1. Introduction
Microorganisms, such as bacteria and yeast, are considered as a vital source of biopigments like carotenoids [
1,
2]. Animals and higher plants are also able to synthesize carotenoids [
3]. Carotenoids are a group of natural pigments present in egg, fish, fruits, and vegetables and can be synthesized by different organisms such as
Rhodotorula [
4,
5]. The yellow, orange, pink, or red carotenoids are secondary metabolites produced by different organisms including yeast
Rhodotorula sp. [
6].
There are a variety of natural sources of carotenoids. Photosynthetic organisms such as cyanobacteria and algae and non-photosynthetic organisms such as yeast, bacteria, and fungi are good natural sources of carotenoids. Additionally, aquatic animals, microalgae, and all of the colorful vegetables and fruits are a major natural sources of carotenoids [
7]. On the other hand, carotenoids are synthesized chemically, through different chemical reactions [
8].
Carotenoids have a significant medical, industrial, food and pharmaceutical importance due to their antioxidative function, which increases their activity against chronic and cancer diseases and also enhance immunity and vision [
9].Carotenoids were reported to have antioxidant, anticancer, and antiobesity effects [
10,
11]. Synthetic carotenoids have a deleterious effect on the environment and public health compared to natural pigments. On the other hand, carotenoids from higher plants and animals suffer from uncontrolled issues related to the weather and geographical problems [
12], while the microbial carotenoids are considered the optimal eco-friendly and economic alternative.
Globally, the demand for using carotenoids as naturally derived pigments is increasing. To fulfill these demands, new biotechnological applications should be used. Scaling up the production of any microbial products can be performed precisely by detecting the optimum natural conditions. Recent studies aim to use high-carotenoid production yeasts such as
Rhodotorula, agro-industrial wastes, and bioreactor design to cover the worldwide demand of naturally synthesized carotenoids [
13].
Over the years, different methods of optimization were developed and each one pretended to be the perfect method. Those methods include Build-Test-Fix [
14], One Factor at a Time (OFAT) [
15], and Design of Experiments (DOE) [
16]. There are two types of DOE experiments. First, factorial designs that test the effects of all factors and the interaction between them by factorial experiment which includes a two-level full factorial design, two-level fractional factorial design [
17], and screening factorial design [
18]. Second, Response Surface Methodology (RSM) that determines the optimal settings of the experimental factors and includes central composites, Box–Behnken designs, 3-level factorials, and Draper–Lin design. RSM explains the interaction effect between the factors in a fermentation process [
19].
Response Surface Methodology (RSM), Plackett–Burman design (PBD), and the bioreactor optimized the production of carotenoids from
Rhodotorula ATL72 [
20,
21]. This study aimed to use the biotechnological process as optimization and a bioreactor to enhance the production of carotenoids by
Rhodotorula ATL72.
2. Materials and Methods
2.1. The Strain and the Produced Pigment
The used strain has been previously isolated and identified by the authors and it was found to be more closely related to
Rhodotorula bloemfonteinensis (EU075187) based on 18S rRNA gene analysis. This locally isolated strain has been found to be a potential high producer of the pink carotenoids pigment [
22].
2.2. Optimization of Various Physico-Chemical Parameters Using Central Composite Design
CCD design was utilized to optimize variable factors such as pH, temperature, and salinity to determine the optimum conditions for the cultivation at the level of the shake flask, through defining the main effects and the interaction between the previous factors on the yeast growth and the production of carotenoids [
23]. Each variable in the CCD matrix has five levels, which were chosen according to previous studies [
22,
23] that showed the effects of these variables on the production of carotenoids, as shown in
Table 1.
To test the effect of each variable, enriched broth media was used which consisted of yeast extract 5 g/L, glucose 10 g/L, and sea water. The experiments were performed in a 50 mL flask and each one was incubated at 180 rpm in a shaking incubator according to what is displayed in the matrix in
Table 1. The growth rate was detected by recording O.D
600, while the carotenoid production was detected by calculating the carotenoid concentration.
2.3. Screening the Medium Components by Placket–Burman Design
The main aim of using Placket–Burman design is to screen the important components of the media concerning their individual major effects not the effects that result from the interaction between different medium components [
24].
Thirteen different kinds of relatively cheap available components of the media were utilized, including glucose, peptone, sucrose, urea, glycerol, malt extract, fructose, molasses, ammonium chloride, whey, yeast extract, potato extract, NH4NO3, and one dummy factor to measure the standard error of the design. All chemicals were purchased from Sigma-Aldrich Egypt, each factor was utilized at a maximum concentration (+) and minimum concentration (−) where the maximum level of all the tested variables is 1 g/L and the minimum value to be tested is the complete absence of the variable.
The major effect of each factor was determined by the (Equation (1)).
where E(Xi) is the effect of the tested factor, Yi+ and Yi− are the calculated responses.
The significance level (
p-value) for each factor was detected using Student’s
t-test
where (SE) is the standard error for the factors. Any factor with
p-value < 0.1 is significant at the 90% confidence level.
The relationship between the significant factors and the response within 2-level factorial design depends on first order polynomial order (Equation (3)) as no interactions could be calculated by this screening design.
where Y is the calculated response, β0 is model intercept, βi is the regression coefficient for each corresponding variable, Xi is the corresponding variable, and K is the number of variables [
25].
The experiments were performed in Erlenmeyer flasks containing 50 mL of basal media (yeast extract 1 g/L and NaCl 35 g/L) besides the components of each trial and incubated in optimized conditions of 180 rpm and 27.5 °C for 96 h. Responses were estimated in terms of OD600 and carotenoid content (mg/L).
2.4. Optimization of the Production Medium by Central Composite Design
After utilizing PBD, to detect the most potential components of the production medium, the optimum concentration of each factor was defined using CCD. The matrix of the central composite design has 5 levels for each factor, 6-star points and center points to detect the curvature. The CCD studied the major effect of each factor and the interaction between them.
A second-order polynomial model was used for the prediction of the optimum components of the production medium for carotenoid production, as shown in (Equation (4)):
where βi represents the regression coefficient of each variable, βii represents the regression coefficient for square effects and βij represents the regression coefficient of the interactions. Using Design Expert 8.0 statistical package (StatEase, Inc., Minneapolis, MN, USA), an analysis of variance (ANOA) was completed.
CCD was used to optimize the potential components of the media through 25 trials with 3 variables and 5 levels as shown in
Table 2 [
26].
Factors with a major positive effect on both growth of yeast and carotenoid production were malt extract, urea, and fructose. Each factor was studied at a maximum concentration (+) and at a minimum concentration (−) where the maximum concentration is expressed as 9.3 g/L and the minimum concentration is expressed as 2.6 g/L.
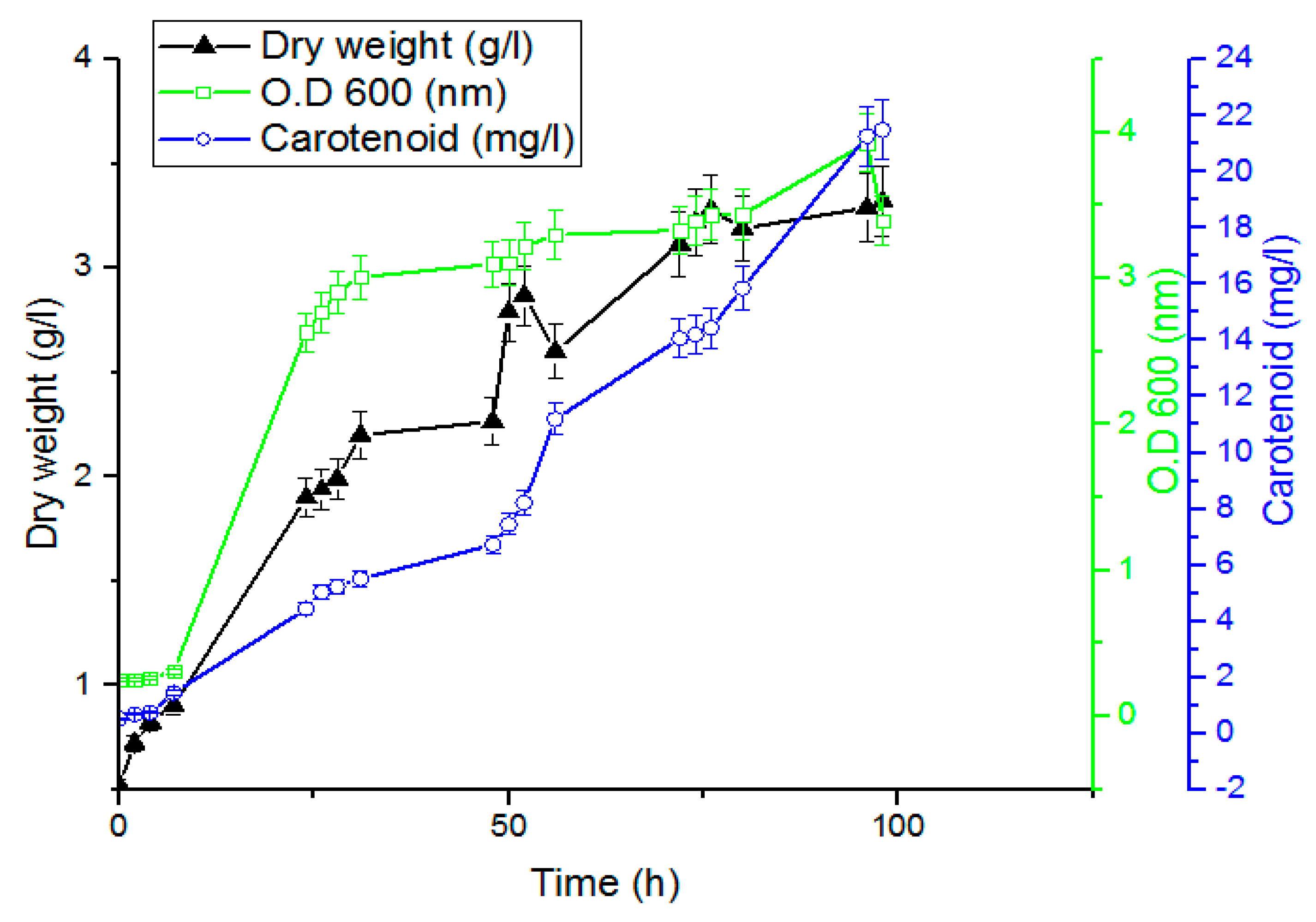
2.5. Time Course of Carotenoid Production by Rhodotorula ATL72
The time course for the growth and carotenoid production was studied by culturing the yeast on the optimized media. In total, 200 mL of overnight seed culture was inoculated with O.D
600 1 nm and incubated at 27.5 °C, 6.7 pH, and 180 rpm for 72 h. Twenty flasks with 100 mL working volume for each were inoculated with 10 mL of the seed culture and incubated at 27.5 °C and 180 rpm for 98 h. Samples were withdrawn at different time intervals for analysis [
27].
2.6. Batch Run in 1 L Bioreactor
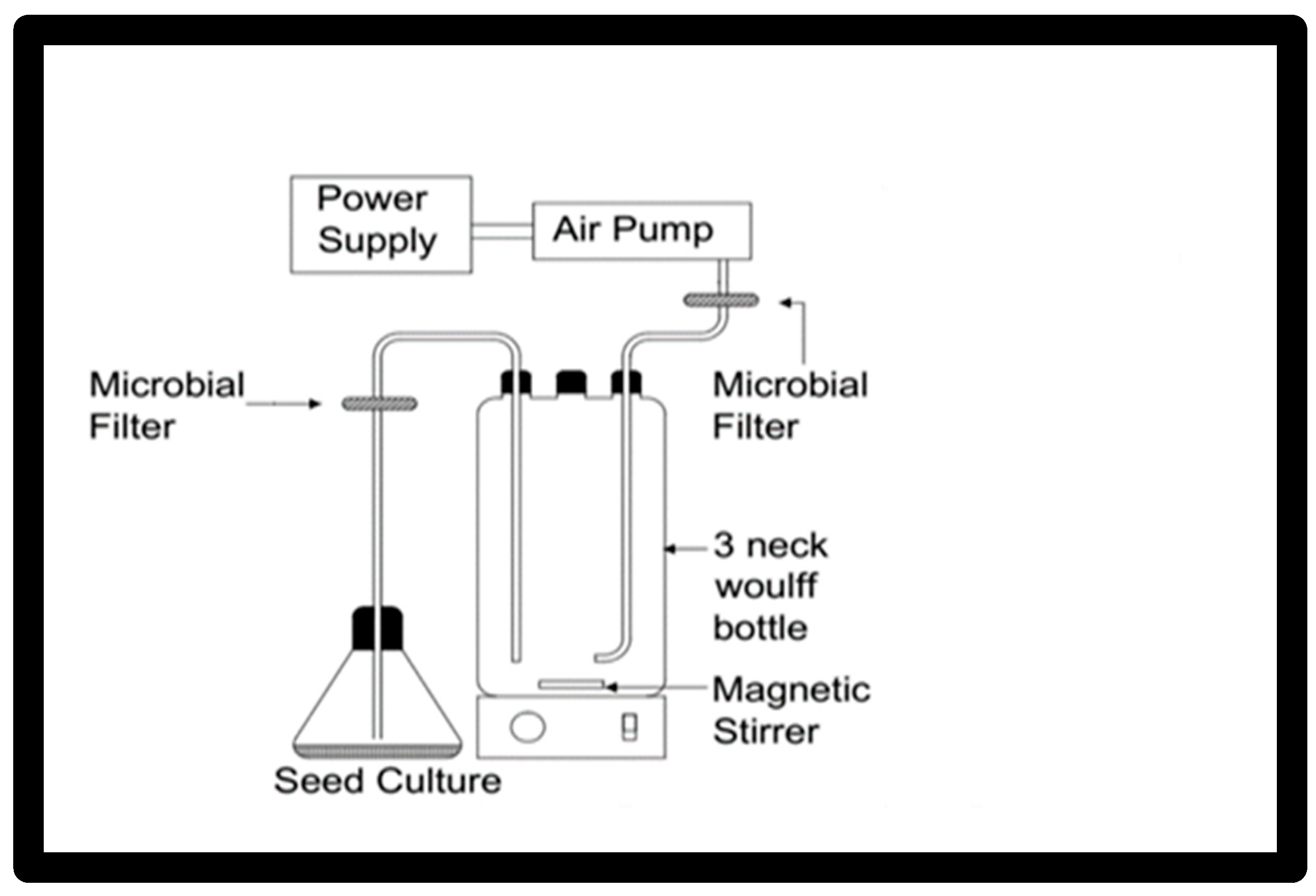
A culture system in
Figure 1 using a 1 L flask, 3-neck Woulff bottle was used for scaling up the growth and the carotenoid production for the yeast
Rhodotorula ATL72. Two lines were fitted at the shoulders of the flask, one for aeration and the other for inoculation and harvesting. Each line was supplied with a microbial filter to prevent contamination. Air flow was supplied using an air pump and sparged to the system by passing through a microbial filter prior to entering into the media. The temperature was adjusted by placing the flask in the incubator at 27.5 °C. The agitation speed was adjusted using a magnetic stirrer at a speed of 250 rpm. The seed culture of 100 mL was prepared from freshly cultured broth, which had the exact concentrations of the nutrients of the production medium. The concentrations of the nutrients in the production medium were the following (g/L): malt extract 3.7, fructose 7.7, urea 9, yeast extract 1, and NaCl 35 and inoculated in 1000 mL of the optimized media. Samples were withdrawn at different time intervals for calculation of the growth rate (optical density at 600 nm) and carotenoid content (mg/L).
Figure 1 displays the proposed bioreactor design.
2.7. Batch Run in the 7 L Bioreactor
A batch run was performed using an Eppendorf–New Brunswick 7 L Rushton turbine Stirred Tank Bioreactor with a working volume of 3 L. The bioreactor was set after sterilization. The seed culture was inoculated by a loopful of fresh plate of the yeast, of age 48 h, and maintained at 27.5 °C, pH 6.7, and 180 rpm. The batch run occurred at 27.5 °C, 180 rpm, uncontrolled pH, and controlled dissolved oxygen (D.O) at 10%. For calculations of the growth and carotenoid content, samples were withdrawn at constant time intervals.
A total of 700 mL of the seed culture was prepared, which had the exact concentrations of the nutrients of the production medium. The concentrations of the nutrients in the production medium were the following (g/L): malt extract 3.7, fructose 7.7, urea 9, yeast extract 1, and NaCl 35.
2.8. Extraction and Determination of Carotenoids
Extraction of carotenoids from yeast was performed according to Sedmak et al. [
28]. The extraction was completed as follows: the yeast biomass was harvested by centrifugation and washed with distilled water three times. Then, the extraction was performed as follows: 5 mL of dimethylsulfoxide (DMSO), acetone, petroleum ether, and 20% NaCl were added serially to the harvested pellet with vortexing [
29,
30,
31].
The total carotenoid was determined by measuring the optical density according to [
28,
30]. The total carotenoid content from the yeast pellet was expressed as volumetric carotenoids (mg/L). The extension coefficient (
) 2680 was used according to the following equation:
where:
A: absorbance at 490, V: total volume: extinction coefficient
All the above procedures were performed under low light to avoid pigment degradation [
26,
32,
33].
4. Discussion
In recent years, the interest in natural pigments has increased worldwide as a safe alternative to synthetic ones. Hence, seeking a natural, economical, and safe alternative for synthetic carotenoids is a must. The production of carotenoids varies between species in the genus
Rhodotorula and is affected by the environmental conditions and the medium components [
31]. This work highlights the factors affecting the production of carotenoids and scaling up of both carotenoid production and cellular growth.
Rhodotorula ATL72 produces a large amount of the carotenoid pigments in the growth medium. Carotenoids are lipid-soluble pigments and vital membrane components, and they are present within the membrane lipid core [
32].
The production of carotenoids in the natural environment is insufficient for commercialization. Thus, microbial production of carotenoids at industrial scale has some criteria for scaling up of the production process, such as consuming several inexpensive carbon and nitrogen sources, pH tolerance, requirement of temperature, developing the cultivation strategies, and utilizing inexpensive downstream process [
33].
Design of Experiments (DOE) is an alternate experiment which confirms testing of all variables and their interactions, and the analysis of DOE provides more reliable and complete information than that from One Factor at a Time (OFAT) experiments which causes a decline in the interactions and provides misleading results [
34].
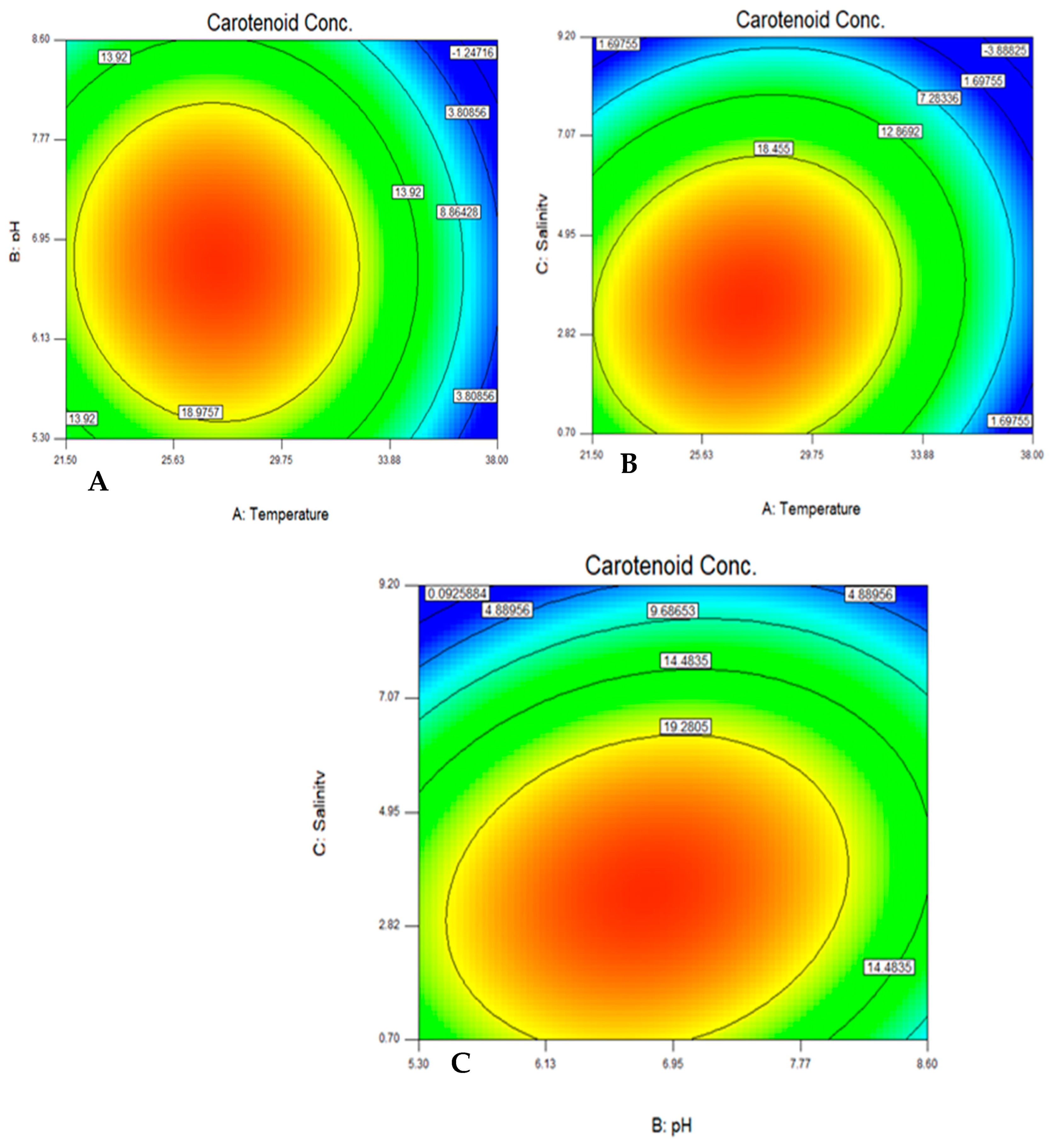
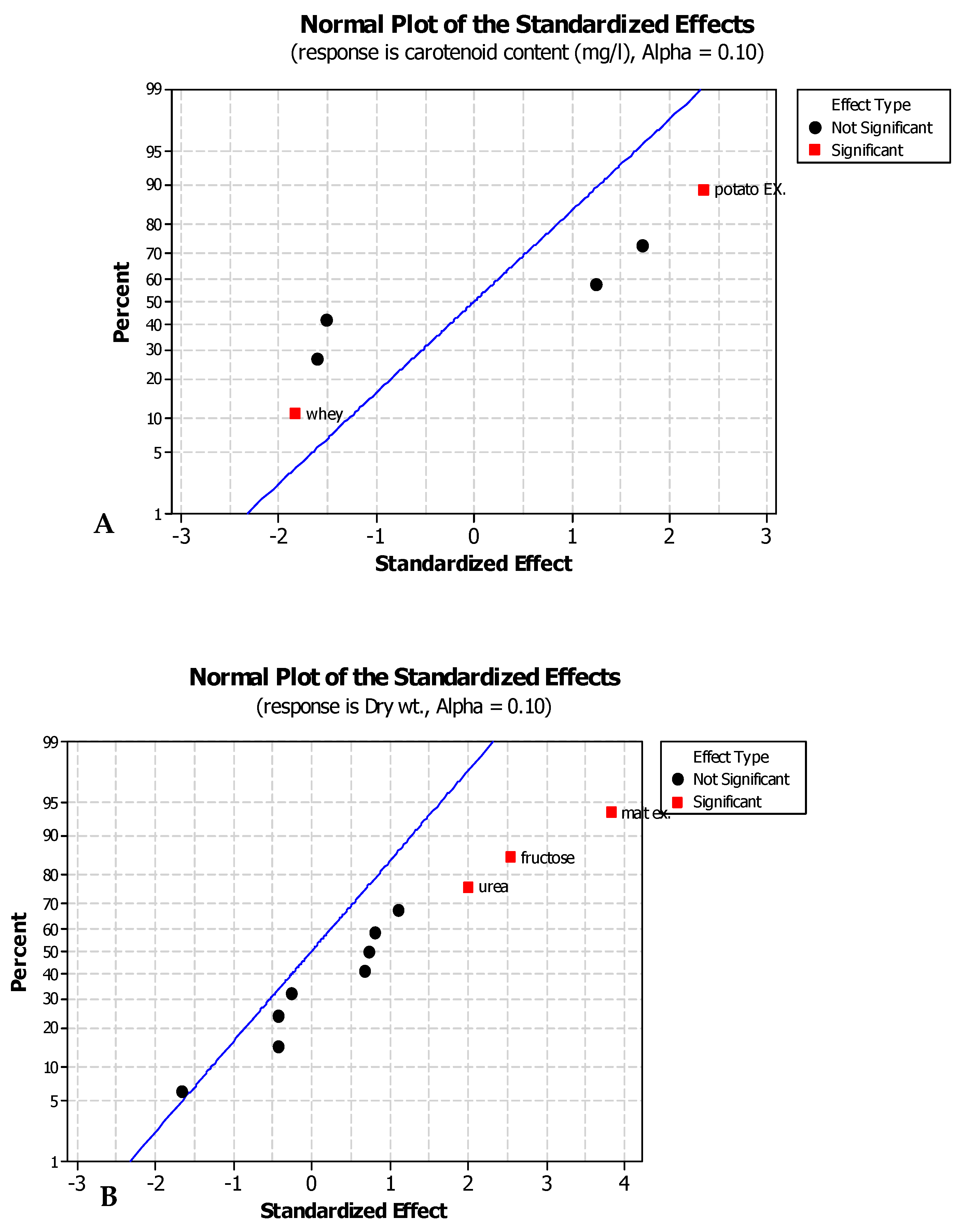
CCD was applied to study the effect of temperature, pH, and salinity on carotenoid production and yeast growth. Then, Plackett–Burman design (PBD) was used for screening the effective carbon and nitrogen sources for the production of carotenoids. Twenty different experiments, each with their characteristic combination, were applied and the responses to be optimized were evaluated at the end of each trial. The maximum amount of carotenoids was found to be 25.14 mg/L at trial numbers 16 and 18. The results were analyzed by a multi-way ANOVA analysis (
Table 4). For indicating the statistical significance of a factor, a
p-value of <0.1 was used as a cut-off point at 90% confidence level. A
p-value is significant when equal to or less than α [
23]. The main effect of temperature was found to be the most significant factor affecting the yeast growth, while the main effects of both pH and salinity were found to be insignificant factors.
The quadratic effects of both temperature and salinity were found to be significant factors, which are affecting the yeast growth. In the case of factors affecting carotenoid production, the main effects of temperature and salinity and the quadratic effects of temperature, salinity, and pH were found to be the most significant factors. On the other hand, the main effect of pH and the interactions between pH, salinity, and temperature were found to be insignificant factors, as shown in
Table 4.
The color contour plots were generated to show the interaction of the tested variables with the carotenoid production and the optimum combination. The optimum range was found to be 27.5 °C, pH 6.7, and salinity 3.5% NaCl, which agreed with what has been reported by [
35].
Rhodotorula spp. can grow in a wide range of temperatures (5–35 °C), but the optimum temperature and its activity depend on the species specificity of the yeast [
36].
Rhodotorula ATL72 produced the maximum amount of carotenoid pigments at 27.5 °C and the rate was reduced as the temperature increased, which is in agreement with the mainstream directions in the literature. Most researchers have reported the optimum range of temperature for carotenoid production to be around 20 °C and decreases as the temperature increases to 30 °C. Bhosale and Gadre [
37] have reported that β-carotene accumulation was found to be higher at 20 °C and decreases as the temperature increases. On the other hand, Muthezhilan et al. [
38] have reported that the optimum conditions of carotenoid production by
Rhodotorula sp. AMBY109 are temperature 34.29 °C, pH 8.54, and salinity 20.15 ppt.
The increase in the pigmentation of
Rhodotorula ATL72 can be attributed to the activity of β-carotene synthase which is more active at low temperature, compared to dehydrogenation and decarboxylation which are responsible for the torulene synthesis [
39].
The optimum pH for the carotenoid production was found to be 6.7, which is confirmed by Wang et al. [
40] who have reported that the optimal pH for the carotenoid production by
Rhodotorula glutinis (
R. glutinis) was 6.7 which indicates the preference of the yeast for a slightly acidic pH near 6 [
41].
There was a significant increase in both the growth and the carotenoid production by
Rhodotorula ATL72 at 3.5% NaCl which agreed with the findings of Kanzy, Nasr, El-Shazly and Barakat [
30] which can be explained by the carotenoids acting as protective agents protecting the cells from free radicals, hence, the biosynthesis of the carotenoids represents a stress response mechanism. Exposure of the cells to mild stress as salt stress leads to overproduction of different substrates and tolerance to higher doses of stress or tolerance to stress by other agents which is known as cross-protection [
42], Therefore, the addition of salts induces carotenogensis by the isolate ATL72.
Statistical experimental designs including Plackett–Burman design (PBD) and central composite design (CCD) were used for optimization and enhancement of the growth and carotenoid production. The media that resulted from using these strategies, supported good growth of the yeast and improved the carotenoid biosynthesis at the same time.
Plackett–Burman design (PBD) was utilized for screening 13 relatively cheap and available carbon and nitrogen sources for their effect on carotenogensis and the yeast growth. Three of them (malt extract, fructose, and urea) showed the most positively significant effects on both carotenoid production and yeast growth.
The most significant factor for carotenogenesis and yeast growth was malt extract as the carbon source having high concentrations of carbohydrates. There is a significant effect of malt extract as reported by [
43,
44]. The second significant factor for carotenoid production and yeast growth was fructose as an additional carbon source as reported by Latha et al. [
45,
46,
47]. The third significant factor was urea as a nitrogen source for carotenoid production and yeast growth as reported by Park et al. [
48].
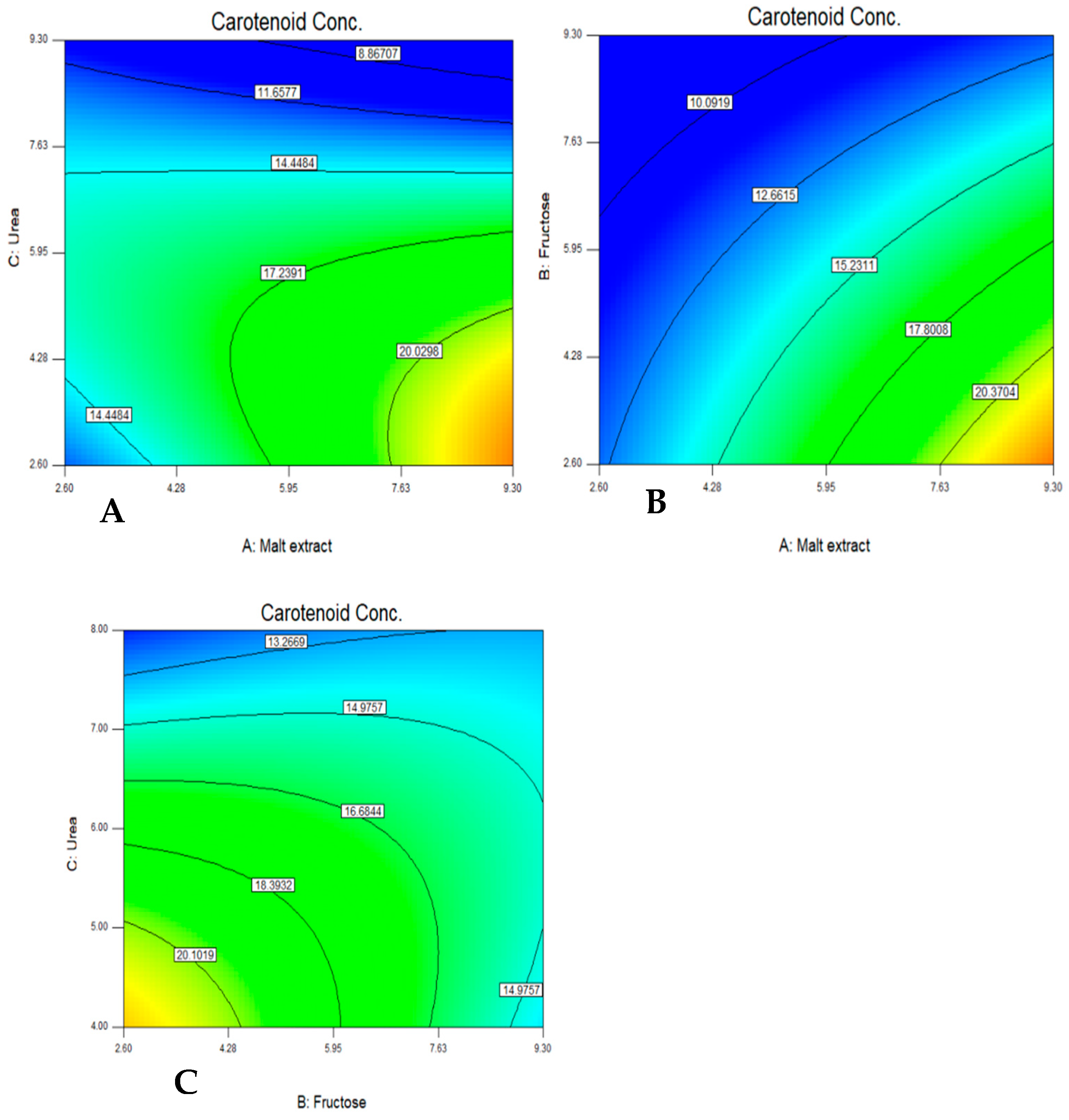
After defining these carbon and nitrogen sources as the most significant medium components for optimizing the carotenoid production, a central composite design matrix was applied to detect the optimum medium compositions and interactions between the variables for optimizing the carotenoid production. The analysis was completed using an ANOVA (
Table 6) and it was found that the optimum medium for carotenoid production was as follows (g/L); malt extract 3.7, fructose 7.7, and urea 9. The maximum experimental response for carotenoid production was 24.6 mg/L. The growth conditions and the medium components were found to be vital to carotenoid production by different strains of
Rhodotorula [
44,
49,
50,
51]. No definite medium was reported to be the optimized one, but it varies depending on the growth conditions and the strain.
The scaling up process from the shake flask to the bioreactor level was completed at two stages; the first stage using a 1 L bioreactor under the optimized medium which increased the productivity to 149.18 mg/L after 119 h. The second stage was completed using a 7 L bioreactor under the optimized medium, showing a remarkable increase in the carotenoid productivity reaching a value of 229.9 mg/L 47 h after inoculating the bioreactor, which represents the stationary phase of the growth. These results agreed with those reported by Malisorn and Suntornsuk [
52] for
Rhodotorula glutinis DM28 while the highest β-carotene production was 201 µg/L after 24 h under the optimized condition of temperature of 30 °C, pH of 6, and dissolved oxygen of 80% in a stirred tank reactor. Meanwhile, Saenge et al. [
53] achieved a maximum total carotenoid production of 125.75 mg/L using batch fermentation and 180.20 mg/L after 120 h using fed-batch fermentation in a 2 L stirred tank bioreactor. The increase in carotenoid production is due to the high aeration rate which agreed with the findings of Varmira et al. [
32,
54].