Aspergillus fumigatus—Host Interactions Mediating Airway Wall Remodelling in Asthma
Abstract
1. Introduction
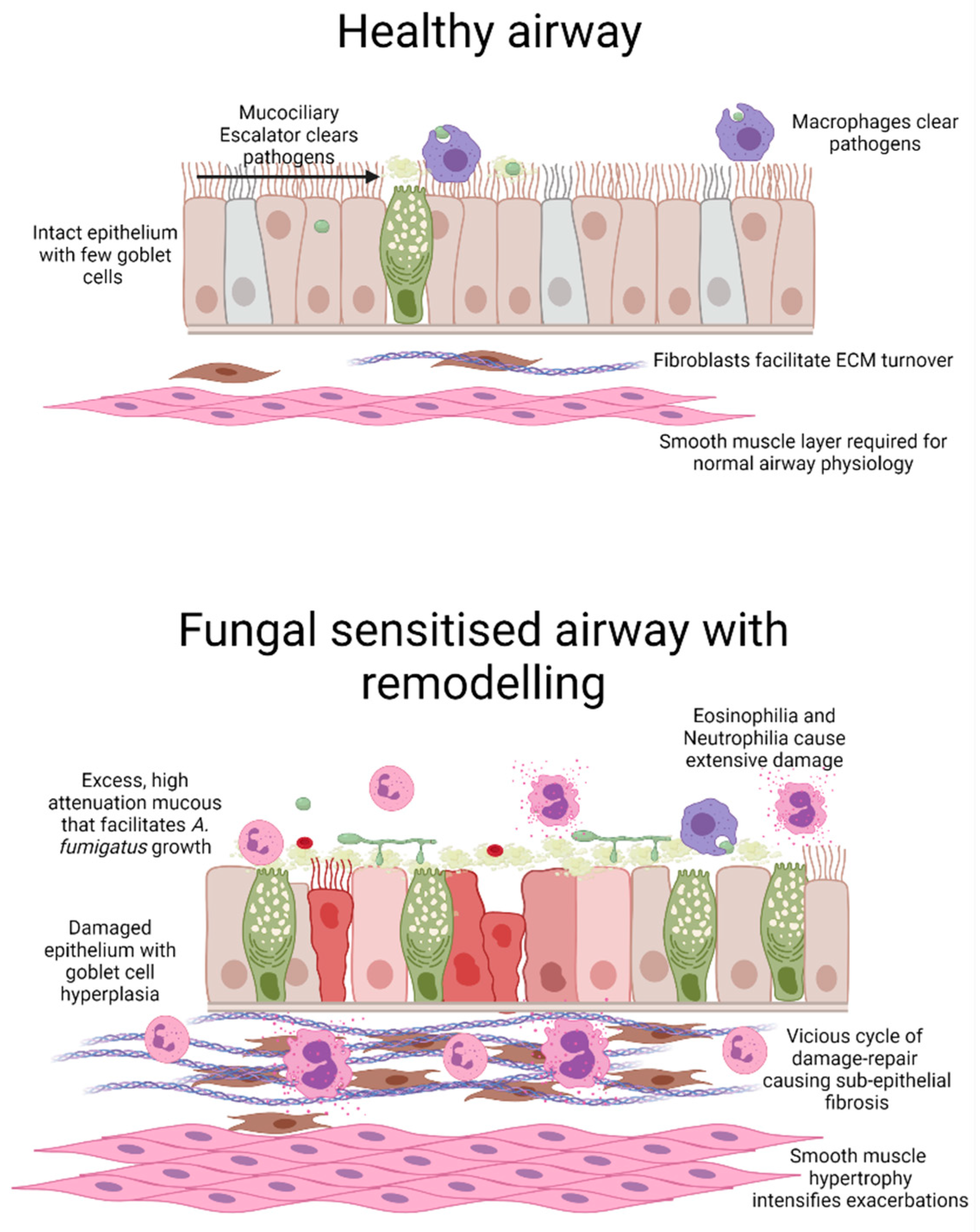
2. Aspergillus fumigatus Sensitised Asthma
3. Interaction of Airway Epithelium with A. fumigatus
4. A. fumigatus-Induced Epithelial Cell Damage
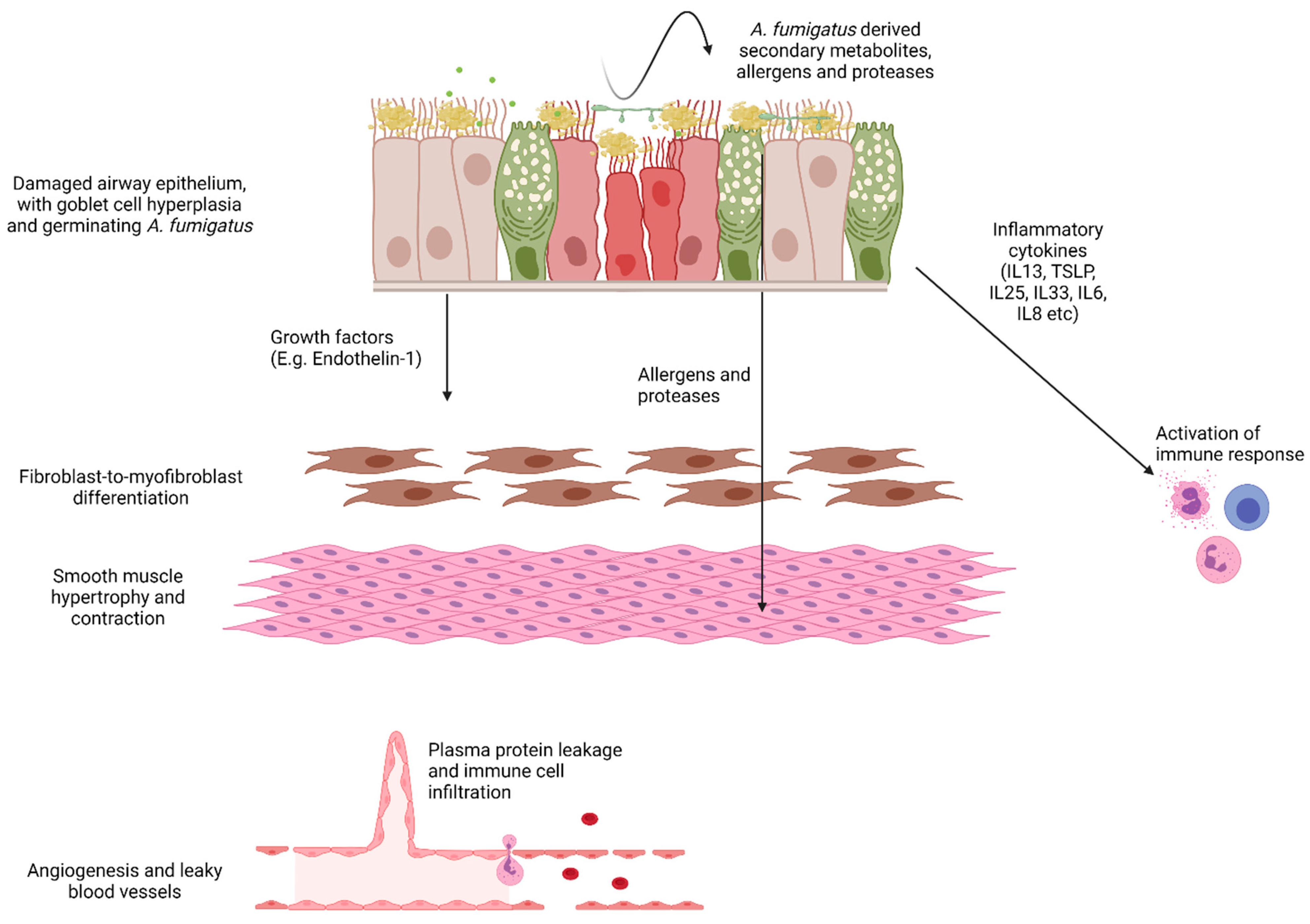
5. A. fumigatus Involvement in Driving Airway Structural Changes
| Mouse Strain | A. Fumigatus Delivery Method | Key Findings | Ref |
|---|---|---|---|
| A. fumigatus conidia-only models | |||
| C57BL6J | Intranasal delivery of 4 × 105 conidia (isolated from household dust samples), three times per week over 18 days. | Relative to fixed conidia, live conidia caused airway hyperreactivity, eosinophilia, elevated IL-4 and IL-17 levels. Both live and fixed conidia caused modest neutrophilia. | [90] |
| BALB/c | Nose-only aerosol challenge with approximately 1 × 105 conidia (strain B-5233/ATCC 13073) twice per week for 4 weeks. | Histology revealed persistence and germination of wild-type but not melanin-deficient conidia (∆alb1) 48 h post-final dose. Mice exposed to the wild-type but not ∆alb1 strain also displayed eosinophilia, neutrophilia Th2/Th17 sensitisation and evidence of subepithelial fibrosis and goblet cell hyperplasia by histology | [88] |
| C57BL6J | Intratracheal delivery of 2.5 × 106 conidia (strain Af293) embedded in agar beads once. | Non-invasive fungal growth in airway lumen coupled with galactomannan detection. Robust inflammation, including Th2, Th17 and neutrophilia. Severe airway remodelling by histology | [89] |
| C57BL6J | Intranasal delivery of 1 × 107 conidia (strain W72310 from ABPA patient or CEA10) seven times over 2 weeks. | W72310 but not CEA10 conidia persisted in the lung and could be detected as late as 28 days post-final exposure associated with eosinophilia, neutrophilia and Th2 sensitisation. Histology indicates subepithelial fibrosis and goblet cell hyperplasia in response to W72310. | [92] |
| C57BL6J | Intranasal delivery of 4 × 105 conidia (strain Af293), three times per week over 18 days | Evidence of Th2 sensitisation, increased subepithelial fibrosis and epithelial thickening coupled with increased Endothelin-1 levels. | [98] |
| Systemic sensitisation followed by A. fumigatus conidia and/or antigen | |||
| CBA/J | Systemic sensitisation by intraperitoneal and subcutaneous delivery of A. fumigatus antigen in Freund’s adjuvant, followed by a weekly challenge with A. fumigatus antigen for 3 weeks. In week 4, mice received an intratracheal dose of 5 × 106 conidia (strain 13073). | Relative to conidia alone, pre-sensitisation caused profound Th2 sensitisation, profound eosinophilia, neutrophilia and peribronchiolar inflammation. Analysis of histopathology showed that pre-sensitisation also caused goblet cell hyperplasia and subepithelial fibrosis. | [93] |
| BALB/c | Systemic sensitisation by intraperitoneal and subcutaneous delivery of A. fumigatus antigen in alum. After 2 weeks, mice received a weekly intranasal dose of A. fumigatus antigen for 3 weeks and 1 week later, an estimated 6.6 × 105 conidia (strain NIH 5233) were delivered by aerosol | Thickening of the epithelium, goblet cell hyperplasia and airway hyperreactivity persisted for at least 7 days post-final dose. Persistence of Th2 sensitisation and subepithelial fibrosis at the 35-day timepoint. | [94] |
| CBA/J | Systemic sensitisation by intraperitoneal and subcutaneous delivery of A. fumigatus antigen in Alum, followed by a weekly intranasal delivery of A. fumigatus antigen for 3 weeks. Finally, mice received a single intratracheal dose of 5 × 106 conidia (strain not specified). | Upregulation of IL-4 and IL-13. Neutralisation of IL-13, but not IL-4 significantly reduced airway hyperresponsiveness, collagen deposition and subepithelial fibrosis as shown by histology. | [97] |
| BALB/c | Systemic intraperitoneal sensitisation with alum and crude A. fumigatus extract (strain not specified) followed by intranasal delivery of crude extract on days 25–27. In a separate group, mice received crude extract eleven times over the course of 5 weeks. | Alp1/Asp f 13 immunoreactivity visible in the submucosa of A. fumigatus sensitised mice. | [99] |
| A. fumigatus extract or culture filtrate models | |||
| BALB/c and C57BL/6 derived genetically altered | Intranasal delivery of A. fumigatus extract (strain not specified), heat-inactivated extract or purified Alp1/Asp f 13, three times per week for 2 weeks. | Compared to mice receiving A. fumigatus extract or purified Alp1, those exposed to heat inactivated extract or Alp 1 showed diminished airway hyperreactivity, Th2 sensitisation, neutrophilia, peribronchiolar inflammation and goblet cell hyperplasia. Eosinophil-deficient and PAR2-deficient mice developed comparable inflammation, neutrophilia, Th2 sensitisation and goblet cell hyperplasia in response to Alp 1 to that found in wild-type mice. | [100] |
| BALB/c | Intranasal delivery of A. fumigatus sterilised and dialysed culture filtrate (CEA10 derived and protease allergen-deficient strains), twice per week for 4 weeks. | Neutrophilia, eosinophilia and Th2 sensitisation coupled with airway hyperreactivity and remodelling. Exposure to culture filtrates lacking protease allergens, Asp f 5 or Asp f 13, significantly reduced the extent of airway wall remodelling | [101] |
| C57BL6J | Intranasal delivery of A. fumigatus sterilised and dialysed culture filtrates (strain Af293), twice per week for 4 weeks. | Extensive inflammation and Th2 sensitisation in parallel with extensive subepithelial fibrosis. Endothelin-1 receptor antagonism prevented A. fumigatus-induced airway wall remodelling. | [98] |
6. Involvement of A. fumigatus-Derived Proteases in Airway Wall Remodelling
7. A. fumigatus-Induced Airway Epithelial-Derived Profibrogenic Factor Production
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Loftus, P.A.; Wise, S.K. Epidemiology and economic burden of asthma. Int. Forum Allergy Rhinol. 2015, 5 (Suppl. S1), S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Mims, J.W. Asthma: Definitions and pathophysiology. Int. Forum Allergy Rhinol. 2015, 5 (Suppl. S1), S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Schatz, M.; Rosenwasser, L. The allergic asthma phenotype. J. Allergy Clin. Immunol. Pract. 2014, 2, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Masaki, K.; Fukunaga, K.; Matsusaka, M.; Kabata, H.; Tanosaki, T.; Mochimaru, T.; Kamatani, T.; Ohtsuka, K.; Baba, R.; Ueda, S.; et al. Characteristics of severe asthma with fungal sensitization. Ann. Allergy Asthma Immunol. 2017, 119, 253–257. [Google Scholar] [CrossRef]
- Zureik, M.; Neukirch, C.; Leynaert, B.; Liard, R.; Bousquet, J.; Neukirch, F. Sensitisation to airborne moulds and severity of asthma: Cross sectional study from European Community respiratory health survey. BMJ 2002, 325, 411–414. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The airway epithelium in asthma. Nat. Med. 2012, 18, 684–692. [Google Scholar] [CrossRef]
- Manuyakorn, W.; Howarth, P.H.; Holgate, S.T. Airway remodelling in asthma and novel therapy. Asian Pac. J. Allergy Immunol. 2013, 31, 3–10. [Google Scholar]
- Fehrenbach, H.; Wagner, C.; Wegmann, M. Airway remodeling in asthma: What really matters. Cell Tissue Res. 2017, 367, 551–569. [Google Scholar] [CrossRef]
- Lezmi, G.; Deschildre, A.; Taam, R.A.; Fayon, M.; Blanchon, S.; Troussier, F.; Mallinger, P.; Mahut, B.; Gosset, P.; De Blic, J. Remodelling and inflammation in preschoolers with severe recurrent wheeze and asthma outcome at school age. Clin. Exp. Allergy 2018, 48, 806–813. [Google Scholar] [CrossRef]
- Kaminska, M.; Foley, S.; Maghni, K.; Storness-Bliss, C.; Coxson, H.; Ghezzo, H.; Lemière, C.; Olivenstein, R.; Ernst, P.; Hamid, Q.; et al. Airway remodeling in subjects with severe asthma with or without chronic persistent airflow obstruction. J. Allergy Clin. Immunol. 2009, 124, 45–51.e4. [Google Scholar] [CrossRef]
- Grenier, P.A.; Fetita, C.I.; Brillet, P.Y. Quantitative computed tomography imaging of airway remodeling in severe asthma. Quant. Imaging Med. Surg. 2016, 6, 76–83. [Google Scholar] [PubMed]
- Gupta, S.; Siddiqui, S.; Haldar, P.; Entwisle, J.J.; Mawby, D.; Wardlaw, A.; Bradding, P.; Pavord, I.; Green, R.H.; Brightling, C. Quantitative analysis of high-resolution computed tomography scans in severe asthma subphenotypes. Thorax 2010, 65, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Aysola, R.S.; Hoffman, E.A.; Gierada, D.; Wenzel, S.; Cook-Granroth, J.; Tarsi, J.; Zheng, J.; Schechtman, K.B.; Ramkumar, T.P.; Cochran, R.; et al. Airway remodeling measured by multidetector CT is increased in severe asthma and correlates with pathology. Chest 2008, 134, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.I.; Colby, T.V.; Muller, N.L. Asthma and associated conditions: High-resolution CT and pathologic findings. AJR Am. J. Roentgenol. 2004, 183, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Coman, I.; Pola-Bibián, B.; Barranco, P.; Vila-Nadal, G.; Dominguez-Ortega, J.; Romero, D.; Villasante, C.; Quirce, S. Bronchiectasis in severe asthma: Clinical features and outcomes. Ann. Allergy Asthma Immunol. 2018, 120, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodriguez, J.A.; Saglani, S.; Rodriguez-Martinez, C.E.; Oyarzun, M.A.; Fleming, L.; Bush, A. The relationship between inflammation and remodeling in childhood asthma: A systematic review. Pediatr. Pulmonol. 2018, 53, 824–835. [Google Scholar] [CrossRef]
- Saglani, S.; Lloyd, C.M. Novel concepts in airway inflammation and remodelling in asthma. Eur. Respir. J. 2015, 46, 1796–1804. [Google Scholar] [CrossRef]
- Chaudhuri, R.; Rubin, A.; Sumino, K.; e Silva, J.R.L.; Niven, R.; Siddiqui, S.; Klooster, K.; McEvoy, C.; Shah, P.L.; Simoff, M.; et al. Safety and effectiveness of bronchial thermoplasty after 10 years in patients with persistent asthma (BT10+): A follow-up of three randomised controlled trials. Lancet Respir Med 2021, 9, 457–466. [Google Scholar] [CrossRef]
- Kosmidis, C.; Denning, D.W. The clinical spectrum of pulmonary aspergillosis. Thorax 2015, 70, 270–277. [Google Scholar] [CrossRef]
- Anees-Hill, S.; Douglas, P.; Pashley, C.H.; Hansell, A.; Marczylo, E.L. A systematic review of outdoor airborne fungal spore seasonality across Europe and the implications for health. Sci. Total Environ. 2021, 151716. [Google Scholar] [CrossRef]
- Rick, E.; Woolnough, K.F.; Seear, P.J.; Fairs, A.; Satchwell, J.; Richardson, M.; Monteiro, W.R.; Craner, M.; Bourne, M.; Wardlaw, A.J.; et al. The airway fungal microbiome in asthma. Clin. Exp. Allergy 2020, 50, 1325–1341. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, M.G.; Chishimba, L.; Niven, R.M.; Bromley, M.; Simpson, A.; Smyth, L.; Denning, D.; Bowyer, P. Corticosteroid treatment is associated with increased filamentous fungal burden in allergic fungal disease. J. Allergy Clin. Immunol. 2018, 142, 407–414. [Google Scholar] [CrossRef]
- Wardlaw, A.J.; Rick, E.-M.; Ozyigit, L.P.; Scadding, A.; Gaillard, E.A.; Pashley, C.H. New Perspectives in the Diagnosis and Management of Allergic Fungal Airway Disease. J. Asthma Allergy 2021, 14, 557–573. [Google Scholar] [CrossRef]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef]
- Agarwal, R.; Aggarwal, A.N.; Gupta, D.; Jindal, S.K. Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with bronchial asthma: Systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 2009, 13, 936–944. [Google Scholar]
- O’Driscoll, B.R.; Hopkinson, L.C.; Denning, D.W. Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions. BMC Pulm. Med. 2005, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Medrek, S.K.; Kao, C.C.; Yang, D.H.; Hanania, N.A.; Parulekar, A.D. Fungal Sensitization Is Associated with Increased Risk of Life-Threatening Asthma. J. Allergy Clin. Immunol. Pract. 2017, 5, 1025–1031.e2. [Google Scholar] [CrossRef] [PubMed]
- Black, P.N.; Udy, A.A.; Brodie, S.M. Sensitivity to fungal allergens is a risk factor for life-threatening asthma. Allergy 2000, 55, 501–504. [Google Scholar] [CrossRef]
- Denning, D.W.; Pashley, C.; Hartl, D.; Wardlaw, A.; Godet, C.; Del Giacco, S.; Delhaes, L.; Sergejeva, S. Fungal allergy in asthma-state of the art and research needs. Clin. Transl. Allergy 2014, 4, 14. [Google Scholar] [CrossRef]
- Agarwal, R.; Gupta, D. Severe asthma and fungi: Current evidence. Med. Mycol. 2011, 49 (Suppl. S1), S150–S157. [Google Scholar] [CrossRef]
- Bendien, S.A.; van Loon-Kooij, S.; Kramer, G.; Huijgen, W.; Altenburg, J.; Ten Brinke, A.; Maitland-van der Zee, A.H. Bronchiectasis in Severe Asthma: Does It Make a Difference? Respiration 2020, 99, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Menzies, D.; Holmes, L.; McCumesky, G.; Prys-Picard, C.; Niven, R. Aspergillus sensitization is associated with airflow limitation and bronchiectasis in severe asthma. Allergy 2011, 66, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Fairs, A.; Agbetile, J.; Hargadon, B.; Bourne, M.; Monteiro, W.R.; Brightling, C.E.; Bradding, P.; Green, R.H.; Mutalithas, K.; Desai, D.; et al. IgE sensitization to Aspergillus fumigatus is associated with reduced lung function in asthma. Am. J. Respir. Crit. Care Med. 2010, 182, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Máiz, L.; Vendrell, M.; Olveira, C.; Girón, R.; Nieto, R.; Martínez-García, M.Á. Prevalence and factors associated with isolation of Aspergillus and Candida from sputum in patients with non-cystic fibrosis bronchiectasis. Respiration 2015, 89, 396–403. [Google Scholar] [CrossRef]
- Sisodia, J.; Bajaj, T. Allergic Bronchopulmonary Aspergillosis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Chetty, A. Pathology of allergic bronchopulmonary aspergillosis. Front. Biosci. 2003, 8, e110–e114. [Google Scholar] [CrossRef]
- Mitchell, T.A.M.; Hamilos, D.L.; Lynch, D.A.; Newell, J.D. Distribution and Severity of Bronchiectasis in Allergic Bronchopulmonary Aspergillosis (ABPA). J. Asthma 2000, 37, 65–72. [Google Scholar] [CrossRef]
- De Soyza, A.; Aliberti, S. Bronchiectasis and Aspergillus: How are they linked? Med. Mycol. 2017, 55, 69–81. [Google Scholar] [CrossRef]
- Angus, R.M.; Davies, M.L.; Cowan, M.D.; McSharry, C.; Thomson, N. Computed tomographic scanning of the lung in patients with allergic bronchopulmonary aspergillosis and in asthmatic patients with a positive skin test to Aspergillus fumigatus. Thorax 1994, 49, 586–589. [Google Scholar] [CrossRef][Green Version]
- Holgate, S.T.; Roberts, G.; Arshad, H.S.; Howarth, P.H.; Davies, D.E. The role of the airway epithelium and its interaction with environmental factors in asthma pathogenesis. Proc. Am. Thorac. Soc. 2009, 6, 655–659. [Google Scholar] [CrossRef]
- Sheppard, D.C. Molecular mechanism of Aspergillus fumigatus adherence to host constituents. Curr. Opin. Microbiol. 2011, 14, 375–379. [Google Scholar] [CrossRef]
- Takahashi-Nakaguchi, A.; Sakai, K.; Takahashi, H.; Hagiwara, D.; Toyotome, T.; Chibana, H.; Watanabe, A.; Yaguchi, T.; Yamaguchi, M.; Kamei, K.; et al. Aspergillus fumigatus adhesion factors in dormant conidia revealed through comparative phenotypic and transcriptomic analyses. Cell Microbiol. 2018, 20, e12802. [Google Scholar] [CrossRef]
- Liu, H.; Lee, M.J.; Solis, N.V.; Phan, Q.T.; Swidergall, M.; Ralph, B.; Ibrahim, A.S.; Sheppard, D.C.; Filler, S.G. Aspergillus fumigatus CalA binds to integrin α(5)β(1) and mediates host cell invasion. Nat. Microbiol. 2016, 2, 16211. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Y.; Chen, F.; Sun, H.; Chen, C.; Zhao, B.-L. Important factors mediates the adhesion of Aspergillus fumigatus to alveolar epithelial cells with E-cadherin. Am. J. Transl. Res. 2016, 8, 2419–2425. [Google Scholar] [PubMed]
- Bertuzzi, M.; Hayes, G.E.; Icheoku, U.J.; Van Rhijn, N.; Denning, D.W.; Osherov, N.; Bignell, E.M. Anti-Aspergillus Activities of the Respiratory Epithelium in Health and Disease. J. Fungi 2018, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Paris, S.; Boisvieux-Ulrich, E.; Crestani, B.; Houcine, O.; Taramelli, D.; Lombardi, L.; Latgé, J.P. Internalization of Aspergillus fumigatus conidia by epithelial and endothelial cells. Infect. Immun. 1997, 65, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Amitani, R.; Kawanami, R. Interaction of Aspergillus with human respiratory mucosa: A study with organ culture model. Med. Mycol. 2009, 47 (Suppl. S1), S127–S131. [Google Scholar] [CrossRef] [PubMed]
- Kerr, S.C.; Fischer, G.J.; Sinha, M.; McCabe, O.; Palmer, J.M.; Choera, T.; Lim, F.Y.; Wimmerova, M.; Carrington, S.D.; Yuan, S.; et al. FleA Expression in Aspergillus fumigatus Is Recognized by Fucosylated Structures on Mucins and Macrophages to Prevent Lung Infection. PLoS Pathog. 2016, 12, e1005555. [Google Scholar] [CrossRef]
- Yang, Z.; Jaeckisch, S.M.; Mitchell, C.G. Enhanced binding of Aspergillus fumigatus spores to A549 epithelial cells and extracellular matrix proteins by a component from the spore surface and inhibition by rat lung lavage fluid. Thorax 2000, 55, 579–584. [Google Scholar] [CrossRef]
- Bromley, I.M.; Donaldson, K. Binding of Aspergillus fumigatus spores to lung epithelial cells and basement membrane proteins: Relevance to the asthmatic lung. Thorax 1996, 51, 1203–1209. [Google Scholar] [CrossRef]
- Bouchara, J.P.; Sanchez, M.; Chevailler, A.; Marot-Leblond, A.; Lissitzky, J.C.; Tronchin, G.; Chabasse, D. Sialic acid-dependent recognition of laminin and fibrinogen by Aspergillus fumigatus conidia. Infect. Immun. 1997, 65, 2717–2724. [Google Scholar] [CrossRef]
- Peñalver, M.C.; O’Connor, J.E.; Martinez, J.P.; Gil, M.L. Binding of human fibronectin to Aspergillus fumigatus conidia. Infect. Immun. 1996, 64, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Coulot, P.; Bouchara, J.P.; Renier, G.; Annaix, V.; Planchenault, C.; Tronchin, G.; Chabasse, D. Specific interaction of Aspergillus fumigatus with fibrinogen and its role in cell adhesion. Infect. Immun. 1994, 62, 2169–2177. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Gautam, P.; Pandit, H.; Singh, Y.; Basir, S.F.; Madan, T. Identification of fibrinogen-binding proteins of Aspergillus fumigatus using proteomic approach. Mycopathologia 2012, 173, 73–82. [Google Scholar] [CrossRef]
- Wasylnka, J.A.; Moore, M.M. Adhesion of Aspergillus species to extracellular matrix proteins: Evidence for involvement of negatively charged carbohydrates on the conidial surface. Infect. Immun. 2000, 68, 3377–3384. [Google Scholar] [CrossRef] [PubMed]
- Aimanianda, V.; Bayry, J.; Bozza, S.; Kniemeyer, O.; Perruccio, K.; Elluru, S.R.; Clavaud, C.; Paris, S.; Brakhage, A.A.; Kaveri, S.V.; et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 2009, 460, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.-K.; Lu, X.; Li, X.; Sun, Q.-Y.; Su, X.; Song, Y.; Sun, H.-M.; Shi, Y. Dectin-1 is inducible and plays a crucial role in Aspergillus-induced innate immune responses in human bronchial epithelial cells. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2755–2764. [Google Scholar] [CrossRef]
- Werner, J.L.; Metz, A.E.; Horn, D.; Schoeb, T.R.; Hewitt, M.M.; Schwiebert, L.M. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J. Immunol. 2009, 182, 4938–4946. [Google Scholar] [CrossRef]
- Carrion Sde, J.; Leal, S.M., Jr.; Ghannoum, M.A.; Aimanianda, V.; Latgé, J.P.; Pearlman, E. The RodA hydrophobin on Aspergillus fumigatus spores masks dectin-1- and dectin-2-dependent responses and enhances fungal survival in vivo. J. Immunol. 2013, 191, 2581–2588. [Google Scholar] [CrossRef]
- Bigot, J.; Guillot, L.; Guitard, J.; Ruffin, M.; Corvol, H.; Balloy, V.; Hennequin, C. Bronchial Epithelial Cells on the Front Line to Fight Lung Infection-Causing Aspergillus fumigatus. Fron. Immunol. 2020, 11, 1041. [Google Scholar] [CrossRef]
- Heijink, I.H.; Kuchibhotla, V.N.S.; Roffel, M.P.; Maes, T.; Knight, D.A.; Sayers, I.; Nawijn, M.C. Epithelial cell dysfunction, a major driver of asthma development. Allergy 2020, 75, 1902–1917. [Google Scholar] [CrossRef]
- Fedorov, I.A.; Wilson, S.J.; Davies, D.; Holgate, S.T. Epithelial stress and structural remodelling in childhood asthma. Thorax 2005, 60, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Xu, A.R.; Chen, S.Y.; Huang, Y.; Han, X.R.; Guan, W.J.; Wang, D.Y.; Zhong, N.S. Aberrant Epithelial Cell Proliferation in Peripheral Airways in Bronchiectasis. Front. Cell Dev. Biol. 2020, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Bowyer, P.; Denning, D.W. Genomic analysis of allergen genes in Aspergillus spp.: The relevance of genomics to everyday research. Med. Mycol. 2007, 45, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Amitani, R.; Taylor, G.; Elezis, E.N.; Llewellyn-Jones, C.; Mitchell, J.; Kuze, F.; Cole, P.J.; Wilson, R. Purification and characterization of factors produced by Aspergillus fumigatus which affect human ciliated respiratory epithelium. Infect. Immun. 1995, 63, 3266–3271. [Google Scholar] [CrossRef] [PubMed]
- Amitani, R.; Murayama, T.; Nawada, R.; Lee, W.; Niimi, A.; Suzuki, K.; Tanaka, E.; Kuze, F. Aspergillus culture filtrates and sputum sols from patients with pulmonary aspergillosis cause damage to human respiratory ciliated epithelium in vitro. Eur. Respir. J. 1995, 8, 1681–1687. [Google Scholar] [CrossRef]
- Cody, D.T., II.; McCaffrey, T.V.; Roberts, G.; Kern, E.B. Effects of Aspergillus fumigatus and Alternaria alternata on human ciliated epithelium in vitro. Laryngoscope 1997, 107 Pt 1, 1511–1514. [Google Scholar] [CrossRef]
- Botterel, F.; Cordonnier, C.; Barbier, V.; Wingerstmann, L.; Liance, M.; Coste, A.; Escudier, E.; Bretagne, S. Aspergillus fumigatus causes in vitro electrophysiological and morphological modifications in human nasal epithelial cells. Histol. Histopathol. 2002, 17, 1095–1101. [Google Scholar]
- Khoufache, K.; Puel, O.; Loiseau, N.; Delaforge, M.; Rivollet, D.; Coste, A.; Cordonnier, C.; Escudier, E.; Botterel, F.; Bretagne, S. Verruculogen associated with Aspergillus fumigatus hyphae and conidia modifies the electrophysiological properties of human nasal epithelial cells. BMC Microbiol. 2007, 7, 5. [Google Scholar] [CrossRef]
- Crameri, R.; Limacher, A.; Weichel, M.; Glaser, A.G.; Zeller, S.; Rhyner, C. Structural aspects and clinical relevance of Aspergillus fumigatus antigens/allergens. Med. Mycol. 2006, 44 Suppl. S1, S261–S267. [Google Scholar] [CrossRef][Green Version]
- Muthu, V.; Singh, P.; Choudhary, H.; Dhooria, S.; Sehgal, I.S.; Prasad, K.T.; Aggarwal, A.N.; Garg, M.; Chakrabarti, A.; Agarwal, R. Role of recombinant Aspergillus fumigatus antigens in diagnosing Aspergillus sensitisation among asthmatics. Mycoses 2020, 63, 928–936. [Google Scholar] [CrossRef]
- Muthu, V.; Singh, P.; Choudhary, H.; Sehgal, I.S.; Dhooria, S.; Prasad, K.T.; Aggarwal, A.N.; Garg, M.; Chakrabarti, A.; Agarwal, R. Diagnostic Cutoffs and Clinical Utility of Recombinant Aspergillus fumigatus Antigens in the Diagnosis of Allergic Bronchopulmonary Aspergillosis. J. Allergy Clin. Immunol. Pract. 2020, 8, 579–587. [Google Scholar] [CrossRef]
- Kespohl, S.; Raulf, M. Mould allergens: Where do we stand with molecular allergy diagnostics?: Part 13 of the series Molecular Allergology. Allergo J. Int. 2014, 23, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.W.; Venaille, T.J.; Mendis, A.H.; McAleer, R. Allergens as proteases: An Aspergillus fumigatus proteinase directly induces human epithelial cell detachment. J. Allergy Clin. Immunol. 1990, 86, 726–731. [Google Scholar] [CrossRef]
- Sharon, H.; Amar, D.; Levdansky, E.; Mircus, G.; Shadkchan, Y.; Shamir, R.; Osherov, N. PrtT-regulated proteins secreted by Aspergillus fumigatus activate MAPK signaling in exposed A549 lung cells leading to necrotic cell death. PLoS ONE 2011, 6, e17509. [Google Scholar] [CrossRef]
- Kogan, T.V.; Jadoun, J.; Mittelman, L.; Hirschberg, K.; Osherov, N. Involvement of secreted Aspergillus fumigatus proteases in disruption of the actin fiber cytoskeleton and loss of focal adhesion sites in infected A549 lung pneumocytes. J. Infect. Dis. 2004, 189, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Daly, P.; Verhaegen, S.; Clynes, M.; Kavanagh, K. Culture filtrates of Aspergillus fumigatus induce different modes of cell death in human cancer cell lines. Mycopathologia 1999, 146, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, D.L.; Merkhofer, R.; Ober, C.; Kujoth, G.C.; Niu, M.; Keller, N.P.; Gern, J.E.; Brockman-Schneider, R.A.; Evans, M.; Jackson, D.J.; et al. Cell TRPV4 Serves as a Damage Sensor Driving Lung Allergic Inflammation. Cell Host Microbe 2020, 27, 614–628.e6. [Google Scholar] [CrossRef]
- Farnell, E.; Rousseau, K.; Thornton, D.J.; Bowyer, P.; Herrick, S.E. Expression and secretion of Aspergillus fumigatus proteases are regulated in response to different protein substrates. Fungal Biol. 2012, 116, 1003–1012. [Google Scholar] [CrossRef]
- Iadarola, P.; Lungarella, G.; Martorana, P.A.; Viglio, S.; Guglielminetti, M.; Korzus, E.; Gorrini, M.; Cavarra, E.; Rossi, A.; Travis, J.; et al. Lung injury and degradation of extracellular matrix components by Aspergillus fumigatus serine proteinase. Exp. Lung Res. 1998, 24, 233–251. [Google Scholar] [CrossRef]
- Larcher, G.; Bouchara, J.P.; Annaix, V.; Symoens, F.; Chabasse, D.; Tronchin, G. Purification and characterization of a fibrinogenolytic serine proteinase from Aspergillus fumigatus culture filtrate. FEBS Lett. 1992, 308, 65–69. [Google Scholar] [CrossRef]
- Gifford, A.H.; Klippenstein, J.R.; Moore, M.M. Serum stimulates growth of and proteinase secretion by Aspergillus fumigatus. Infect. Immun. 2002, 70, 19–26. [Google Scholar] [CrossRef]
- Kauffman, H.F.; Tomee, J.; van de Riet, M.A.; Timmerman, A.J.; Borger, P. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J. Allergy Clin. Immunol. 2000, 105 Pt 1, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Tomee, J.F.C.; Wierenga, A.T.J.; Hiemstra, P.; Kauffman, H.F. Proteases from Aspergillus fumigatus induce release of proinflammatory cytokines and cell detachment in airway epithelial cell lines. J. Infect. Dis. 1997, 176, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.; Namvar, S.; Gago, S.; Labram, B.; Bowyer, P.; Richardson, M.; Herrick, S. Differential Proinflammatory Responses to Aspergillus fumigatus by Airway Epithelial Cells In Vitro Are Protease Dependent. J. Fungi 2021, 7, 468. [Google Scholar] [CrossRef] [PubMed]
- Kurup, V.P.; Grunig, G. Animal models of allergic bronchopulmonary aspergillosis. Mycopathologia 2002, 153, 165–177. [Google Scholar] [CrossRef]
- Desoubeaux, G.; Cray, C. Animal Models of Aspergillosis. Comp. Med. 2018, 68, 109–123. [Google Scholar]
- Buskirk, A.D.; Green, B.J.; Lemons, A.; Nayak, A.P.; Goldsmith, W.T.; Kashon, M.L.; Anderson, S.E.; Hettick, J.M.; Templeton, S.P.; Germolec, D.R.; et al. A murine inhalation model to characterize pulmonary exposure to dry Aspergillus fumigatus conidia. PLoS ONE 2014, 9, e109855. [Google Scholar] [CrossRef]
- Urb, M.; Snarr, B.D.; Wojewodka, G.; Lehoux, M.; Lee, M.J.; Ralph, B.; Divangahi, M.; King, I.L.; McGovern, T.K.; Martin, J.G.; et al. Evolution of the Immune Response to Chronic Airway Colonization with Aspergillus fumigatus Hyphae. Infect. Immun. 2015, 83, 3590–3600. [Google Scholar] [CrossRef]
- Porter, P.C.; Roberts, L.; Fields, A.; Knight, M.; Qian, Y.; Delclos, G.L.; Han, S.; Kheradmand, F.; Corry, D.B. Necessary and sufficient role for T helper cells to prevent fungal dissemination in allergic lung disease. Infect. Immun. 2011, 79, 4459–4471. [Google Scholar] [CrossRef]
- Bertuzzi, M.; Van Rhijn, N.; Krappmann, S.; Bowyer, P.; Bromley, M.; Bignell, E. On the lineage of Aspergillus fumigatus isolates in common laboratory use. Med. Mycol. 2020, 59, 7–13. [Google Scholar] [CrossRef]
- Jones, J.T.; Liu, K.W.; Wang, X.; Kowalski, C.H.; Ross, B.S.; Mills, K.A.M.; Kerkaert, J.D.; Hohl, T.M.; Lofgren, L.A.; Stajich, J.E. Aspergillus fumigatus Strain-Specific Conidia Lung Persistence Causes an Allergic Broncho-Pulmonary Aspergillosis-Like Disease Phenotype. mSphere 2021, 6, e01250-20. [Google Scholar] [CrossRef] [PubMed]
- Hogaboam, C.M.; Blease, K.; Mehrad, B.; Steinhauser, M.L.; Standiford, T.J.; Kunkel, S.L.; Lukacs, N.W. Chronic airway hyperreactivity, goblet cell hyperplasia, and peribronchial fibrosis during allergic airway disease induced by Aspergillus fumigatus. Am. J. Pathol. 2000, 156, 723–732. [Google Scholar] [CrossRef]
- Hoselton, S.A.; Samarasinghe, A.E.; Seydel, J.M.; Schuh, J.M. An inhalation model of airway allergic response to inhalation of environmental Aspergillus fumigatus conidia in sensitized BALB/c mice. Med. Mycol. 2010, 48, 1056–1065. [Google Scholar] [CrossRef]
- Ghosh, S.; Hoselton, S.A.; Asbach, S.V.; Steffan, B.N.; Wanjara, S.B.; Dorsam, G.P.; Schuh, J.M. B lymphocytes regulate airway granulocytic inflammation and cytokine production in a murine model of fungal allergic asthma. Cell Mol. Immunol. 2015, 12, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Kurup, V.P.; Guo, J.; Murali, P.S.; Choi, H.; Fink, J.N. Immunopathologic responses to Aspergillus antigen in interleukin-4 knockout mice. J. Lab Clin. Med. 1997, 130, 567–575. [Google Scholar] [CrossRef]
- Blease, K.; Jakubzick, C.; Westwick, J.; Lukacs, N.; Kunkel, S.L.; Hogaboam, C.M. Therapeutic effect of IL-13 immunoneutralization during chronic experimental fungal asthma. J. Immunol. 2001, 166, 5219–5224. [Google Scholar] [CrossRef]
- Labram, B.; Namvar, S.; Hussell, T.; Herrick, S.E. Endothelin-1 mediates Aspergillus fumigatus-induced airway inflammation and remodelling. Clin. Exp. Allergy 2019, 49, 861–873. [Google Scholar] [CrossRef]
- Balenga, N.A.; Klichinsky, M.; Xie, Z.; Chan, E.C.; Zhao, M.; Jude, J.; Laviolette, M.; Panettieri, R.A., Jr.; Druey, K.M. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat. Commun. 2015, 6, 6763. [Google Scholar] [CrossRef]
- Redes, J.L.; Basu, T.; Ram-Mohan, S.; Ghosh, C.C.; Chan, E.C.; Sek, A.; Zhao, M.; Krishnan, R.; Rosenberg, H.F.; Druey, K.M. Aspergillus fumigatus-Secreted Alkaline Protease 1 Mediates Airways Hyperresponsiveness in Severe Asthma. Immunohorizons 2019, 3, 368–377. [Google Scholar]
- Namvar, S.; Warn, P.; Farnell, E.; Bromley, M.; Fraczek, M.; Bowyer, P.; Herrick, S. Aspergillus fumigatus proteases, Asp f 5 and Asp f 13, are essential for airway inflammation and remodelling in a murine inhalation model. Clin. Exp. Allergy 2015, 45, 982–993. [Google Scholar] [CrossRef]
- Oguma, T.; Asano, K.; Tomomatsu, K.; Kodama, M.; Fukunaga, K.; Shiomi, T.; Ohmori, N.; Ueda, S.; Takihara, T.; Shiraishi, Y. Induction of mucin and MUC5AC expression by the protease activity of Aspergillus fumigatus in airway epithelial cells. J. Immunol. 2011, 187, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Basu, T.; Seyedmousavi, S.; Sugui, J.A.; Balenga, N.; Zhao, M.; Kwon Chung, K.J.; Biardel, S.; Laviolette, M.; Druey, K.M. Aspergillus fumigatus alkaline protease 1 (Alp1/Asp f13) in the airways correlates with asthma severity. J. Allergy Clin. Immunol. 2018, 141, 423–425.e7. [Google Scholar] [CrossRef] [PubMed]
- Ossovskaya, V.S.; Bunnett, N.W. Protease-activated receptors: Contribution to physiology and disease. Physiol. Rev. 2004, 84, 579–621. [Google Scholar] [CrossRef] [PubMed]
- Yike, I. Fungal proteases and their pathophysiological effects. Mycopathologia 2011, 171, 299–323. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.E.; Kita, H. The role of protease activation of inflammation in allergic respiratory diseases. J. Allergy Clin. Immunol. 2004, 114, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Skorkowska, K.; Adamiec, R. The biological role of protease-activated receptors in angiology. The present view. Int. Angiol. 2005, 24, 215–220. [Google Scholar]
- Ebeling, C.; Forsythe, P.; Ng, J.; Gordon, J.R.; Hollenberg, M.; Vliagoftis, H. Proteinase-activated receptor 2 activation in the airways enhances antigen-mediated airway inflammation and airway hyperresponsiveness through different pathways. J. Allergy Clin. Immunol. 2005, 115, 623–630. [Google Scholar] [CrossRef]
- Knight, D.A.; Lim, S.; Scaffidi, A.K.; Roche, N.; Chung, K.F.; Stewart, G.A.; Thompson, P.J. Protease-activated receptors in human airways: Upregulation of PAR-2 in respiratory epithelium from patients with asthma. J. Allergy Clin. Immunol. 2001, 108, 797–803. [Google Scholar] [CrossRef]
- Moretti, S.; Bellocchio, S.; Bonifazi, P.; Bozza, S.; Zelante, T.; Bistoni, F.; Romani, L. The contribution of PARs to inflammation and immunity to fungi. Mucosal Immunol. 2008, 1, 156–168. [Google Scholar] [CrossRef]
- Wu, X.; Lee, B.; Zhu, L.; Ding, Z.; Chen, Y. Exposure to mold proteases stimulates mucin production in airway epithelial cells through Ras/Raf1/ERK signal pathway. PLoS ONE 2020, 15, e0231990. [Google Scholar] [CrossRef]
- Holgate, S.T.; Holloway, J.; Wilson, S.; Bucchieri, F.; Puddicombe, S.; Davies, D.E. Epithelial-mesenchymal communication in the pathogenesis of chronic asthma. Proc. Am. Thorac. Soc. 2004, 1, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Smartt, H.; Holgate, S.T.; Roche, W.R. Growth factors secreted by bronchial epithelial cells control myofibroblast proliferation: An in vitro co-culture model of airway remodeling in asthma. Lab Investig. 1999, 79, 395–405. [Google Scholar] [PubMed]
- Ojiaku, C.A.; Yoo, E.J.; Panettieri, R.A., Jr. Transforming Growth Factor β1 Function in Airway Remodeling and Hyperresponsiveness. The Missing Link? Am. J. Respir. Cell Mol. Biol. 2017, 56, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Skrgat, S.; Malovrh, M.M.; Sarc, I.; Šilar, M.; Dimitric, V.; Korosec, P. TSLP as biomarker in asthma patients. Eur. Respir. J. 2015, 46 (Suppl. S59), PA3868. [Google Scholar]
- Burgess, J.K.; Jonker, M.R.; Berg, M.; Ten Hacken, N.T.H.; Meyer, K.B.; van den Berge, M.; Nawijn, M.C.; Heijink, I.H. Periostin: Contributor to abnormal airway epithelial function in asthma? Eur. Respir. J. 2021, 57, 2001286. [Google Scholar] [CrossRef]
- Cheng, D.; Xue, Z.; Yi, L.; Shi, H.; Zhang, K.; Huo, X.; Bonser, L.R.; Zhao, J.; Xu, Y.; Erle, D.; et al. Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive asthma. Am. J. Respir. Crit. Care Med. 2014, 190, 639–648. [Google Scholar] [CrossRef]
- Drake, L.Y.; Kita, H. IL-33: Biological properties, functions, and roles in airway disease. Immunol. Rev. 2017, 278, 173–184. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Lv, Z.; Li, Y.; Chen, Y.; Huang, K.; Corrigan, C.J.; Ying, S. Elevated Expression of IL-33 and TSLP in the Airways of Human Asthmatics In Vivo: A Potential Biomarker of Severe Refractory Disease. J. Immunol. 2018, 200, 2253. [Google Scholar] [CrossRef]
- Khosravi, A.R.; Shokri, H.; Hassan Al-Heidary, S.; Ghafarifar, F. Evaluation of murine lung epithelial cells (TC-1 JHU-1) line to develop Th2-promoting cytokines IL-25/IL-33/TSLP and genes Tlr2/Tlr4 in response to Aspergillus fumigatus. J. Mycol. Med. 2018, 28, 349–354. [Google Scholar] [CrossRef]
- Verma, M.; Liu, S.; Michalec, L.; Sripada, A.; Gorska, M.M.; Alam, R. Experimental asthma persists in IL-33 receptor knockout mice because of the emergence of thymic stromal lymphopoietin-driven IL-9(+) and IL-13(+) type 2 innate lymphoid cell subpopulations. J. Allergy Clin. Immunol. 2018, 142, 793–803.e8. [Google Scholar] [CrossRef]
- Gordon, E.D.; Sidhu, S.S.; Wang, Z.E.; Woodruff, P.G.; Yuan, S.; Solon, M.C.; Conway, S.J.; Huang, X.; Locksley, R.M.; Fahy, J.V. A protective role for periostin and TGF-beta in IgE-mediated allergy and airway hyperresponsiveness. Clin. Exp. Allergy 2012, 42, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Khor, Y.H.; Teoh, A.K.Y.; Lam, S.M.; Mo, D.C.Q.; Weston, S.; Reid, D.W.; Walters, E.H. Increased vascular permeability precedes cellular inflammation as asthma control deteriorates. Clin. Exp. Allergy 2009, 39, 1659–1667. [Google Scholar] [CrossRef]
- Hoshino, M.; Nakamura, Y.; Hamid, Q.A. Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. J. Allergy Clin. Immunol. 2001, 107, 1034–1038. [Google Scholar] [CrossRef]
- Filep, J.G.; Sirois, M.G.; Rousseau, A.; Fournier, A.; Sirois, P. Effects of endothelin-1 on vascular permeability in the conscious rat: Interactions with platelet-activating factor. Br. J. Pharmacol. 1991, 104, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Redington, A.E.; Springall, D.R.; Ghatei, M.A.; Lau, L.C.; Bloom, S.R.; Holgate, S.T.; Polak, J.M.; Howarth, P.H. Endothelin in bronchoalveolar lavage fluid and its relation to airflow obstruction in asthma. Am. J. Respir. Crit. Care Med. 1995, 151, 1034–1039. [Google Scholar] [PubMed]
- Brims, F.J.H.; Chauhan, A.J.; Higgins, B.; Shute, J.K. Coagulation factors in the airways in moderate and severe asthma and the effect of inhaled steroids. Thorax 2009, 64, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Wagers, S.S.; Norton, R.J.; Rinaldi, L.M.; Bates, J.H.; Sobel, B.E.; Irvin, C.G. Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness. J. Clin. Investig. 2004, 114, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Panchabhai, T.S.; Mukhopadhyay, S.; Sehgal, S.; Bandyopadhyay, D.; Erzurum, S.C.; Mehta, A.C. Plugs of the Air Passages: A Clinicopathologic Review. Chest 2016, 150, 1141–1157. [Google Scholar] [CrossRef]
- de Giorgio-Miller, A.; Bottoms, S.; Laurent, G.; Carmeliet, P.; Herrick, S. Fibrin-induced skin fibrosis in mice deficient in tissue plasminogen activator. Am. J. Pathol. 2005, 167, 721–732. [Google Scholar] [CrossRef]
- Shi-Wen, X.; Chen, Y.; Denton, C.P.; Eastwood, M.; Renzoni, E.; Bou-Gharios, G.; Pearson, J.D.; Dashwood, M.; Du Bois, R.M.; Black, C.M.; et al. Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol. Biol. Cell 2004, 15, 2707–2719. [Google Scholar] [CrossRef]
- Millien, V.O.; Lu, W.; Shaw, J.; Yuan, X.; Mak, G.; Roberts, L.; Song, L.Z.; Knight, J.M.; Creighton, C.J.; Luong, A.; et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science 2013, 341, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Dasari, P.; Shopova, I.; Stroe, M.; Wartenberg, D.; Martin-Dahse, H.; Beyersdorf, N.; Hortschansky, P.; Dietrich, S.; Cseresnyés, Z.; Figge, M.T.; et al. Aspf2 From Aspergillus fumigatus Recruits Human Immune Regulators for Immune Evasion and Cell Damage. Front. Immunol. 2018, 9, 1635. [Google Scholar] [CrossRef]
- Salo, P.M.; Arbes, S.J., Jr.; Sever, M.; Jaramillo, R.; Cohn, R.D.; London, S.J.; Zeldin, D.C. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J. Allergy Clin. Immunol. 2006, 118, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ramirez, G.; Barber, D.; Tome-Amat, J.; Garrido-Arandia, M.; Diaz-Perales, A. Alternaria as an Inducer of Allergic Sensitization. J. Fungi 2021, 7, 838. [Google Scholar] [CrossRef] [PubMed]
- Pashley, C.H.; Wardlaw, A.J. Allergic fungal airways disease (AFAD): An under-recognised asthma endotype. Mycopathologia 2021, 186, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Celakovska, J.; Vankova, R.; Bukac, J.; Cermakova, E.; Andrys, C.; Krejsek, J. Atopic Dermatitis and Sensitisation to Molecular Components of Alternaria, Cladosporium, Penicillium, Aspergillus, and Malassezia-Results of Allergy Explorer ALEX 2. J. Fungi 2021, 7, 183. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namvar, S.; Labram, B.; Rowley, J.; Herrick, S. Aspergillus fumigatus—Host Interactions Mediating Airway Wall Remodelling in Asthma. J. Fungi 2022, 8, 159. https://doi.org/10.3390/jof8020159
Namvar S, Labram B, Rowley J, Herrick S. Aspergillus fumigatus—Host Interactions Mediating Airway Wall Remodelling in Asthma. Journal of Fungi. 2022; 8(2):159. https://doi.org/10.3390/jof8020159
Chicago/Turabian StyleNamvar, Sara, Briony Labram, Jessica Rowley, and Sarah Herrick. 2022. "Aspergillus fumigatus—Host Interactions Mediating Airway Wall Remodelling in Asthma" Journal of Fungi 8, no. 2: 159. https://doi.org/10.3390/jof8020159
APA StyleNamvar, S., Labram, B., Rowley, J., & Herrick, S. (2022). Aspergillus fumigatus—Host Interactions Mediating Airway Wall Remodelling in Asthma. Journal of Fungi, 8(2), 159. https://doi.org/10.3390/jof8020159






