Ibrexafungerp, a Novel Triterpenoid Antifungal in Development for the Treatment of Mold Infections
Abstract
1. Introduction
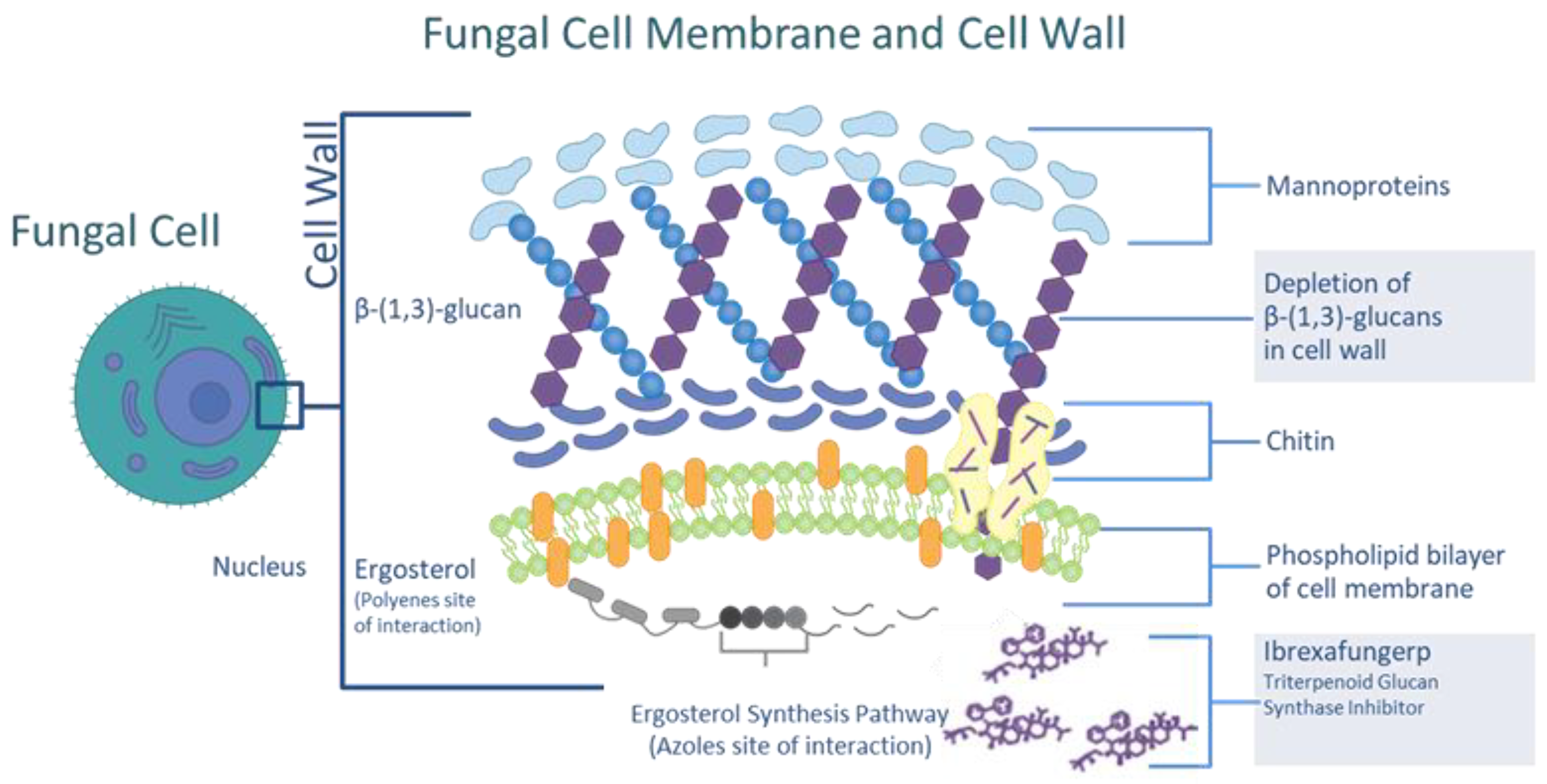
2. Ibrexafungerp Mechanism of Action
3. Pharmacokinetics
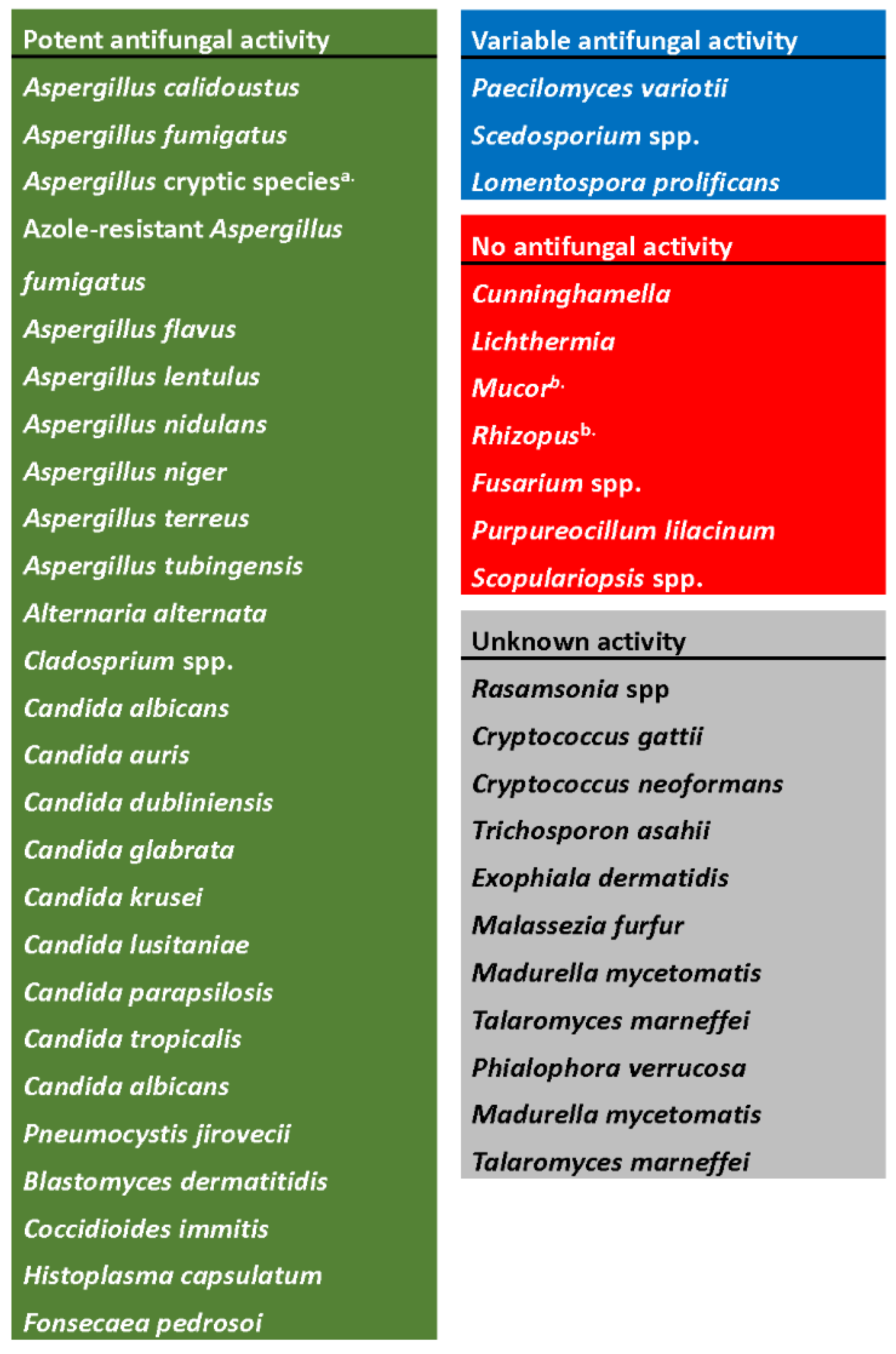
4. In Vitro Activity against Aspergillus species
- a.
- Includes A. fumigatiaffinis, A. thermomutatus, A. udagawae, A. hiratsukae, A. felis, A. citrinoterreus, A. carneus, A. aureoterreus, A. hortai, A. kevei, A. insuetus, A. ochraceus, and A. sclerotiorum.
- b.
- No in vitro activity, although in vivo activity has been reported in animal models.
5. In Vivo Activity against Aspergillus spp. in Animal Models of Infection
6. In Vitro Activity against Other Molds
7. In Vivo Activity against Other Molds
8. Ongoing Clinical Investigations with Ibrexafungerp for Invasive Mold Infections
9. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacobs, S.E.; Zagaliotis, P.; Walsh, T.J. Novel antifungal agents in clinical trials. F1000Research 2021, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P. Emerging fungal infections: New species, new names, and antifungal resistance. Clin. Chem. 2022, 68, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Seiler, G.T.; Ostrosky-Zeichner, L. Investigational agents for the treatment of resistant yeasts and molds. Curr. Fungal Infect. Rep. 2021, 15, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Muldoon, E.G.; Strek, M.E.; Patterson, K.C. Allergic and Noninvasive Infectious Pulmonary Aspergillosis Syndromes. Clin. Chest Med. 2017, 38, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, C.; Shah, A. Allergic Aspergillus sinusitis and its association with allergic bronchopulmonary aspergillosis. Asia Pac. Allergy 2011, 1, 130–137. [Google Scholar] [CrossRef]
- Lamoth, F.; Alexander, B.D. Antifungal activities of SCY-078 (MK-3118) and standard antifungal agents against clinical non-Aspergillus mold isolates. Antimicrob. Agents Chemother. 2015, 59, 4308–4311. [Google Scholar] [CrossRef]
- Kosmidis, C.; Denning, D.W. The clinical spectrum of pulmonary aspergillosis. Thorax 2015, 70, 270–277. [Google Scholar] [CrossRef]
- Rubio, P.M.; Sevilla, J.; González-Vicent, M.; Lassaletta, A.; Cuenca-Estrella, M.; Díaz, M.A.; Riesco, S.; Madero, L. Increasing incidence of invasive aspergillosis in pediatric hematology oncology patients over the last decade: A retrospective single centre study. J. Pediatr. Hematol. Oncol. 2009, 31, 642–646. [Google Scholar] [CrossRef]
- Bassetti, M.; Bouza, E. Invasive mould infections in the ICU setting: Complexities and solutions. J. Antimicrob. Chemother. 2017, 72 (Suppl. S1), i39–i47. [Google Scholar] [CrossRef]
- Cadena, J.; Thompson, G.R., 3rd; Patterson, T.F. Aspergillosis: Epidemiology, Diagnosis, and Treatment. Infect. Dis. Clin. N. Am. 2021, 35, 415–434. [Google Scholar] [CrossRef]
- Thompson, G.R., 3rd; Young, J.H. Aspergillus Infections. N. Engl. J. Med. 2021, 385, 1496–1509. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Executive Summary: Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, 433–442. [Google Scholar] [CrossRef]
- Spellberg, B.J.; Edwards, J.E., Jr.; Ibrahim, A.S. Novel perspectives on mucormycosis: Pathophysiology, presentation, and management. Clin. Microbiol. Rev. 2005, 18, 556–569. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Spellberg, B.; Edwards, J.E., Jr. Iron acquisition: A novel perspective on mucormycosis pathogenesis and treatment. Curr. Opin. Infect. Dis. 2008, 21, 620–625. [Google Scholar] [CrossRef]
- Singh, P.; Arora, S.; Mittal, N.; Singh, A.; Verma, R.; Sharma, S.; Goyal, S. Diabetes and rhino-orbito-cerebral mucormycosis—A deadly duo. J. Diabetes Metab. Disord. 2021, 20, 201–207. [Google Scholar] [CrossRef]
- Hamed, H.; Madia, R.; Ladadweh, H.; Falana, H.; Abu Khalil, A.D. Fatal mucormycosis post COVID-19 infection in uncontrolled diabetes with misuse of glucocorticoids and antibiotics. Infect. Drug Resist. 2022, 15, 1121–1126. [Google Scholar] [CrossRef]
- Stone, N.; Gupta, N.; Schwartz, I. Mucormycosis: Time to address this deadly fungal infection. Lancet Microbe 2021, 2, e343–e344. [Google Scholar] [CrossRef]
- Son, H.J.; Song, J.S.; Choi, S.; Jung, J.; Kim, M.J.; Chong, Y.P.; Kim, S.H. Risk factors for mortality in patients with pulmonary mucormycosis. Mycoses 2020, 63, 729–736. [Google Scholar] [CrossRef]
- Mehta, S.; Pandey, A. Rhino-orbital mucormycosis associated with COVID-19. Cureus 2020, 12, e10726. [Google Scholar] [CrossRef]
- Garre, V. Recent advances and future directions in the understanding of mucormycosis. Front. Cell Infect. Microbiol. 2022, 12, 850581. [Google Scholar] [CrossRef]
- Hoenigl, M.; Salmanton-García, J.; Walsh, T.J.; Nucci, M.; Neoh, C.F.; Jenks, J.D.; Cornely, O.A. Global guideline for the diagnosis and management of rare mould infections: An initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect. Dis. 2021, 21, e246–e257. [Google Scholar] [CrossRef] [PubMed]
- Marr, K. Combination antifungal therapy: Where are we now, and where are we going? Oncology 2004, 18 (Suppl. S7), 24–29. [Google Scholar] [PubMed]
- Merck and Co., Inc. Caspofungin (Approved Product Labeling). 2001. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/206110lbl.pdf (accessed on 21 September 2022).
- Wring, S.; Borroto-Esoda, K.; Solon, E.; Angulo, D. SCY-078, a novel fungicidal agent, demonstrates distribution to tissues associated with fungal infections during mass balance studies with intravenous and oral [14C]SCY-078 in albino and pigmented rats. Antimicrob. Agents Chemother. 2019, 63, e02119-18. [Google Scholar] [CrossRef] [PubMed]
- Schwebke, J.R.; Sobel, R.; Gersten, J.K.; Sussman, S.A.; Lederman, S.N.; Jacobs, M.A.; Sobel, J.D. Ibrexafungerp Versus Placebo for Vulvovaginal Candidiasis Treatment: A Phase 3, Randomized, Controlled Superiority Trial (VANISH 303). Clin. Infect. Dis. 2022, 74, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Sobel, R.; Nyirjesy, P.; Ghannoum, M.A.; Delchev, D.A.; Azie, N.E.; Angulo, D.; Sobel, J.D. Efficacy and safety of oral ibrexafungerp for the treatment of acute vulvovaginal candidiasis: A global phase 3, randomised, placebo-controlled superiority study (VANISH 306). BJOG Int. J. Obstet. Gynaecol. 2022, 129, 412–420. [Google Scholar] [CrossRef]
- McCarthy, M.W. Pharmacokinetics and Pharmacodynamics of Ibrexafungerp. Drugs R D 2022, 22, 9–13. [Google Scholar] [CrossRef]
- Wring, S.A.; Randolph, R.; Park, S.; Abruzzo, G.; Chen, Q.; Flattery, A.; Borroto-Esoda, K. Preclinical pharmacokinetics and pharmacodynamic target of SCY-078, a first-in-class orally active antifungal glucan synthesis inhibitor, in murine models of disseminated candidiasis. Antimicrob. Agents Chemother. 2017, 61, e02068-16. [Google Scholar] [CrossRef]
- Davis, M.R.; Donnelley, M.A.; Thompson, G.R. Ibrexafungerp: A novel oral glucan synthase inhibitor. Med. Mycol. 2020, 58, 579–592. [Google Scholar] [CrossRef]
- Wring, S.; Murphy, G.; Atiee, G.; Corr, C.; Hyman, M.; Willett, M.; Angulo, D. Clinical pharmacokinetics and drug-drug interaction potential for coadministered SCY-078, an oral fungicidal glucan synthase inhibitor, and tacrolimus. Clin. Pharmacol. Drug Dev. 2019, 8, 60–69. [Google Scholar] [CrossRef]
- SCYNEXIS, Inc. Ibrexafungerp (Approved Product Labeling). 2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214900s000lbl.pdf (accessed on 21 September 2022).
- Murphy, G.; Darpo, B.; Marbury, T.; Hyman, M.; Angulo, D. Lack of an effect of SCY-078 a novel antifungal agent on QTc interval in healthy subjects. In Abstract 172, Proceedings of the ASM Microbe, New Orleans, LA, USA, 1–5 June 2017; SCYNEXIS, Inc.: Jersey City, NJ, USA.
- Hoenigl, M.; Sprute, R.; Egger, M.; Arastehfar, A.; Cornely, O.A.; Krause, R.; Jenks, J.D. The Antifungal Pipeline: Fosmanogepix, Ibrexafungerp, Olorofim, Opelconazole, and Rezafungin. Drugs 2021, 81, 1703–1729. [Google Scholar] [CrossRef]
- Bowman, J.C.; Hicks, P.S.; Kurtz, M.B.; Rosen, H.; Schmatz, D.M.; Liberator, P.A.; Douglas, C.M. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob. Agents Chemother. 2002, 46, 3001–3012. [Google Scholar] [CrossRef]
- Odds, F.C.; Brown, A.J.; Gow, N.A. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Ghannoum, M.; Long, L.; Larkin, E.L.; Isham, N.; Sherif, R.; Borroto-Esoda, K.; Angulo, D. Evaluation of the antifungal activity of the novel oral glucan synthase inhibitor SCY-078, singly and in combination, for the treatment of invasive aspergillosis. Antimicrob. Agents Chemother. 2018, 62, e00244-18. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Messer, S.A.; Motyl, M.R.; Jones, R.N.; Castanheira, M. In vitro activity of a new oral glucan synthase inhibitor (MK-3118) tested against Aspergillus spp. by CLSI and EUCAST broth microdilution methods. Antimicrob. Agents Chemother. 2013, 57, 1065–1068. [Google Scholar] [CrossRef]
- Jiménez-Ortigosa, C.; Paderu, P.; Motyl, M.R.; Perlin, D.S. Enfumafungin derivative MK-3118 shows increased in vitro potency against clinical echinocandin-resistant Candida Species and Aspergillus species isolates. Antimicrob. Agents Chemother. 2014, 58, 1248–1251. [Google Scholar] [CrossRef]
- Ghannoum, M.; Long, L.; Sherif, R.; Abidi, F.Z.; Borroto-Esoda, K.; Barat, S.; Wiederhold, N. Determination of antifungal activity of SCY-078, a novel glucan synthase inhibitor, against a broad panel of rare pathogenic fungi. In Proceedings of the ASM Microbe, Chicago, IL, USA, 18 June 2020. [Google Scholar]
- Petraitis, V.; Petraitiene, R.; Katragkou, A.; Maung BB, W.; Naing, E.; Kavaliauskas, P.; Walsh, T.J. Combination therapy with ibrexafungerp (formerly SCY-078), a first-in-class triterpenoid inhibitor of (1→3)-β-D-glucan synthesis, and isavuconazole for treatment of experimental invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 2020, 64, e02429-19. [Google Scholar] [CrossRef]
- Jagadeesan, V.; Driscoll, E.; Hao, B. In Vitro Evaluation of Combination of Ibrexafungerp and Azoles against Aspergillus spp. Isolated from Lung Transplant Recipients. In Proceedings of the ASM Microbe 2019, San Francisco, CA, USA, 20–24 June 2019. [Google Scholar]
- Rivero-Menendez, O.; Soto-Debran, J.C.; Cuenca-Estrella, M.; Alastruey-Izquierdo, A. In Vitro Activity of ibrexafungerp against a collection of clinical isolates of Aspergillus, including cryptic species and Cyp51A mutants, using EUCAST and CLSI methodologies. J. Fungi 2021, 7, 232. [Google Scholar] [CrossRef]
- CLSI. Method for Antifungal Disk Diffusion Susceptibility Testing of Nondermatophyte Filamentous Fungi, 1st ed.; CLSI Guideline M51; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2010. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Eucast Definitive Document E.Def 9.4 Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Conidia Forming Moulds; European Committee on Antimicrobial Susceptibility Testing: Stockholm, Sweden, 2022. [Google Scholar]
- Rautemaa-Richardson, R.; Bazaz, R.; Cornely, O.A. Outcomes of Oral Ibrexafungerp by Pathogen from two open-label studies of patients with serious fungal infections (FURI and CARES). In Proceedings of the 10th Trends in Medical Mycology, Aberdeen, UK, 18 June 2021. [Google Scholar]
- Borroto-Esoda, K.; Barat, S.; Angulo, D.; Holden, K.; Warn, P. SCY-078 demonstrates significant antifungal activity in a murine model of invasive aspergillosis. Open Forum Infect. Dis. 2017, 4, S472. [Google Scholar] [CrossRef]
- Petraitis, V.; Kavaliauskas, P.; Planciuniene, R. In vitro activity of ibrexafungerp in combination with isavuconazole or amphotericin B against medically important molds. In Proceedings of the ASM Microbe 2018, Atlanta, GA, USA, 6 June 2018. [Google Scholar]
- Gebremariam, T.; Alkhazraji, S.; Gu, Y. Efficacy assessment of ibrexafungerp in the neutropenic mouse model of pulmonary mucormycosis. In Proceedings of the 32nd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Lisbon, Portugal, 23 April 2022. [Google Scholar]

| Tissue | Tissue:Plasma Ratio |
|---|---|
| Bone | 1.3 |
| Bone marrow (femur) | 36 |
| Brain (cerebrum) | 0.1 |
| Esophagus | 6 |
| Eye (uvea) | 117 |
| Heart (myocardium) | 10 |
| Kidney (cortex) | 25 |
| Liver | 56.5 |
| Lung | 26.5 |
| Lymph node | 38 |
| Oral mucosa | 5.5 |
| Salivary gland | 22.5 |
| Skin (non-pigmented) | 11.3 |
| Spleen | 75.6 |
| Urinary bladder | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angulo, D.A.; Alexander, B.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A.; Hoenigl, M.; Ibrahim, A.S.; Ghannoum, M.A.; King, T.R.; Azie, N.E.; Walsh, T.J. Ibrexafungerp, a Novel Triterpenoid Antifungal in Development for the Treatment of Mold Infections. J. Fungi 2022, 8, 1121. https://doi.org/10.3390/jof8111121
Angulo DA, Alexander B, Rautemaa-Richardson R, Alastruey-Izquierdo A, Hoenigl M, Ibrahim AS, Ghannoum MA, King TR, Azie NE, Walsh TJ. Ibrexafungerp, a Novel Triterpenoid Antifungal in Development for the Treatment of Mold Infections. Journal of Fungi. 2022; 8(11):1121. https://doi.org/10.3390/jof8111121
Chicago/Turabian StyleAngulo, David A., Barbara Alexander, Riina Rautemaa-Richardson, Ana Alastruey-Izquierdo, Martin Hoenigl, Ashraf S. Ibrahim, Mahmoud A. Ghannoum, Thomas R. King, Nkechi E. Azie, and Thomas J. Walsh. 2022. "Ibrexafungerp, a Novel Triterpenoid Antifungal in Development for the Treatment of Mold Infections" Journal of Fungi 8, no. 11: 1121. https://doi.org/10.3390/jof8111121
APA StyleAngulo, D. A., Alexander, B., Rautemaa-Richardson, R., Alastruey-Izquierdo, A., Hoenigl, M., Ibrahim, A. S., Ghannoum, M. A., King, T. R., Azie, N. E., & Walsh, T. J. (2022). Ibrexafungerp, a Novel Triterpenoid Antifungal in Development for the Treatment of Mold Infections. Journal of Fungi, 8(11), 1121. https://doi.org/10.3390/jof8111121










