Allergy to Fungi in Veterinary Medicine: Alternaria, Dermatophytes and Malassezia Pay the Bill!
Abstract
:1. Introduction
2. Most Relevant Fungi in Health
3. Fungi as Sources of Allergens
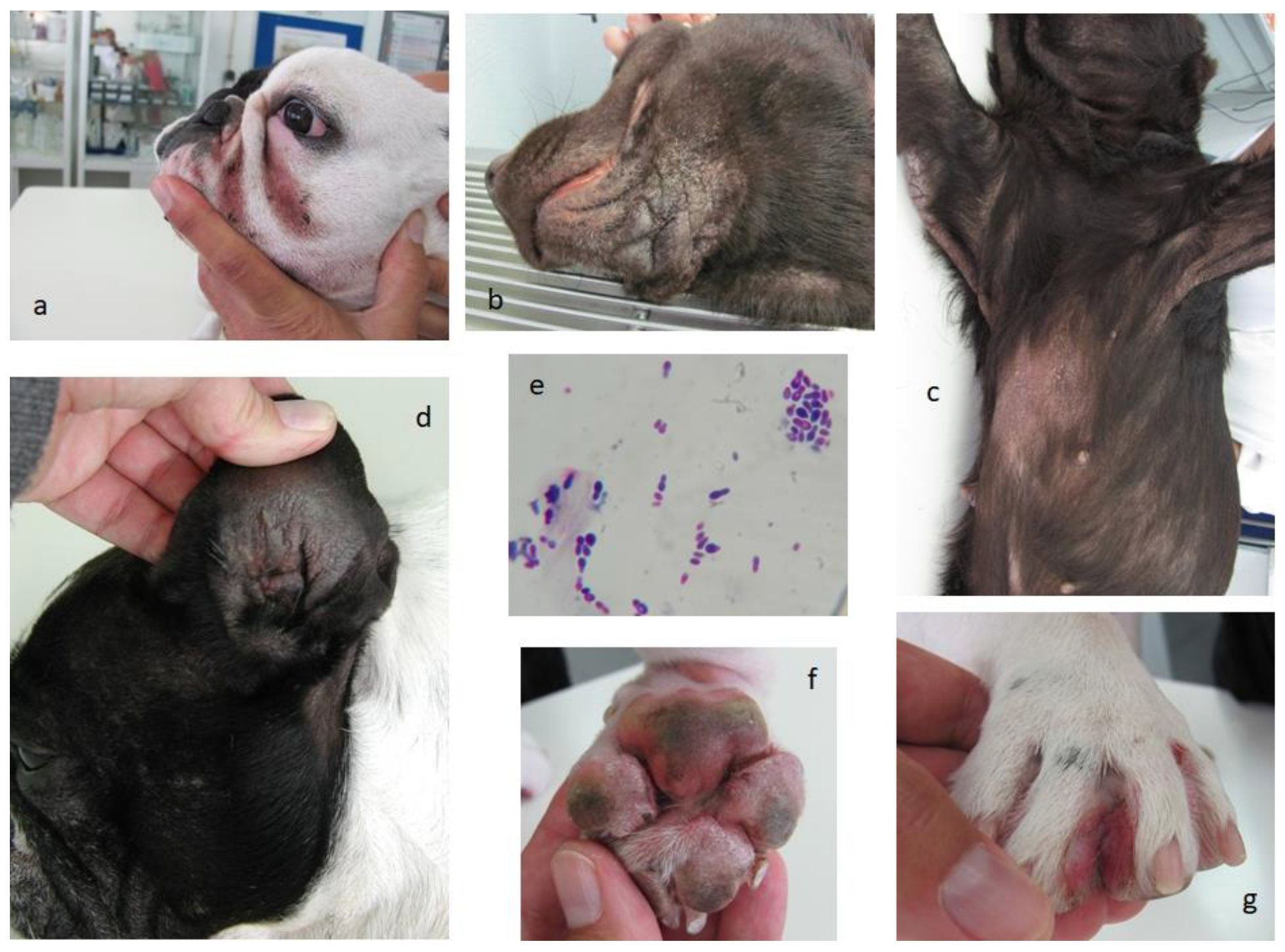
4. Malassezia, a Complex Big Issue in Animal Allergy
5. Main Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.Y.; Kumar, S.; Hedges, S.B. Divergence time estimates for the early history of animal phyla and the origin of plants, animals and fungi. Proc. Royal Soc. B 1999, 266, 163–171. [Google Scholar] [CrossRef]
- Donoghue, M.J.; Cracraft, J. Assembling the Tree of Life; Oxford University Press: Oxford, UK, 2004; p. 187. [Google Scholar]
- De Lucca, A.J. Harmful fungi in both Agriculture and Medicine. Rev. Iberoam Micol. 2007, 24, 3–13. [Google Scholar] [CrossRef]
- Moriello, K.A.; Coyner, K.; Paterson, S.; Mignon, B. Diagnosis and treatment of dermatophytosis in dogs and cats. Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet Dermatol. 2017, 28, 266–268. [Google Scholar] [CrossRef]
- Yafetto, L.; Carroll, L.; Cui, Y.; Davis, D.J.; Fischer, M.W.; Henterly, A.C.; Kessler, J.D.; Kilroy, H.A.; Shidler, J.B.; Stolze-Rybczynski, J.L.; et al. The fastest flights in nature: Highspeed spore discharge mechanisms among fungi. PLoS ONE 2008, 3, e3237. [Google Scholar] [CrossRef] [PubMed]
- Akan, M.; Hazirogiu, R.; Ihan, Z.; Sareyyupogiu, B.; Tunca, R. A case of aspergillosis in a broiler breeder flock. Avian Dis. 2002, 46, 497–501. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Wang, Y.; Chai, T.; Cai, Y.; Li, N. Pathogenicity and immune responses of Aspergillus fumigatus infection in chickens. Front Vet. Sci. 2020, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Maurya, V.; Gugnami, H.C.; Sarnia, P.U.; Madan, T.; Shah, A. Sensitization to Aspergillus antigens and occurrence of allergic bronchopulmonary aspergillosis in patients with asthma. Chest 2005, 127, 1252–1259. [Google Scholar] [CrossRef]
- Shah, A.; Panjabi, O. Allergic bronchopulmonary aspergillosis: A review of a disease with a worldwide distribution. J. Asthma. 2002, 39, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Van der Westhuizen, L.; Shephard, G.S.; Snyman, S.D.; Abel, S.; Swanevelder, S.; Gelderblom, W.O. Inhibition of sphingolipid biosynthesis in rat primary hepatocyte cultures by fumonisin B1 and other structurally related compounds. Food Chem. Toxicol. 1998, 36, 497–503. [Google Scholar] [CrossRef]
- Paixão, A.; Caldeira, J.; Leocádio, J.; Martins, L. The Importance of Skin Barrier Integrity for the Prevention of Veterinary Allergy. Rev. Por.T. Imunoalergologia. 2022, in press. [Google Scholar]
- Romano, C.; Paccagnini, E.; Difonzo, D. Onychomycosis caused by Alternaria spp. in Tuscany from 1985 to 1999. Mycoses 2001, 44, 73–76. [Google Scholar] [CrossRef]
- Romero, C.; Vanzi, L.; Massi, D.; Difonzo, E.M. Subcutaneous alternariosis. Mycoses 2005, 48, 408–412. [Google Scholar]
- Zahra, L.V.; Mallia, D.; Hardie, J.G.; Bezzina, A.; Fenech, T. Case report: Keratomycosis due to Alternaria alternata in a diabetic patient. Mycoses 2002, 45, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Morrison, V.A.; Mc Glave, P.B. Mucormycosis in the BMT population. Bone Marrow Transpl. 1993, 11, 383–388. [Google Scholar]
- Barnes, C. Fungi and Atopy. Clin. Rev. Allergy Immunol. 2019, 57, 439–448. [Google Scholar] [CrossRef]
- Čelakovská, J.; Bukač, J.; Ettler, K.; Vaneckova, J.; Ettlerova, K.; Krejsek, J. Sensitisation to outdoor and indoor fungi in atopic dermatitis patients and the relation to the occurrence of food allergy to peanuts and walnuts. Mycoses 2018, 61, 698–703. [Google Scholar] [CrossRef]
- Saberi-Riseh, R.; Javan-Nikkhah, M.; Heidarian, R.; Hosseini, S.; Soleimani, P. Detection of fungal infectious agent of wheat grains in store-pits of Markazi province, Iran. Commun. Agric. Appl. Biol. Sci. 2004, 69, 541–554. [Google Scholar]
- Lacey, J.; Dutkiewicz, J. Bioaerosols and occupational lung disease. J. Aerosol. Sci. 1994, 25, 1371–1404. [Google Scholar] [CrossRef]
- Verma, J.; Singh, B.P.; Sridhara, S.; Gaur, S.N.; Arora, N. Purification and characterization of a cross-reactive 45-kD major allergen of Fusarium solani. Int. Arch. Allergy. Immunol. 2003, 130, 193–199. [Google Scholar] [CrossRef]
- Muhammed, M.; Anagnostou, T.; Desalermos, A.; Kourkoumpetis, T.K.; Carneiro, H.A.; Glavis-Bloom, J.; Coleman, J.J.; Mylonakis, E. Fusarium infection report of 26 cases and review of 97 cases from the literature. Medicine 2013, 92, 305–316. [Google Scholar] [CrossRef]
- O’Neil, O.E.; McOants, M.L.; Salvaggio, J.E.; Lehrer, S.B. Fusarium solani: Prevalence of skin reactivity and antigenic allergenic analysis. J. Allergy Clin. Immunol. 1986, 77, 842–849. [Google Scholar] [CrossRef]
- Martins, L.; Pires, E.; Inácio, F. CD4 T-Cell Cytokine Response to Mite Recombinant Tropomyosin in Mite, Snail and Shrimp Allergic Patients. Internet J. Asthma. Allergy Immunol. 2009, 7, 1. [Google Scholar]
- Wilhernus, K.R.; Jones, D.B. Curvularia keratitis. Tr. Am. Ophthalmol. Soc. 2001, 99, 111–132. [Google Scholar]
- Fernandez, M.; Noyola, D.E.; Rossman, S.N.; Edwards, M.S. Cutaneous phaeohyphomycosis caused by Curvularia lunata and a review of Curvularia infections in pediatrics. Pediatr. Infect Dis. J. 1999, 18, 727–731. [Google Scholar] [CrossRef]
- Gupta, R.; Singh, B.P.; Sridhara, S.; Gaur, S.N.; Chaudhary, V.K.; Arora, N. Allergens of Curvularia lunata during cultivation in different media. J. Allergy Clin. Immunol. 1999, 4, 857–862. [Google Scholar] [CrossRef]
- Gupta, R.; Singh, B.P.; Sridhara, S.; Gaur, S.N.; Kunar, R.; Chaudhary, V.K.; Arora, N. Identification of cross-reactive proteins amongst different Curvularia species. Int. Arch. Allergy Immunol. 2002, 127, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Singh, B.P.; Sridhara, S.; Gaur, S.N.; Kumar, R.; Ghaudhary, V.K.; Arora, N. Allergenic cross-reactivity of Curvularia lunata with other airborne fungal species. Allergy 2002, 57, 636–640. [Google Scholar] [CrossRef]
- Strzok, E.; Siepker, C.; Armwood, A.; Howerth, E.; Smith, J.; Banovic, F. Successful treatment of cutaneous Curvularia geniculata, Nocardia niigatensis, and viral papillomatosis in a dog during the therapeutic management of immune-mediated hemolytic anemia. Front Vet. Sci. 2019, 6, 249. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kim, H.; Jang, H.; Park, H. Sensitization rates of causative allergens for dogs with atopic dermatitis: Detection of canine allergen-specific IgE. J. Vet. Med. Sci. 2014, 15, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Spano, M.; Zuliani, D.; Peano, A.; Bertazzolo, W. Cladosporium cladosporioides-complex infection in a mixed-breed dog. Vet. Clin. Pathol. 2018, 47, 150–153. [Google Scholar] [CrossRef]
- Dauvillier, J.; Ter Woort, F.; van Erck-Westergren, E. Fungi in respiratory samples of horses with inflammatory airway disease. J. Vet. Intern. Med. 2019, 33, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Jarrah, S.A.; Zanetti, C.C.; Maruyama, F.H.; Ito, A.T.; Rosa, J.M.A.; Colodel, E.M.; Lima, S.R.; Nakazato, L.; Dutra, V. Cladosporium cladosporioides isolated from a cat with squamous cell carcinoma. Arq. Bras. Med. Vet. Zootec. 2017, 69, 377–380. [Google Scholar] [CrossRef]
- Mariani, C.L.; Platt, S.R.; Scase, T.J.; Howerth, E.W.; Chrisman, C.L.; Clemmons, R.M. Cerebral phaeohyphomycosis caused by Cladosporium spp. in two domestic shorthair cats. J. Am. Anim. Hosp. Assoc. 2002, 38, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, O. Molds and Respiratory Allergy–Part 1. MOJ Immunol. 2015, 2, 00045. [Google Scholar] [CrossRef]
- Meason-Smith, C.; Diesel, A.; Patterson, A.P.; Older, C.E.; Mansell, J.M.; Suchodolski, J.S.; Hoffmann, A.R. What is living on your dog’s skin? Characterization of the canine cutaneous mycobiota and fungal dysbiosis in canine allergic dermatitis. FEMS Microbiol. Ecol. 2015, 91, fiv139. [Google Scholar] [CrossRef]
- Stone, N.; Gupta, N.; Schwartz, I. Mucormycosis: Time to address this deadly fungal infection. Microbe 2021, 2, e343–e344. [Google Scholar] [CrossRef]
- Cafarchia, C.; Figueredo, L.A.; Otranto, D. Fungal diseases of horses. Vet. Microbiol. 2013, 167, 215–234. [Google Scholar] [CrossRef]
- Youn, H.Y.; Kang, H.S.; Bhang, D.H.; Kim, M.K.; Hwang, C.Y.; Han, H.R. Allergens causing atopic diseases in canine. J. Vet. Sci. 2002, 3, 335–341. [Google Scholar] [CrossRef]
- Lund, A.; DeBoer, D.J. Immunoprophylaxis of dermatophytosis in animals. Mycopathologia 2008, 166, 407–424. [Google Scholar] [CrossRef]
- Medeiros, F.; Crepaldi, N.; Tognoli, L.; Pereira, R.E.P. Dermatófitos–Revisão de literatura. Rev. Cient Eletrônica. Med. Vet. 2009, 12. [Google Scholar]
- Seyedmousavi, S.; Bosco, S.d.G.; de Hoog, S.; Ebel, F.; Elad, D.; Gomes, R.R.; Jacobsen, I.D.; Jensen, H.E.; Martel, A.; Mignon, B.; et al. Fungal infections in animals: A patchwork of different situations. Med. Mycol. 2018, 56, S165–S187. [Google Scholar] [CrossRef] [PubMed]
- Yurayart, C.; Chindamporn, A.; Suradhat, S.; Tummaruk, P.; Kajiwara, S.; Prapasarakul, N. Comparative analysis of the frequency, distribution and population sizes of yeasts associated with seborrheic dermatitis and healthy skin. Vet. Microbiol. 2011, 148, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.S.; Bettenay, S.V.; Shipstone, M. Cutaneous candidiasis in a dog caused by Candida guilliermondii. Vet. Rec. 2002, 150, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Willems, N.; Houwers, D.J.; Schlotter, Y.M.; Theelen, B.; Boekhout, T. Disseminated candidiasis in a young, previously healthy, dog and review of literature. Mycopathologia 2017, 182, 591–596. [Google Scholar] [CrossRef]
- Woodfolk, J. Allergy and dermatophytes. Clin. Microbiol. Rev. 2005, 18, 30–43. [Google Scholar] [CrossRef]
- Salo, P.M.; Arbes, S.J., Jr.; Jaramillo, R.; Calatroni, A.; Weir, C.H.; Sever, M.L.; Hoppin, J.A.; Rose, K.M.; Liu, A.H.; Gergen, P.J.; et al. Prevalence of allergic sensitization in the United States: Results from the National Health and Nutrition Examination Survey (NHANES) 2005–2006. J. Allergy Clin. Immunol. 2014, 134, 350–359. [Google Scholar] [CrossRef]
- Amado, M.; Portnoy, J.M.; Barnes, C. Fungal cross-allergenicity in specific IgE testing. J. Allergy Clin. Immunol. 2014, 133 (Suppl. 2), AB92. [Google Scholar] [CrossRef]
- Williams, P.B.; Barnes, C.S.; Portnoy, J.M. Environmental allergens workgroup. Innate and adaptive immune response to fungal products and allergens. J. Allergy Clin. Immunol. Pract. 2016, 4, 386–395. [Google Scholar] [CrossRef]
- Crameri, R.; Weichel, M.; Fluckiger, S.; Glaser, A.G.; Rhyner, C. Fungal allergies: A yet unsolved problem. Chem. Immunol. Allergy 2006, 91, 121–133. [Google Scholar]
- Green, B.J.; Tovey, E.R.; Sercombe, J.K.; Blachere, F.M.; Beezhold, D.H.; Schmechel, D. Airborne fungal fragments and allergenicity. Med. Mycol. 2006, 44 (Suppl. 1), S245–S255. [Google Scholar] [CrossRef]
- Allergome–The Platform for Allergen Knowledge. Available online: https://www.allergome.org/ (accessed on 20 January 2022).
- O’Hollaren, M.T.; Yunginger, J.W.; Offord, K.P.; Somers, M.J.; O’Connell, E.J.; Ballard, D.J.; Sachs, M.I. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N. Engl. J. Med. 1991, 324, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Pfefferle, P.I.; Keber, C.U.; Cohen, R.M.; Garn, H. The Hygiene hypothesis–Learning from but not living in the past. Front. Immunol. 2021, 12, 635935. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, J.M.; Barnes, C.S.; Kennedy, K. Importance of mold allergy in asthma. Curr. Allergy Asthma. Rep. 2008, 8, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Jara, D.; Portnoy, J.; Dhar, M.; Barnes, C. Relation of indoor and outdoor airborne fungal spore levels in the Kansas City metropolitan area. Allergy Asthma. Proc. 2017, 38, 130–135. [Google Scholar] [CrossRef]
- Mueller, R.S.; Janda, J.; Jensen-Jarolim, E.; Rhyner, C.; Marti, E. Allergens in veterinary medicine. Allergy 2016, 71, 27–35. [Google Scholar] [CrossRef]
- Dirscherl, P.; Grabner, A.; Buschmann, H. Responsiveness of basophil granulocytes of horses suffering from chronic obstructive pulmonary disease to various allergens. Vet. Immunol. Immunopathol. 1993, 38, 217–227. [Google Scholar] [CrossRef]
- Schmallenbach, K.H.; Rahman, I.; Sasse, H.H.; Dixon, P.M.; Halliwell, R.E.; McGorum, B.C.; Crameri, R.; Miller, H.R. Studies on pulmonary and systemic Aspergillus fumigatus-specific IgE and IgG antibodies in horses affected with chronic obstructive pulmonary disease (COPD). Vet. Immunol. Immunopathol. 1998, 66, 245–256. [Google Scholar] [CrossRef]
- Kunzle, F.; Gerber, V.; Van Der Haegen, A.; Wampfler, B.; Straub, R.; Marti, E. IgE-bearing cells in bronchoalveolar lavage fluid and allergen-specific IgE levels in sera from RAO-affected horses. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2007, 54, 40–47. [Google Scholar] [CrossRef]
- Eder, C.; Crameri, R.; Mayer, C.; Eicher, R.; Straub, R.; Gerber, H.; Lazary, S.; Marti, E. Allergen-specific IgE levels against crude mould and storage mite extracts and recombinant mould allergens in sera from horses affected with chronic bronchitis. Vet. Immunol. Immunopathol. 2000, 73, 241–253. [Google Scholar] [CrossRef]
- Scharrenberg, A.; Gerber, V.; Swinburne, J.E.; Wilson, A.D.; Klukowska-Rotzler, J.; Laumen, E.; Marti, E. IgE, IgGa, IgGb and IgG(T) serum antibody levels in offspring of two sires affected with equine recurrent airway obstruction. Anim. Genet. 2010, 41 (Suppl. 2), 131–137. [Google Scholar] [CrossRef]
- Leocádio, J.G.; Galhós, A.; Damásio, L.; Leal, C.R.; Fino, T.; Martins, L.M. Horse sensitization and allergy to mold, pollen, dust and storage mites, and culicoides in a horse population from southern Portugal. In Proceedings of the European Academy of Allergy and Clinical Immunology (EAACI) Congress, Lisbon, Portugal, 1–5 June 2019. [Google Scholar]
- Martins, L.M. Results from Veterinary Immunoallergology Pilot Research; University of Évora: Évora, Portugal, 2019; (unpublished work). [Google Scholar]
- Tizard, I.R.; Sydney, W.J. The microbiota regulates immunity and immunologic diseases in dogs and cats. Vet. Clin. North. Am. Small Anim. Pract. 2017, 48, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, J.C. The microbiome in early life: Implications for health outcomes. Nat. Med. 2016, 22, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Hohenbichler, J.; Spindler, V.; Pahlke, G.; Rychlik, M.; Del Favero, G.; Marko, D. Immunomodulatory potential of combined Alternaria alternata mycotoxins in non-cancerous epithelial colon cells. In Proceedings of the 56th Congress of the European Societies of Toxicology (EUROTOX 2021), Toxicology of the Next Generation, Copenhagen, Denmark, 27 September–1 October 2021. [Google Scholar]
- Hohenbichler, J.; Aichinger, G.; Rychlik, M.; Del Favero, G.; Marko, D. Alternaria alternata Toxins Synergistically Activate the Aryl Hydrocarbon Receptor Pathway in vitro. Biomolecules 2020, 10, 1018. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multi–sensitized atopy and T-cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr. Allergy Immunol. 2016, 27 (Suppl. 23), 1–250. [Google Scholar] [CrossRef] [PubMed]
- Hensel, P.; Santoro, D.; Favrot, C.; Hill, P.; Griffin, C. Canine atopic dermatitis: Detailed guideline for diagnosis and allergen identification. BMC Vet. Res. 2015, 11, 196. [Google Scholar] [CrossRef]
- Martins, L.M. Results from Veterinary Immunoallergology Outpatient Consultation; University of Évora: Évora, Portugal, 2022; (unpublished work). [Google Scholar]
- Bond, R.; Morris, D.O.; Guillot, J.; Bensignor, E.J.; Robson, D.; Mason, K.V.; Kano, R.; Hill, P.B. Biology, diagnosis and treatment of Malassezia dermatitis in dogs and cats. Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 2020, 31, 27.e4. [Google Scholar] [CrossRef]
- Di Tommaso, M.; Luciani, A.; Crisi, P.E.; Beschi, M.; Rosi, P.; Rocconi, F.; Miglio, A. Detection of serum allergen-specific IgE in atopic dogs tested in northern Italy: Preliminary study. Animals 2021, 11, 358. [Google Scholar] [CrossRef]
- Farver, K.; Morris, D.O.; Shofer, F.; Esch, B. Humoral Measurement of type-1 hypersensitivity reactions to a commercial Malassezia allergen. Vet. Dermatol. 2005, 16, 261–268. [Google Scholar] [CrossRef]
- Cecci, G.M.; Cardoso, M.L.; Bento, O.; Martins, L.M. Allergy approach to a dog population from a veterinary dermatology consultation at the tropical inland city of Londrina, Paraná, Brazil. In Proceedings of the European Academy of Allergy and Clinical Immunology (EAACI) Congress, Lisbon, Portugal, 1–5 June 2019. [Google Scholar]
- Esteves de Campos, I.; Ferreiro Pinto, C.; Munhoz Severino, A.C.; Bento, O.; Antunes, C.; Costa, A.R.; Martins, L.M. Dermatological and allergy approach to a dog population from a veterinary consultation at the tropical coastal city of São Paulo, Brazil. In Proceedings of the European Academy of Allergy and Clinical Immunology (EAACI) Congress, Lisbon, Portugal, 1–5 June 2019. [Google Scholar]
- Martins, L.; Bento, O.; Inacio, F. Veterinary allergy diagnosis: Past, present and future perspectives. Allergo J. Int. 2016, 25, 20–32. [Google Scholar] [CrossRef]
- Han, C.; Chan, W.Y.; Hill, P.B. Prevalence of positive reactions in intradermal and IgE serological allergy tests in dogs from south Australia, and the subsequent outcome of allergen-specific immunotherapy. Aust. Vet. J. 2020, 98, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Furiani, N.; Scarampella, F.; Noli, C.; Ordeix, L. A retrospective study of 486 intradermal tests performed on atopic dogs in Northern Italy. Veterinaria (Cremona) 2009, 23, 41–46. [Google Scholar]
- Balappanavar, B.R.; Vasanth, M.S. Clinico-diagnostic and therapeutic management of canine malasseziosis. Intas. Polivet. 2013, 14, 353–357. [Google Scholar]
- Guillot, J.; Bond, R. Malassezia yeasts in veterinary dermatology: An updated overview. Front. Cell Infect Microbiol. 2020, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Mazzilli, S.; Lanna, C.; Cosio, T.; Palumbo, V.; Cesaroni, G.; Lozzi, F.; Diluvio, L.; Bianchi, L. The effectiveness of a new topical formulation containing GSH-C4 and hyaluronic acid in seborrheic dermatitis: Preliminary results of an exploratory pilot study. Clin. Cosmet. Investig. Dermatol. 2019, 12, 881–885. [Google Scholar] [CrossRef]
- Pin, D.; Pendaries, V.; Alassane, K.S.; Froment, C.; Amalric, N.; Cardiergues, M.; Serre, G.; Haftek, M.; Vidémont, E.; Simon, M. Refined Immunochemical Characterization in healthy dog skin of the epidermal cornification proteins, filaggrin, and corneodesmosin. J. Histochem. Cytochem. 2019, 67, 85–97. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, Z.; Guo, Y.; Song, L.; Weatherhead, J.E.; Huang, X.; Zeng, Y.; Bimler, L.; Chang, C.-Y.; Knight, J.M.; et al. Candida albicans elicits protective allergic responses via platelet mediated T helper 2 and T helper 17 cell polarization. Immunity 2021, 54, 2595–2610.e7. [Google Scholar] [CrossRef]
- Montagnoli, C.; Bozza, S.; Gaziano, R.; Zelante, T.; Bonifazi, P.; Moretti, S.; Bellocchio, S.; Pitzurra, L.; Romani, L. Immunity and tolerance to Aspergillus fumigatus. Novartis Found Symp. 2006, 279, 66. [Google Scholar]
| Sensitizing Species | ||||
|---|---|---|---|---|
| Allergens/Molecular Weight (kDa) | Relevant for | Recommended Extract Concentration/mL | References | |
| Alternaria alternada | Alt a 1 (30) | Dog; horse | 1000–8000 PNU(#)/100 µg(##) | [59,63,72] |
| Aspergillus fumigatus | Asp f 7 (27.4); Asp f 8 (11); Asp f 9 (34) | Dog; horse | 1000–8000 PNU(#)/100 µg(##) | [59,60,61,62,63,64] |
| Malassezia sp. | Dog | 100 µg (##) | [72,73,74,75,76,77,78] | |
| Malassezia globosa | MGL_1304 (26) | Horse | [61] | |
| Aspergillus mix (*) | Dog; horse | 1000–8000 PNU(#)/100 µg(##) | [63,72] | |
| Fungi mix (**) | Dog; horse | 1000–8000 PNU(#)/100 µg(##) | [63,72] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, L.M.L. Allergy to Fungi in Veterinary Medicine: Alternaria, Dermatophytes and Malassezia Pay the Bill! J. Fungi 2022, 8, 235. https://doi.org/10.3390/jof8030235
Martins LML. Allergy to Fungi in Veterinary Medicine: Alternaria, Dermatophytes and Malassezia Pay the Bill! Journal of Fungi. 2022; 8(3):235. https://doi.org/10.3390/jof8030235
Chicago/Turabian StyleMartins, Luís Miguel Lourenço. 2022. "Allergy to Fungi in Veterinary Medicine: Alternaria, Dermatophytes and Malassezia Pay the Bill!" Journal of Fungi 8, no. 3: 235. https://doi.org/10.3390/jof8030235
APA StyleMartins, L. M. L. (2022). Allergy to Fungi in Veterinary Medicine: Alternaria, Dermatophytes and Malassezia Pay the Bill! Journal of Fungi, 8(3), 235. https://doi.org/10.3390/jof8030235






