome Analysis of Two Ascophyllum nodosum Extract Biostimulants: Same Seaweed but Different. J. Agric. Food Chem. 2016, 64, 2980–2989. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant Properties of Seaweed Extracts in Plants: Implications towards Sustainable Crop Production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Ertani, A.; Francioso, O.; Tinti, A.; Schiavon, M.; Pizzeghello, D.; Nardi, S. Evaluation of seaweed extracts from Laminaria and Ascophyllum nodosum spp. As biostimulants in Zea mays L. using a combination of chemical, biochemical and morphological approaches. Front. Plant Sci. 2018, 9, 428. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [Green Version]
- El-Sharkawy, H.H.A.; Abbas, M.S.; Soliman, A.S.; Ibrahim, S.A.; El-Nady, I.A.I. Synergistic effect of growth-promoting microorganisms on bio-control of Fusarium oxysporum f. sp. pisi, growth, yield, physiological and anatomical characteristics of pea plants. Pestic. Biochem. Physiol. 2021, 178, 104939. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, H.; Zou, C.; Li, Y.; Chen, Y.; Wang, Z.; Jiang, Y.; Liu, A.; Zhao, P.; Wang, M.; et al. Combined inoculation with multiple arbuscular mycorrhizal fungi improves growth, nutrient uptake and photosynthesis in cucumber seedlings. Front. Microbiol. 2017, 8, 2516. [Google Scholar] [CrossRef] [PubMed]
- Muneer, M.A.; Wang, P.; Zaib-un-Nisa; Lin, C.; Ji, B. Potential role of common mycorrhizal networks in improving plant growth and soil physicochemical properties under varying nitrogen levels in a grassland ecosystem. Glob. Ecol. Conserv. 2020, 24, e01352. [Google Scholar] [CrossRef]
- Sato, T.; Hachiya, S.; Inamura, N.; Ezawa, T.; Cheng, W.; Tawaraya, K. Secretion of acid phosphatase from extraradical hyphae of the arbuscular mycorrhizal fungus Rhizophagus clarus is regulated in response to phosphate availability. Mycorrhiza 2019, 29, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Chanratana, M.; Kim, K.; Seshadri, S.; Sa, T. Impact of Arbuscular Mycorrhizal Fungi on Photosynthesis, Water Status, and Gas Exchange of Plants Under Salt Stress–A Meta-Analysis. Front. Plant Sci. 2019, 10, 457. [Google Scholar] [CrossRef]
- Pons, S.; Fournier, S.; Chervin, C.; Bécard, G.; Rochange, S.; Dit Frey, N.F.; Pagès, V.P. Phytohormone production by the arbuscular mycorrhizal fungus Rhizophagus irregularis. PLoS ONE 2020, 15, e0240886. [Google Scholar] [CrossRef]

| Items | Results |
|---|---|
| Water-soluble | 100% |
| Moisture | 3% |
| pH | 8 |
| Alginic acid | 18.9% |
| Organic matter | 50% |
| Nitrogen (N) | 1.8% |
| Phosphorus pentoxide (P2O5) | 2.4% |
| Potassium oxide (K2O) | 18.7% |
| Betaine | 62 ppm |
| Cytokinin | 200 ppm |
| Indole-3-acetic acid (IAA) | 50 ppm |
| Gibberellin | 18 ppm |
| Abscisic acid (ABA) | 20 ppm |
| Ethylene | 9 ppm |
| Polyamine | 10 ppm |
| Amino Acid | 2% |
| Mannitol | 4% |
| Sulfur (S) | 1% |
| Calcium (Ca) | 1% |
| Magnesium (Mg) | 0.2% |
| Sodium (Na) | 1% |
| Boron (B) | 0.2% |
| Iron (Fe) | 200 ppm |
| Manganese (Mn) | 2 ppm |
| Zinc (Zn) | 27 ppm |
| Copper (Cu) | 7 ppm |
| Gene Description | Abbrev. | Accession No. | Sequence (5′-3′) |
|---|---|---|---|
| Jasmonate and ethylene-responsive factor 3 | JERF3-F JERF3-R | AY383630 | GCCATTTGCCTTCTCTGCTTC GCAGCAGCATCCTTGTCTGA |
| Chitinase class II | CHI ΙΙ-F CHI ΙΙ-R | U30465 | GCGTTGTGGTTCTGGATGACA CAGCGGCAGAATCAGCAACA |
| Peroxidase | POD-F POD-R | X94943 | CCTTGTTGGTGGGCACACAA GGCCACCAGTGGAGTTGAAA |
| Elongation factor 1-α | EF1-α-F EF1-α-R | EC959059 | GAACTGGGTGCTTGATAGGC |
| AACCAAAATATCCGGAGTAAAAGA |
| Treatment | Shoot Height (cm) | Root Length (cm) | Shoot Dry Weight (g) | Root Dry Weight (g) | Number of Leaves/Plant | Leaf Area (cm2) |
|---|---|---|---|---|---|---|
| C | 45.0 ± 3.6 d | 5.3 ± 0.6 ef | 1.1 ± 0.3 e | 0.12 ± 0.02 e | 5.3 ± 1.0 cd | 37.7 ± 3.4 cd |
| P | 34.3 ± 4.2 e | 2.7 ± 0.3 g | 0.7 ± 0.1 f | 0.08 ± 0.01 f | 4.0 ± 0.5 d | 31.5 ± 2.5 f |
| CM | 50.7 ± 4.6 bc | 13.2 ± 0.9 a | 2.5 ± 0.4 a | 0.20 ± 0.03 b | 10.0 ± 1.2 a | 55.1 ± 4.3 a |
| SW1 | 47.0 ± 3.5 cd | 6.3 ± 0.6 de | 1.6 ± 0.2 d | 0.11 ± 0.05 e | 6.3 ± 0.5 bcd | 39.8 ± 3.6 c |
| SW2 | 47.7 ± 2.7 bc | 7.3 ± 0.4 cd | 1.8 ± 0.3 bcd | 0.16 ± 0.04 cd | 6.7 ± 0.4 bcd | 44.7 ± 3.8 b |
| SW3 | 49.0 ± 3.1 bc | 7.7 ± 0.7 cd | 2.2 ± 0.4 bc | 0.17 ± 0.03 c | 7.7 ± 0.6 abcd | 46.6 ± 3.4 b |
| PF | 42.3 ± 3.6 d | 4.7 ± 0.6 fg | 1.0 ± 0.4 e | 0.10 ± 0.04 ef | 5.3 ± 0.6 cd | 34.7 ± 2.7 de |
| PM | 49.0 ± 2.6 bc | 9.1 ± 0.4 bcd | 2.2 ± 0.6 bc | 0.19 ± 0.08 bc | 8.7 ± 0.7 abc | 36.2 ± 2.6 d |
| PFM | 47.3 ± 3.3 cd | 7.4 ± 0.3 cde | 1.6 ± 0.4 d | 0.13 ± 0.03 de | 6.3 ± 0.7 bcd | 35.3 ± 2.6 de |
| SW1M | 47.0 ± 4.1 cd | 8.0 ± 0.4 cd | 1.8 ± 0.3 bcd | 0.19 ± 0.04 bc | 8.3 ± 1.1 abc | 37.9 ± 2.7 cd |
| SW2M | 49.3 ± 3.7 bc | 9.0 ± 0.5 bcd | 2.3 ± 0.4 ab | 0.20 ± 0.05 b | 8.7 ± 1.2 abc | 44.7 ± 3.4 b |
| SW3M | 57.7 ± 4.2 a | 11.7 ± 1.0 ab | 2.5 ± 0.8 a | 0.24 ± 0.06 a | 9.7 ± 1.3 ab | 57.1 ± 2.9 a |
| PSW1 | 48.0 ± 2.8 bc | 5.0 ± 0.8 ef | 1.5 ± 0.6 d | 0.13 ± 0.04 de | 6.0 ± 0.6 bcd | 32.1 ± 2.8 f |
| PSW2 | 52.3 ± 3.7 bc | 5.3 ± 0.8 ef | 1.9 ± 0.5 bc | 0.14 ± 0.03 de | 6.3 ± 0.7 bcd | 36.7 ± 2.6 d |
| PSW3 | 55.0 ± 4.8 ab | 6.7 ± 0.5 de | 2.0 ± 0.4 b | 0.16 ± 0.05 cd | 7.3 ± 0.4 abcd | 39.6 ± 3.4 c |
| PSW1M | 49.3 ± 4.6 bc | 7.0 ± 0.4 de | 1.6 ± 0.5 d | 0.16 ± 0.04 cd | 7.3 ± 0.6 abcd | 33.6 ± 3.3 ef |
| PSW2M | 54.3 ± 3.4 ab | 7.7 ± 0.6 cde | 2.1 ± 0.6 bc | 0.17 ± 0.04 c | 7.7 ± 0.5 abcd | 36.9 ± 3.6 d |
| PSW3M | 55.7 ± 3.8 ab | 10.0 ± 0.4 bc | 2.2 ± 0.6 bc | 0.18 ± 0.06 bc | 8.7 ± 0.8 abc | 40.8 ± 4.0 c |
| Treatment | No. of Pods/Plant | Pod Weight (g) | Pod Length (cm) | Pod Width (cm) | Yield/Plant (g) | No. of Seeds/Pod |
|---|---|---|---|---|---|---|
| C | 2.0 ± 0.8 c | 3.0 ± 0.2 cd | 5.2 ± 0.3 bc | 1.3 ± 0.10 b | 6.0 ± 1.0 h | 3.3 ± 0.6 bc |
| P | 1.3 ± 0.6 d | 2.1 ± 0.3 e | 4.5 ± 0.8 c | 1.1 ± 0.06 c | 3.1 ± 1.4 i | 2.3 ± 0.5 c |
| CM | 3.3 ± 0.5 ab | 4.8 ± 0.6 ab | 6.2 ± 0.3 ab | 1.4 ± 0.07 ab | 16.1 ± 3.5 b | 5.7 ± 0.7 ab |
| SW1 | 2.3 ± 0.5 bc | 4.2 ± 0.5 b | 6.1 ± 0.4 ab | 1.4 ± 0.04 ab | 9.5 ± 1.1 fg | 4.0 ± 0.9 abc |
| SW2 | 2.7 ± 0.6 bc | 4.2 ± 0.4 b | 6.3 ± 0.6 ab | 1.4 ± 0.05 ab | 11.3 ± 1.3 f | 5.0 ± 0.8 ab |
| SW3 | 3.0 ± 0.9 ab | 5.1 ± 0.5 a | 6.5 ± 0.4 a | 1.4 ± 0.06 ab | 15.4 ± 1.5 cd | 5.7 ± 0.7 ab |
| PF | 2.0 ± 0.7 c | 2.9 ± 0.2 d | 5.1 ± 0.5 bc | 1.3 ± 0.07 b | 5.8 ± 0.9 h | 3.3 ± 0.5 bc |
| PM | 3.0 ± 0.4 ab | 4.7 ± 0.6 ab | 5.9 ± 0.7 ab | 1.4 ± 0.06 ab | 13.8 ± 1.5 d | 5.0 ± 0.6 ab |
| PFM | 3.0 ± 0.6 ab | 3.6 ± 0.3 c | 6.2 ± 0.6 ab | 1.4 ± 0.06 ab | 10.8 ± 1.0 f | 5.3 ± 0.6 ab |
| SW1M | 3.3 ± 0.7 ab | 4.4 ± 0.4 ab | 6.3 ± 0.5 ab | 1.4 ± 0.05 ab | 15.3 ± 2.1 cd | 4.7 ± 0.5 abc |
| SW2M | 3.9 ± 0.5 a | 4.6 ± 0.4 ab | 6.5 ± 0.7 a | 1.5 ± 0.09 a | 16.4 ± 2.2 b | 5.3 ± 0.4 ab |
| SW3M | 4.3 ± 0.9 a | 5.5 ± 0.8 a | 6.8 ± 0.8 a | 1.5 ± 0.07 a | 24.0 ± 3.6 a | 6.0 ± 0.5 a |
| PSW1 | 2.3 ± 0.5 bc | 3.9 ± 0.8 bc | 6.1 ± 0.7 ab | 1.4 ± 0.08 ab | 8.7 ± 1.2 g | 5.0 ± 0.7 ab |
| PSW2 | 3.7 ± 0.4 ab | 4.2 ± 0.6 b | 6.2 ± 0.4 ab | 1.4 ± 0.05 ab | 15.6 ± 1.7 bc | 5.3 ± 0.6 ab |
| PSW3 | 3.3 ± 0.8 ab | 4.6 ± 0.4 ab | 6.3 ± 0.5 ab | 1.4 ± 0.04 ab | 15.7 ± 1.4 bc | 5.7 ± 0.5 ab |
| PSW1M | 3.0 ± 0.6 ab | 4.1 ± 0.3 b | 6.0 ± 0.4 ab | 1.4 ± 0.06 ab | 12.3 ± 1.3 e | 5.0 ± 0.6 ab |
| PSW2M | 3.0 ± 0.4 ab | 4.6 ± 0.6 ab | 6.2 ± 0.6 ab | 1.5 ± 0.07 a | 13.8 ± 1.7 d | 6.0 ± 0.8 a |
| PSW3M | 3.7 ± 0.5 ab | 5.0 ± 0.8 a | 6.3 ± 0.5 ab | 1.5 ± 0.04 a | 18.4 ± 2.0 ab | 6.0 ± 0.7 a |
| Treatment | Phenolic Content (mg.g−1 fwt) | Peroxidase (∆A470 min−1 g−1 fwt) | Polyphenol Oxidase (∆A420 min−1 g−1 fwt) | Electrolyte Leakage (%) | Soluble Solids Content (°Brix) |
|---|---|---|---|---|---|
| C | 394.6 ± 7.8 i | 1.02 ± 0.08 h | 1.03 ± 0.07 i | 55.3 ± 1.4 f | 15.3 ± 0.8 bc |
| P | 568.9 ± 10.1 gh | 1.41 ± 0.0.3 efg | 1.33 ± 0.06 gh | 118.4 ± 1.9 a | 10.0 ± 0.4 d |
| CM | 584.3 ± 6.5 fgh | 1.89 ± 0.06 d | 1.54 ± 0.07 def | 55.5 ± 1.4 f | 17.0 ± 0.7 ab |
| SW1 | 583.6 ± 8.1 fgh | 1.31 ± 0.04 fg | 1.20 ± 0.04 hi | 55.7 ± 1.1 f | 16.5 ± 0.8 ab |
| SW2 | 605.1 ± 11.2 fgh | 1.36 ± 0.05 fg | 1.24 ± 0.06 h | 55.9 ± 0.9 f | 16.7 ± 0.6 ab |
| SW3 | 623.7 ± 9.6 fgh | 1.39 ± 0.05 efg | 1.28 ± 0.07 h | 55.9 ± 1.2 f | 18.6 ± 0.7 a |
| PF | 531.6 ± 7.4 h | 1.30 ± 0.07 g | 1.27 ± 0.05 h | 87.2 ± 1.4 cd | 13.1 ± 0.9 c |
| PM | 900.9 ± 12.3 cd | 2.04 ± 0.05 cd | 1.69 ± 0.07 bcd | 86.9 ± 1.4 cd | 16.8 ± 0.5 ab |
| PFM | 690.5 ± 8.9 ef | 1.98 ± 0.08 cd | 1.54 ± 0.09 def | 87.4 ± 0.9 cd | 16.7 ± 1.0 ab |
| SW1M | 642.4 ± 10.1 fgh | 1.42 ± 0.03 efg | 1.33 ± 0.04 gh | 55.8 ± 1.0 f | 17.2 ± 0.9 ab |
| SW2M | 657.8 ± 6.9 fg | 1.53 ± 0.07 ef | 1.35 ± 0.03 fgh | 55.9 ± 0.9 f | 17.7 ± 1.2 ab |
| SW3M | 677.8 ± 7.4 efg | 1.60 ± 0.08 e | 1.39 ± 0.04 efgh | 57.5 ± 1.2 f | 18.5 ± 1.6 a |
| PSW1 | 796.7 ± 11.0 de | 1.96 ± 0.08 cd | 1.51 ± 0.07 defg | 97.5 ± 1.4 b | 14.6 ± 0.7 b |
| PSW2 | 878.9 ± 11.8 cd | 1.96 ± 0.09 cd | 1.56 ± 0.05 de | 86.5 ± 1.1 cd | 15.9 ± 1.0 abc |
| PSW3 | 945.6 ± 10.5 c | 2.13 ± 0.06 abc | 1.64 ± 0.06 cd | 85.5 ± 1.2 d | 15.9 ± 0.4 abc |
| PSW1M | 1091.2 ± 18.8 b | 2.12 ± 0.07 bcd | 1.80 ± 0.07 abc | 89.7 ± 1.3 c | 16.5 ± 0.8 ab |
| PSW2M | 1158.7 ± 20.2 b | 2.32 ± 0.06 ab | 1.86 ± 0.08 ab | 85.4 ± 1.4 d | 17.1 ± 0.7 ab |
| PSW3M | 1346.4 ± 23.6 a | 2.36 ± 0.07 a | 1.97 ± 0.06 a | 78.1 ± 1.0 e | 17.4 ± 1.0 ab |
| Treatment | Chl. a (mg g−1 fwt) | Chl. b (mg g−1 fwt) | Carotenoids (mg g−1 fwt) | Total Pigments (mg g−1 fwt) |
|---|---|---|---|---|
| C | 2.34 ± 0.4 cd | 0.71 ± 0.08 ab | 0.50 ± 0.06 a | 3.55 ± 0.9 cde |
| P | 0.62 ± 0.2 f | 0.24 ± 0.03 bc | 0.02 ± 0.004 b | 0.88 ± 0.03 h |
| CM | 2.60 ± 0.7 bc | 1.02 ± 0.04 a | 0.51 ± 0.04 a | 4.13 ± 0.8 b |
| SW1 | 2.34 ± 0.6 cd | 0.75 ± 0.03 ab | 0.43 ± 0.03 ab | 3.52 ± 0.6 cde |
| SW2 | 2.72 ± 0.5 bc | 0.84 ± 0.04 ab | 0.42 ± 0.04 ab | 3.98 ± 0.6 bc |
| SW3 | 3.44 ± 0.7 a | 0.93 ± 0.06 ab | 0.61 ± 0.05 a | 4.98 ± 0.5 a |
| PF | 1.75 ± 0.4 de | 0.71 ± 0.04 ab | 0.33 ± 0.05 ab | 2.79 ± 0.7 ef |
| PM | 2.01 ± 0.6 d | 0.55 ± 0.04 bc | 0.42 ± 0.03 ab | 2.98 ± 0.8 e |
| PFM | 2.22 ± 0.6 cd | 0.54 ± 0.04 bc | 0.44 ± 0.04 ab | 3.20 ± 0.4 de |
| SW1M | 2.61 ± 0.5 bc | 0.84 ± 0.06 ab | 0.25 ± 0.02 ab | 3.70 ± 0.5 cd |
| SW2M | 2.90 ± 0.5 ab | 0.86 ± 0.05 ab | 0.36 ± 0.04 ab | 4.12 ± 0.8 bc |
| SW3M | 3.66 ± 0.4 a | 1.12 ± 0.06 a | 0.61 ± 0.03 a | 5.39 ± 1.0 a |
| PSW1 | 1.14 ± 0.6 e | 0.53 ± 0.05 bc | 0.20 ± 0.04 ab | 1.87 ± 0.3 g |
| PSW2 | 1.23 ± 0.4 e | 0.53 ± 0.06 bc | 0.24 ± 0.03 ab | 2.00 ± 0.4 g |
| PSW3 | 2.33 ± 0.9 cd | 0.64 ± 0.04 b | 0.43 ± 0.04 ab | 3.40 ± 0.6 cde |
| PSW1M | 2.35 ± 0.6 cd | 0.55 ± 0.07 bc | 0.26 ± 0.03 ab | 3.16 ± 0.5 e |
| PSW2M | 2.72 ± 0.9 bc | 0.63 ± 0.04 b | 0.25 ± 0.04 ab | 3.60 ± 0.8 cde |
| PSW3M | 2.74 ± 0.8 bc | 0.74 ± 0.08 ab | 0.33 ± 0.05 ab | 3.81 ± 0.6 bcd |
| Treatment | FC (%) | IC (%) | FA (%) |
|---|---|---|---|
| C | 0 d | 0 f | 0 e |
| P | 0 d | 0 f | 0 e |
| CM | 88.3 ± 3.7 a | 50.7 ± 2.4 a | 20.6 ± 1.4 a |
| SW1 | 0 d | 0 f | 0 e |
| SW2 | 0 d | 0 f | 0 e |
| SW3 | 0 d | 0 f | 0 e |
| PF | 0 d | 0 f | 0 e |
| PM | 69.4 ± 1.1 b | 34.5 ± 1.6 c | 12.4 ± 0.9 cd |
| PFM | 52.1 ± 0.6 c | 26.4 ± 1.2 e | 9.7 ± 0.4 d |
| SW1M | 85.6 ± 2.5 a | 46.4 ± 1.8 ab | 18.7 ± 0.9 ab |
| SW2M | 83.9 ± 1.8 a | 40.9 ± 2.1 b | 17.9 ± 1.0 ab |
| SW3M | 86.4 ± 1.1 a | 44.5 ± 0.9 b | 18.4 ± 0.8 ab |
| PSW1 | 0 d | 0 f | 0 e |
| PSW2 | 0 d | 0 f | 0 e |
| PSW3 | 0 d | 0 f | 0 e |
| PSW1M | 70.2 ± 0.8 b | 30.7 ± 0.9 d | 11.8 ± 0.8 cd |
| PSW2M | 72.8 ± 1.0 b | 33.4 ± 1.1 c | 12.0 ± 0.7 cd |
| PSW3M | 69.6 ± 0.9 b | 35.1 ± 1.0 c | 12.2 ± 0.5 cd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashad, Y.M.; El-Sharkawy, H.H.A.; Elazab, N.T. Ascophyllum nodosum Extract and Mycorrhizal Colonization Synergistically Trigger Immune Responses in Pea Plants against Rhizoctonia Root Rot, and Enhance Plant Growth and Productivity. J. Fungi 2022, 8, 268. https://doi.org/10.3390/jof8030268
Rashad YM, El-Sharkawy HHA, Elazab NT. Ascophyllum nodosum Extract and Mycorrhizal Colonization Synergistically Trigger Immune Responses in Pea Plants against Rhizoctonia Root Rot, and Enhance Plant Growth and Productivity. Journal of Fungi. 2022; 8(3):268. https://doi.org/10.3390/jof8030268
Chicago/Turabian StyleRashad, Younes M., Hany H. A. El-Sharkawy, and Nahla T. Elazab. 2022. "Ascophyllum nodosum Extract and Mycorrhizal Colonization Synergistically Trigger Immune Responses in Pea Plants against Rhizoctonia Root Rot, and Enhance Plant Growth and Productivity" Journal of Fungi 8, no. 3: 268. https://doi.org/10.3390/jof8030268
APA StyleRashad, Y. M., El-Sharkawy, H. H. A., & Elazab, N. T. (2022). Ascophyllum nodosum Extract and Mycorrhizal Colonization Synergistically Trigger Immune Responses in Pea Plants against Rhizoctonia Root Rot, and Enhance Plant Growth and Productivity. Journal of Fungi, 8(3), 268. https://doi.org/10.3390/jof8030268







