Chronic Pulmonary Aspergillosis: Burden, Clinical Characteristics and Treatment Outcomes at a Large Australian Tertiary Hospital
Abstract
:1. Introduction
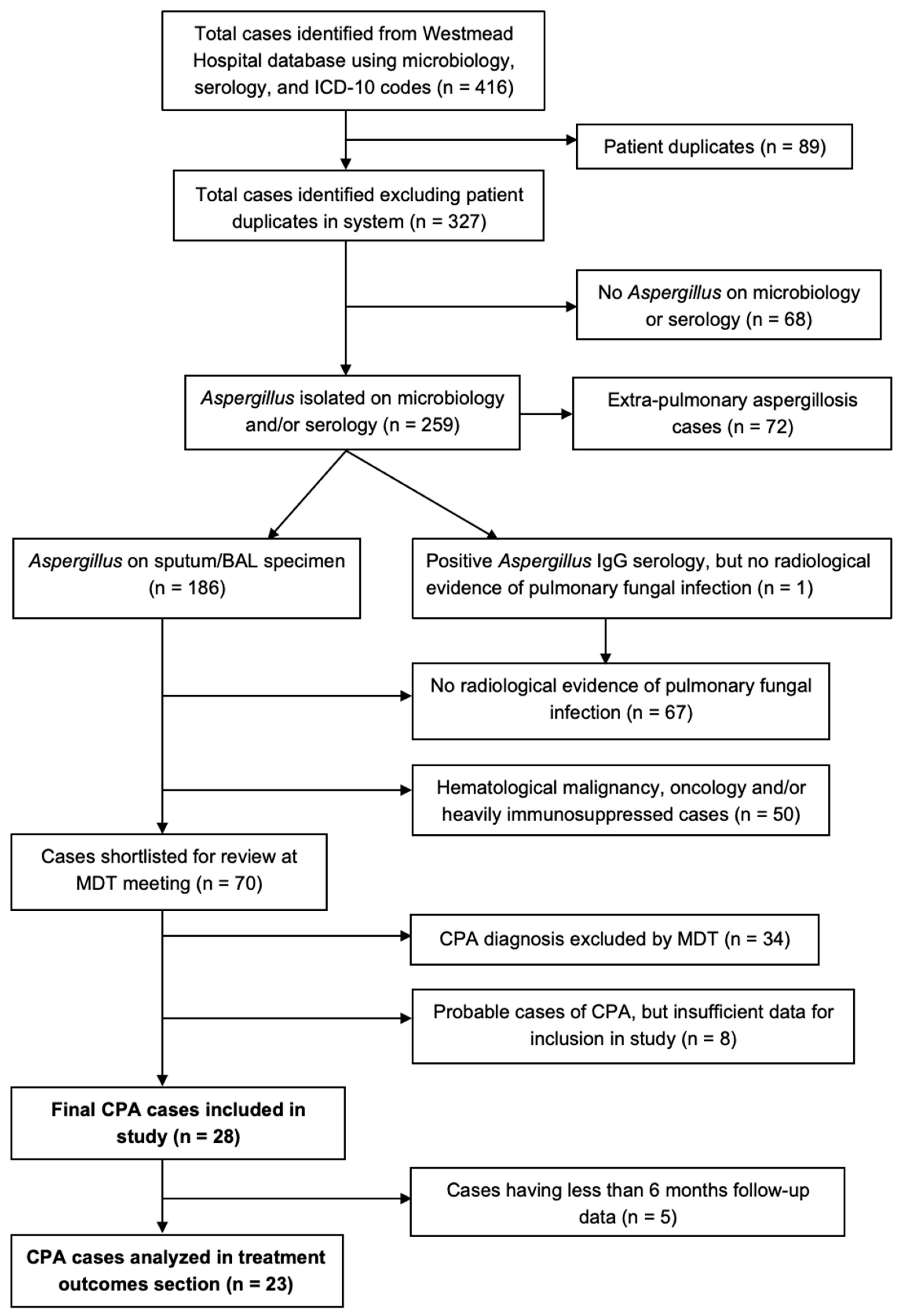
2. Materials and Methods
- -
- Allergic broncho-pulmonary aspergillosis (ABPA),
- -
- Extrapulmonary aspergillosis,
- -
- Clear alternative diagnoses,
- -
- Severely immunocompromised (hematological malignancy, chemotherapy and transplant) patients.
- (1)
- Three months or more of pulmonary or constitutional symptoms (cough, sputum production, dyspnea, weight loss, hemoptysis), except in cases of SAIA where the clinical course is 1–3 months, or simple aspergilloma which may be asymptomatic,
- (2)
- Serological/microbiological evidence of Aspergillus infection,
- (3)
- Compatible radiological features,
- (4)
- Exclusion of conditions which may mimic CPA (chronic cavitary pulmonary histoplasmosis, paracoccidioidomycosis, coccidioidomycosis and active pulmonary TB),
- (5)
- Exclusion of major immunosuppressing conditions or current use of immunosuppressant medications (including chemotherapy and corticosteroid use >7.5 mg prednisone/day for >3 months). Value of corticosteroid use >7.5 mg/day for >3 months was arbitrarily set by the authors as being above the minimum threshold of acceptable immunosuppression for inclusion in this study.
- (6)
- The presence of at least one marker of inflammation (white cell count (WCC) > 11.0 × 109 cells/L, serum C-reactive protein (CRP) > 10 mg/L or elevated erythrocyte sedimentation rate (ESR) >30 mm/h) (stated WCC, CRP and ESR values were set as the cut-offs by the authors for inclusion in this study).
3. Results
3.1. General Characteristics
3.2. Clinical Symptoms
3.3. Laboratory Examinations
3.4. Radiological Examinations
3.5. Prognosis and Treatment Outcomes
4. Discussion
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kosmidis, C.; Denning, D.W. The clinical spectrum of pulmonary aspergillosis. Thorax 2015, 70, 270–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, G.; Novak-Frazer, L. Chronic Pulmonary Aspergillosis—Where Are We? and Where Are We Going? J. Fungi 2016, 2, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowes, D.; Al-shair, K.; Newton, P.J.; Morris, J.; Harris, C.; Rautemaa-richardson, R.; Denning, D.W. Predictors of mortality in chronic pulmonary aspergillosis. Eur. Respir. J. 2017, 49, 1601062. [Google Scholar] [CrossRef]
- Camara, B.; Reymond, E.; Saint-Raymond, C.; Roth, H.; Brenier-Pinchart, M.-P.; Pinel, C.; Cadranel, J.; Ferretti, G.; Pelloux, H.; Pison, C. Characteristics and outcomes of chronic pulmonary aspergillosis: A retrospective analysis of a tertiary hospital registry. Clin. Respir. J. 2015, 9, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, H.; Kou, L.; Lv, W.; Lu, J.; Li, J. Clinical features and diagnosis of chronic pulmonary aspergillosis in Chinese patients. Medicine 2017, 96, e8315. [Google Scholar] [CrossRef]
- Salzer, H.J.F.; Heyckendorf, J.; Kalsdorf, B.; Rolling, T.; Lange, C. Characterization of patients with chronic pulmonary aspergillosis according to the new ESCMID/ERS/ECMM and IDSA guidelines. Mycoses 2017, 60, 136–142. [Google Scholar] [CrossRef]
- Denning, D.W.; Cadranel, J.; Beigelman-aubry, C.; Ader, F.; Chakrabarti, A.; Blot, S.; Ullmann, A.J. Chronic pulmonary aspergillosis: Rationale and clinical guidelines for diagnosis and management. Eur. Respir. J. 2016, 47, 45–68. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Jhun, B.W.O.O.; Jeon, K.; Eom, J.S.; Lee, J.I.H.; Suh, G.E.E.Y.; Kwon, O.J.; Koh, W. Clinical characteristics and treatment outcomes of chronic pulmonary aspergillosis. Med. Mycol. 2013, 51, 811–817. [Google Scholar] [CrossRef]
- Western Sydney Local Health District (WSLHD). Western Sydney Local Health District 2017–2018 Year in Review. 2018; p. 6. Available online: https://www.wslhd.health.nsw.gov.au/Education-Portal/Research/Research-Categories/Centre-for-Infectious-Diseases-and-Microbiology-Public-Health/Publications/wslhd-year-in-review (accessed on 5 May 2021).
- Ohara, S.; Tazawa, Y.; Tanai, C.; Tanaka, Y.; Noda, H.; Horiuchi, H.; Usui, K. Clinical characteristics of patients with Aspergillus species isolation from respiratory samples: Comparison of chronic pulmonary aspergillosis and colonization. Respir. Investig. 2016, 54, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Barac, A.; Kosmidis, C.; Alastruey-izquierdo, A.; Salzer, H.J.F. Chronic pulmonary aspergillosis update: A year in review. Med. Mycol. 2019, 57, S104–S109. [Google Scholar] [CrossRef] [PubMed]
- Norton, S.; Bag, S.K.; Cho, J.-G.; Heron, N.; Assareh, H.; Pavaresh, L.; Corbett, S.; Marais, B.J. Detailed characterisation of the tuberculosis epidemic in Western Sydney: A descriptive epidemiological study. ERJ Open Res. 2019, 5, 00211–02018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongomin, F.; Harris, C.; Hayes, G.; Kosmidis, C.; Denning, D.W. Twelve-month clinical outcomes of 206 patients with chronic pulmonary aspergillosis. PLoS ONE 2018, 13, e0193732. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Company, J.; Martín, M.T.; Goterris-Bonet, L.; Martinez-Marti, A.; Sampol, J.; Roldán, E.; Almirante, B.; Ruiz-Camps, I. Chronic pulmonary aspergillosis in a tertiary care centre in Spain: A retrospective, observational study. Mycoses 2019, 62, 765–772. [Google Scholar] [CrossRef]
- Maitre, T.; Cottenet, J.; Godet, C.; Roussot, A.; Abdoul Carime, N.; Ok, V.; Parrot, A.; Bonniaud, P.; Quantin, C.; Cadranel, J. Chronic pulmonary aspergillosis: Prevalence, favouring pulmonary diseases and prognosis. Eur. Respir. J. 2021, 58, 2003345. [Google Scholar] [CrossRef]
- Godet, C.; Laurent, F.; Bergeron, A.; Ingrand, P. CT Imaging Assessment of Response to Treatment in Chronic Pulmonary Aspergillosis. Chest 2016, 150, 139–147. [Google Scholar] [CrossRef]
- Smith, N.L.; Denning, D.W. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur. Respir. J. 2011, 37, 865–872. [Google Scholar] [CrossRef] [Green Version]
- Maghrabi, F.; Denning, D.W. The Management of Chronic Pulmonary Aspergillosis: The UK National Aspergillosis Centre Approach. Curr. Fungal Infect. Rep. 2017, 11, 242–251. [Google Scholar] [CrossRef] [Green Version]
- Izumikawa, K. Recent advances in chronic pulmonary aspergillosis. Respir. Investig. 2016, 54, 85–91. [Google Scholar] [CrossRef]
- Chan, J.F.; Lau, S.K.; Wong, S.C.; To, K.K.; So, S.Y.; Leung, S.S.; Chan, S.; Pang, C.; Xiao, C.; Hung, I.F.; et al. A 10-year study reveals clinical and laboratory evidence for the ‘semi-invasive’ properties of chronic pulmonary aspergillosis. Emerg. Microbes Infect. 2016, 5, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, K.; Ohshima, N.; Suzuki, J.; Kawashima, M.; Okuda, K.; Sato, R.; Suzukawa, M.; Nagai, H.; Matsui, H.; Ohta, K. Evaluation of clinical characteristics and prognosis of chronic pulmonary aspergillosis depending on the underlying lung diseases: Emphysema vs prior tuberculosis. J. Infect. Chemother. 2015, 21, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Takazono, T.; Izumikawa, K. Recent Advances in Diagnosing Chronic Pulmonary Aspergillosis. Front. Microbiol. 2018, 9, 1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehgal, I.S.; Choudhary, H.; Dhooria, S.; Nath, A.; Mandeep, A.; Arunaloke, G.; Ritesh, C. Diagnostic cutoff of Aspergillus fumigatus-specific IgG in the diagnosis of chronic pulmonary aspergillosis. Mycoses 2018, 61, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Alastruey-Izquierdo, A.; Cadranel, J.; Flick, H.; Godet, C.; Hennequin, C.; Hoenigl, M.; Kosmidis, C.; Lange, C.; Munteanu, O.; Page, I.; et al. Treatment of Chronic Pulmonary Aspergillosis: Current Standards and Future Perspectives. Respiration 2018, 96, 159–170. [Google Scholar] [CrossRef] [PubMed]


| Total (n = 28) | CCPA (n = 17) | SA (n = 4) | CNPA/SAIA (n = 3) | CFPA (n = 3) | AN (n = 1) | |
|---|---|---|---|---|---|---|
| Median age (years), (IQR) | 60 (57–66) | 60 (58–65) | 52 (38–68) | 72 (50–74) | 59 (58–59) | 60 (NA) |
| Gender, n (%) | ||||||
| Male | 17 (60.7%) | 12 (66.7%) | 0 | 2 (66.7%) | 2 (100%) | 1 (100%) |
| Primary predisposing factor, n (%) | ||||||
| Chronic obstructive pulmonary disease (COPD) | 9 (32.1%) | 7 | 0 | 1 | 0 | 1 |
| Prior tuberculosis (TB) | 5 (17.9%) | 4 | 0 | 0 | 1 | 0 |
| Mild iatrogenic immunosuppression | 5 (17.9%) | 1 | 1 | 2 | 1 | 0 |
| Alcoholism | 3 (10.7%) | 2 | 1 | 0 | 0 | 0 |
| Small airways disease | 2 (7.1%) | 1 | 1 | 0 | 0 | 0 |
| Pulmonary interstitial fibrosis | 1 (3.6%) | 1 | 0 | 0 | 0 | 0 |
| Diabetes | 1 (3.6%) | 0 | 1 | 0 | 0 | 0 |
| Asthma | 1 (3.6%) | 1 | 0 | 0 | 0 | 0 |
| ABPA | 1 (3.6%) | 1 | 0 | 0 | 0 | 0 |
| Symptoms Present | Total (n = 28) | CCPA (n = 17) | SA (n = 4) | CFPA (n = 3) | SAIA (n = 3) | AN (n = 1) |
|---|---|---|---|---|---|---|
| Cough, n (%) | 22 (78.6%) | 12 (70.6%) | 3 (75%) | 3 (100%) | 3 (100%) | 1 (100%) |
| Sputum production, n (%) | 16 (57.1%) | 10 (58.8%) | 2 (50%) | 1 (33.3%) | 3 (100%) | 0 |
| Dyspnea, n (%) | 13 (46.4%) | 9 (52.9%) | 1 (25%) | 0 | 2 (66.7%) | 1 (100%) |
| Weight loss, n (%) | 6 (21.4%) | 5 (29.4%) | 0 | 0 | 1 (33.3%) | 0 |
| Hemoptysis, n (%) | 2 (7.1%) | 2 (11.8%) | 0 | 0 | 0 | 0 |
| Fever/chills, n (%) | 2 (7.1%) | 1 (5.9%) | 0 | 0 | 1 (33.3%) | 0 |
| Asymptomatic, n (%) | 1 (3.6%) | 0 | 1 (25%) | 0 | 0 | 0 |
| Total (n = 28) | CCPA (n = 17) | SA (n = 4) | CFPA (n = 3) | SAIA (n = 3) | AN (n = 1) | |
|---|---|---|---|---|---|---|
| Median WCC (×109/L) (n = 17) | 17.2 | 13.5 | 16.8 | 17.2 | 21.8 | NA |
| (IQR) | (13.2–20.9) | (12.8–21.3) | (11.0–18.4) | (NA) | (20.9–22.7) | (NA) |
| (Range) | (5.2–27.6) | (11.7–27.6) | (5.2–20) | (NA) | (20–23.6) | (NA) |
| Median CRP (mg/L) (n = 27) | 71 | 68.5 | 59 | 91 | 114 | 25 |
| (IQR) | (33.5–139.2) | (44–182.2) | (37–178.5) | (51.5–107) | (71–127.5) | (NA) |
| (Range) | (12–298) | (22–287) | (15–298) | (12–123) | (28–141) | (NA) |
| Positive Aspergillus Sputum culture, n (%) | 13 (46.4%) | 9 (52.9%) | 1 (25%) | 0 | 3 (100%) | 0 |
| Positive Aspergillus BAL culture, n (%) | 16 (57.1%) | 11 (64.7%) | 2 (50%) | 1 (33.3%) | 1 (33.3%) | 1 (100%) |
| Positive Aspergillus BAL PCR, n (%) | 15 (53.6%) | 10 (58.8%) | 2 (50%) | 3 (100%) | 0 | 0 |
| Hyphae seen on histology, n (%) | 9 (32.1%) | 4 (23.5%) | 3 (75%) | 1 (33.3%) | 0 | 1 (100%) |
| Total (n = 28) | CCPA (n = 17) | SA (n = 4) | CFPA (n = 3) | SAIA (n = 3) | AN (n = 1) | |
|---|---|---|---|---|---|---|
| Mycetoma/s, n (%) | 25 (89.3%) | 17 (100%) | 4 (100%) | 3 (100%) | 1 (33.3%) | 0 |
| Consolidation, n (%) | 20 (71.4%) | 14 (82.3%) | 0 | 3 (100%) | 2 (66.7%) | 1 (100%) |
| Pleural thickening, n (%) | 19 (67.9%) | 12 (70.6%) | 1 (25%) | 3 (100%) | 2 (66.7%) | 1 (100%) |
| Multiple cavities ± variable wall thickness, n (%) | 16 (57.1%) | 12 (70.6%) | 0 | 2 (66.7%) | 2 (66.7%) | 0 |
| Emphysema, n (%) | 16 (57.1%) | 11 (64.7%) | 1 (25%) | 2 (66.7%) | 1 (33.3%) | 1 (100%) |
| Bronchiectasis, n (%) | 15 (53.6%) | 9 (52.9%) | 3 (75%) | 3 (100%) | 0 | 0 |
| Fibrosis, n (%) | 15 (53.6%) | 11 (64.7%) | 1 (25%) | 2 (66.7%) | 1 (33.3%) | 0 |
| Single cavity ± variable wall thickness, n (%) | 11 (39.3%) | 5 (29.4%) | 4 (100%) | 1 (33.3%) | 1 (33.3%) | 0 |
| Bronchiolar nodules, n (%) | 5 (17.9%) | 5 (29.4%) | 0 | 0 | 0 | 0 |
| Halo sign, n (%) | 2 (7.1%) | 0 | 0 | 0 | 2 (66.7%) | 0 |
| Aspergillus nodule, n (%) | 1 (3.6%) | 0 | 0 | 0 | 0 | 1 (100%) |
| Antifungal Therapy | |
|---|---|
| Itraconazole | 15 (65.2%) |
| Voriconazole | 8 (34.8%) |
| Posaconazole | 3 (13.0%) |
| No antifungal therapy | 4 (17.4%) |
| Median delay to antifungal treatment (months) | 0.7 (IQR: 0–8.1; Range: 0–33) |
| Median duration of antifungal treatment (months) | 10.5 (IQR: 6.5–20.7; Range: 1–44) |
| Adjunctive surgical resection | 2 (8.7%) |
| Outcomes after treatment at 6 months | |
| Improvement in symptoms | 11 (47.8%) |
| Improvement in chest CT appearance | 5 (21.7%) |
| Deterioration in symptoms | 8 (34.8%) |
| Deterioration in chest CT appearance | 11 (47.8%) |
| Dead | 2 (8.7%) |
| Outcomes after treatment at 12 months | |
| Improvement in symptoms | 5 (21.7%) |
| Improvement in chest CT appearance | 3 (13.0%) |
| Deterioration in symptoms | 7 (30.4%) |
| Deterioration in chest CT appearance | 6 (26.1%) |
| Dead | 5 (21.7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Despois, O.; Chen, S.C.-A.; Gilroy, N.; Jones, M.; Wu, P.; Beardsley, J. Chronic Pulmonary Aspergillosis: Burden, Clinical Characteristics and Treatment Outcomes at a Large Australian Tertiary Hospital. J. Fungi 2022, 8, 110. https://doi.org/10.3390/jof8020110
Despois O, Chen SC-A, Gilroy N, Jones M, Wu P, Beardsley J. Chronic Pulmonary Aspergillosis: Burden, Clinical Characteristics and Treatment Outcomes at a Large Australian Tertiary Hospital. Journal of Fungi. 2022; 8(2):110. https://doi.org/10.3390/jof8020110
Chicago/Turabian StyleDespois, Olivier, Sharon C-A. Chen, Nicole Gilroy, Michael Jones, Peter Wu, and Justin Beardsley. 2022. "Chronic Pulmonary Aspergillosis: Burden, Clinical Characteristics and Treatment Outcomes at a Large Australian Tertiary Hospital" Journal of Fungi 8, no. 2: 110. https://doi.org/10.3390/jof8020110
APA StyleDespois, O., Chen, S. C.-A., Gilroy, N., Jones, M., Wu, P., & Beardsley, J. (2022). Chronic Pulmonary Aspergillosis: Burden, Clinical Characteristics and Treatment Outcomes at a Large Australian Tertiary Hospital. Journal of Fungi, 8(2), 110. https://doi.org/10.3390/jof8020110






