Design and Validation of qPCR-Specific Primers for Quantification of the Marketed Terfezia claveryi and Terfezia crassiverrucosa in Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Environmental Sampling
2.2. Soil DNA Extraction
2.3. Design of Specific Primers for Turmas
2.4. Quantitative Real-Time PCR Conditions
2.5. Statistical Analysis
3. Results and Discussion
3.1. In Silico Primer Screening
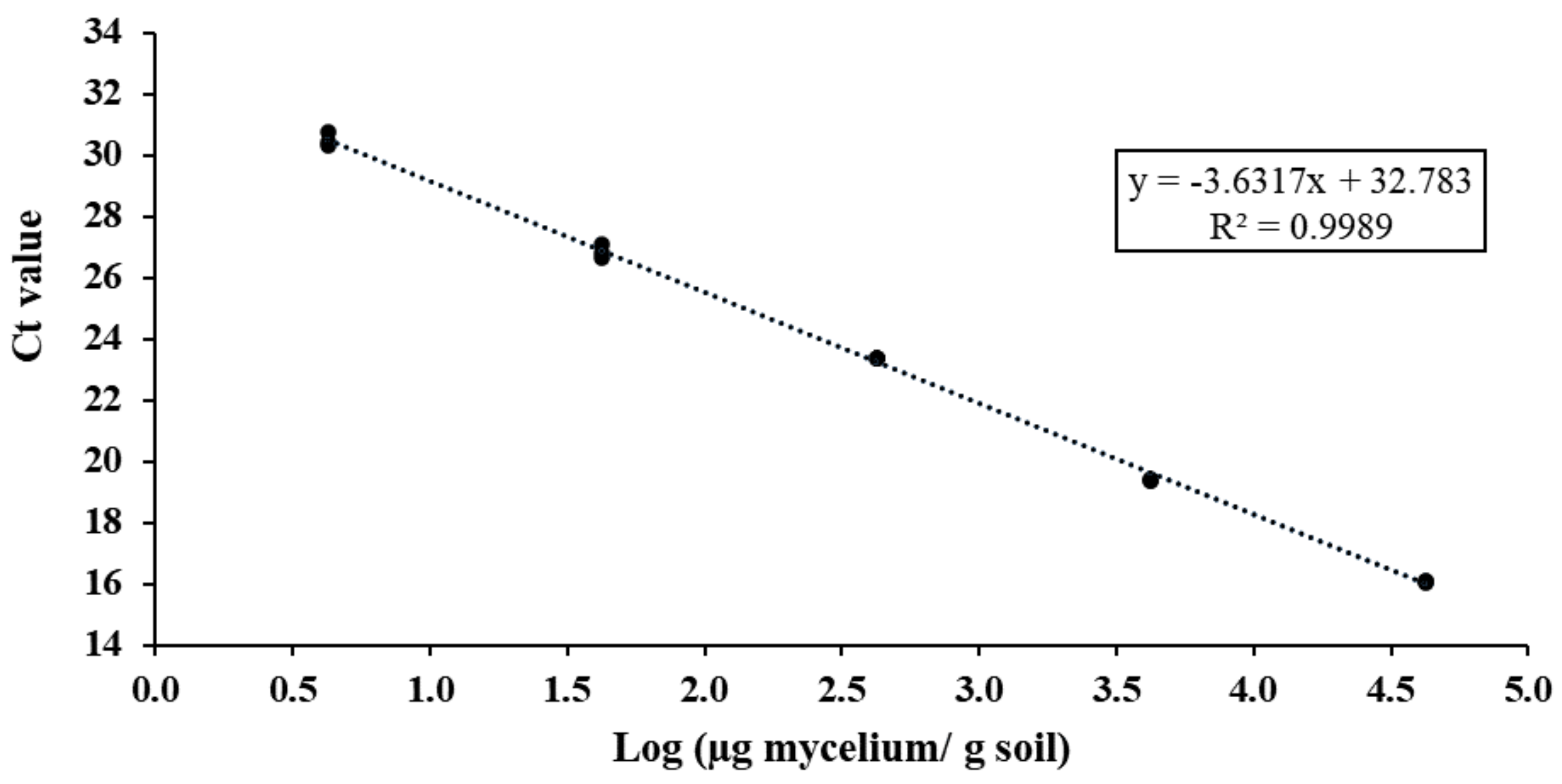
3.2. Selection and Validation of qPCR-Specific Primers
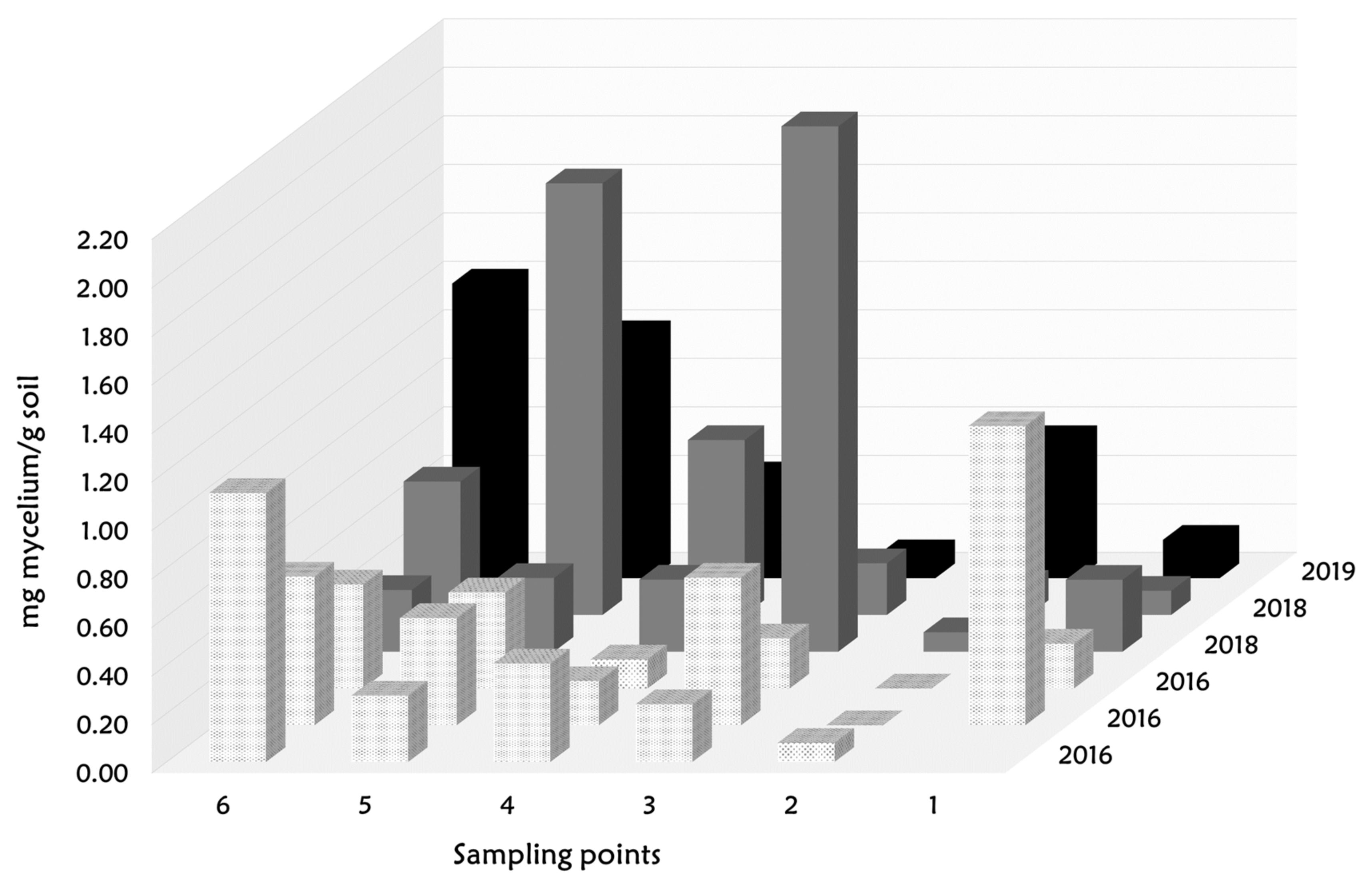
3.3. Spatial Dynamic of Turmas Mycelium in a Desert Truffle Orchard
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morte, A.; Pérez-Gilabert, M.; Gutiérrez, A.; Arenas, F.; Marqués-Gálvez, J.E.; Bordallo, J.J.; Rodríguez, A.; Berná, L.M.; Lozano-Carrillo, C.; Navarro-Ródenas, A. Basic and Applied Research for Desert Truffle Cultivation. In Mycorrhiza-Eco-Physiology, Secondary Metabolites, Nanomaterials; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer: Cham, Switzerland, 2017; pp. 23–42. [Google Scholar]
- Bradai, L.; Bissati, S.; Chenchouni, H. Desert Truffles of the North Algerian Sahara: Diversity and Bioecology. Emir. J. Food Agric. 2014, 26, 425–435. [Google Scholar] [CrossRef]
- Shavit, E. The History of Desert Truffle Use. In Desert Truffles. Soil Biology; Kagan-Zur, V., Roth-Bejerano, N., Sitrit, Y., Morte, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 217–241. [Google Scholar]
- Honrubia, M.; Gutiérrez, A.; Morte, A. Desert Truffle Plantation from South-East Spain. In Proceedings of the Edible Mycorrhizal Mushrooms and Their Cultivation: Proceedings of the Second International Conference on Edible Mycorrhizal Mushrooms, Christchurch, New Zealand, 3–6 July 2001; pp. 3–5. [Google Scholar]
- Morte, A.; Gutiérrez, A.; Ródenas, A.N. Advances in Desert Truffle Mycorrhization and Cultivation. In Mushrooms, Humans and Nature in a Changing World. Perspectives from Ecological, Agricultural and Social Sciences; Pérez-Moreno, J., Guerin-Laguette, A., Arzú, R.F., Yu, F.-Q., Eds.; Springer: Cham, Switzerland, 2020; pp. 205–219. [Google Scholar]
- Volpato, G.; Rossi, D.; Dentoni, D. A Reward for Patience and Suffering: Ethnomycology and Commodification of Desert Truffles among Sahrawi Refugees and Nomads of Western Sahara. Econ. Bot. 2013, 67, 147–160. [Google Scholar] [CrossRef]
- Murcia, M.A.; Martínez-Tomé, M.; Jiménez, A.M.; Vera, A.M.; Honrubia, M.; Parras, P. Antioxidant Activity of Edible Fungi (Truffles and Mushrooms): Losses during Industrial Processing. J. Food Prot. 2002, 65, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Murcia, M.A.; Martínez-Tomé, M.; Vera, A.; Morte, A.; Gutierrez, A.; Honrubia, M.; Jiménez, A.M. Effect of Industrial Processing on Desert Truffles Terfezia claveryi Chatin and Picoa juniperi Vittadini): Proximate Composition and Fatty Acids. J. Sci. Food Agric. 2003, 83, 535–541. [Google Scholar] [CrossRef]
- Wang, S.; Marcone, M.F. The Biochemistry and Biological Properties of the World’s Most Expensive Underground Edible Mushroom: Truffles. Food Res. Int. 2011, 44, 2567–2581. [Google Scholar] [CrossRef]
- Martínez-Tomé, M.; Maggi, L.; Jiménez-Monreal, A.M.; Murcia, M.A.; Marí, J.A.T. Nutritional and Antioxidant Properties of Terfezia and Picoa. In Desert Truffles. Soil Biology; Kagan-Zur, V., Roth-Bejerano, N., Sitrit, M.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 261–273. [Google Scholar]
- Patel, S.; Rauf, A.; Khan, H.; Khalid, S.; Mubarak, M.S. Potential Health Benefits of Natural Products Derived from Truffles: A Review. Trends Food Sci. Technol. 2017, 70, 1–8. [Google Scholar] [CrossRef]
- Dahham, S.S.; Al-Rawi, S.S.; Ibrahim, A.H.; Abdul Majid, A.S.; Abdul Majid, A.M.S. Antioxidant, Anticancer, Apoptosis Properties and Chemical Composition of Black Truffle Terfezia claveryi. Saudi J. Biol. Sci. 2018, 25, 1524–1534. [Google Scholar] [CrossRef]
- Morte, A.; Arenas, F.; Marqués-Gálvez, J.E.; Berna, L.M.; Guarnizo-Serrudo, Á.L.; Gutierrez, A.; Rodriguez, A.; Navarro-Ródenas, A. Turmiculture Project: Desert Truffle Crop against Climate Change and for Rural Development. In Proceedings of the X International Workshop of Edible Mycorrhizal Mushrooms (IWEMM10), Suwa City, Japan, 20–29 October 2019. [Google Scholar]
- Andrino, A.; Navarro-Ródenas, A.; Marqués-Gálvez, J.E.; Morte, A. The Crop of Desert Truffle Depends on Agroclimatic Parameters during Two Key Annual Periods. Agron. Sustain. Dev. 2019, 39, 51. [Google Scholar] [CrossRef]
- Marqués-Gálvez, J.E.; Morte, A.; Navarro-Ródenas, A. Spring Stomatal Response to Vapor Pressure Deficit as a Marker for Desert Truffle Fruiting. Mycorrhiza 2020, 30, 503–512. [Google Scholar] [CrossRef]
- Marqués-Gálvez, J.E.; Navarro-Ródenas, A.; Peguero-Pina, J.J.; Arenas, F.; Guarnizo, A.L.; Gil-Pelegrín, E.; Morte, A. Elevated Atmospheric CO2 Modifies Responses to Water-Stress and Flowering of Mediterranean Desert Truffle Mycorrhizal Shrubs. Physiol. Plant 2020, 170, 537–549. [Google Scholar] [CrossRef]
- Morte, A.; Arenas, F.; Marqués-Gálvez, J.E.; Andrino, A.; Guarnizo, Á.L.; Gutiérrez, A.; Berná, L.M.; Pérez-Gilabert, M.; Rodríguez, A.; Navarro-Ródenas, A. Desert Truffles (Terfezia spp.) Breeding. In Advances in Plant Breeding Strategies: Vegetable Crops; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer: Cham, Switzerland, 2021; pp. 479–504. ISBN 978-3-030-66969-0. [Google Scholar]
- Arenas, F.; Navarro-Ródenas, A.; Marqués-Gálvez, J.E.; Ghignone, S.; Mello, A.; Morte, A. Different Patterns in Root and Soil Fungal Diversity Drive Plant Productivity of the Desert Truffle Terfezia claveryi in Plantation. Environ. Microbiol. 2021, 23, 5917–5933. [Google Scholar] [CrossRef]
- Hall, I.R.; Yun, W.; Amicucci, A. Cultivation of Edible Ectomycorrhizal Mushrooms. Trends Biotechnol. 2003, 21, 433–438. [Google Scholar] [CrossRef]
- Zambonelli, A.; Iotti, M.; Boutahir, S.; Lancellotti, E.; Perini, C.; Pacioni, G. Ectomycorrhizal Fungal Communities of Edible Ectomycorrhizal Mushrooms. In Edible Ectomycorrhizal Mushrooms: Current Knowledge and Future Prospects; Zambonelli, A., Bonito, G.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 105–124. [Google Scholar]
- Navarro-Ródenas, A.; Berná, L.M.; Lozano-Carrillo, C.; Andrino, A.; Morte, A. Beneficial Native Bacteria Improve Survival and Mycorrhization of Desert Truffle Mycorrhizal Plants in Nursery Conditions. Mycorrhiza 2016, 26, 769–779. [Google Scholar] [CrossRef]
- Anderson, I.C.; Cairney, J.W.G. Diversity and Ecology of Soil Fungal Communities: Increased Understanding through the Application of Molecular Techniques. Environ. Microbiol. 2004, 6, 769–779. [Google Scholar] [CrossRef]
- Séjalon-Delmas, N.; Roux, C.; Martins, M.; Kulifaj, M.; Bécard, G.; Dargent, R. Molecular Tools for the Identification of Tuber melanosporum in Agroindustry. J. Agric. Food Chem. 2000, 48, 2608–2613. [Google Scholar] [CrossRef]
- Zarivi, O.; Cesare, P.; Ragnelli, A.M.; Aimola, P.; Leonardi, M.; Bonfigli, A.; Colafarina, S.; Poma, A.M.; Miranda, M.; Pacioni, G. Validation of Reference Genes for Quantitative Real-Time PCR in Périgord Black Truffle (Tuber melanosporum) Developmental Stages. Phytochemistry 2015, 116, 78–86. [Google Scholar] [CrossRef]
- Leonardi, M.; Ascione, S.; Pacioni, G.; Cesare, P.; Pacioni, M.L.; Miranda, M.; Zarivi, O. The Challenge for Identifying the Fungi Living inside Mushrooms: The Case of Truffle Inhabiting Mycelia. Plant Biosyst. 2018, 152, 1002–1010. [Google Scholar] [CrossRef]
- Rizzello, R.; Zampieri, E.; Vizzini, A.; Autino, A.; Cresti, M.; Bonfante, P.; Mello, A. Authentication of Prized White and Black Truffles in Processed Products Using Quantitative Real-Time PCR. Food Res. Int. 2012, 48, 792–797. [Google Scholar] [CrossRef]
- Jomura, M.; Kuwayama, T.; Soma, Y.; Yamaguchi, M.; Komatsu, M.; Maruyama, Y. Mycelial Biomass Estimation and Metabolic Quotient of Lentinula edodes Using Species-specific QPCR. PLoS ONE 2020, 15, e0232049. [Google Scholar] [CrossRef]
- Suz, L.M.; Martín, M.P.; Colinas, C. Detection of Tuber melanosporum DNA in Soil. FEMS Microbiol. Lett. 2006, 254, 251–257. [Google Scholar] [CrossRef]
- Bertini, L.; Rossi, I.; Zambonelli, A.; Amicucci, A.; Sacchi, A.; Cecchini, M.; Gregori, G.; Stocchi, V. Molecular Identification of Tuber magnatum Ectomycorrhizae in the Field. Microbiol. Res. 2006, 161, 59–64. [Google Scholar] [CrossRef]
- Hortal, S.; Pera, J.; Galipienso, L.; Parladé, J. Molecular Identification of the Edible Ectomycorrhizal Fungus Lactarius deliciosus in the Symbiotic and Extraradical Mycelium Stages. J. Biotechnol. 2006, 126, 123–134. [Google Scholar] [CrossRef]
- Suz, L.M.; Martín, M.P.; Oliach, D.; Fischer, C.R.; Colinas, C. Mycelial Abundance and Other Factors Related to Truffle Productivity in Tuber melanosporum-Quercus ílex Orchards. FEMS Microbiol. Lett. 2008, 285, 72–78. [Google Scholar] [CrossRef]
- Parladé, J.; De la Varga, H.; De Miguel, A.M.; Sáez, R.; Pera, J. Quantification of Extraradical Mycelium of Tuber melanosporum in Soils from Truffle Orchards in Northern Spain. Mycorrhiza 2013, 23, 99–106. [Google Scholar] [CrossRef]
- Queralt, M.; Parladé, J.; Pera, J.; De Miguel, A.M. Seasonal Dynamics of Extraradical Mycelium and Mycorrhizas in a Black Truffle (Tuber melanosporum) Plantation. Mycorrhiza 2017, 27, 565–576. [Google Scholar] [CrossRef]
- Iotti, M.; Leonardi, M.; Oddis, M.; Salerni, E.; Baraldi, E.; Zambonelli, A. Development and Validation of a Real-Time PCR Assay for Detection and Quantification of Tuber magnatum in Soil. BMC Microbiol. 2012, 12, 93. [Google Scholar] [CrossRef]
- Iotti, M.; Leonardi, M.; Lancellotti, E.; Salerni, E.; Oddis, M.; Leonardi, P.; Perini, C.; Pacioni, G.; Zambonelli, A. Spatio-Temporal Dynamic of Tuber magnatum Mycelium in Natural Truffle Grounds. PLoS ONE 2014, 9, e115921. [Google Scholar] [CrossRef]
- Iotti, M.; Leonardi, P.; Vitali, G.; Zambonelli, A. Effect of Summer Soil Moisture and Temperature on the Vertical Distribution of Tuber magnatum Mycelium in Soil. Biol. Fertil. Soils 2018, 54, 707–716. [Google Scholar] [CrossRef]
- Gryndler, M.; Trilčová, J.; Hršelová, H.; Streiblová, E.; Gryndlerová, H.; Jansa, J. Tuber aestivum Vittad. Mycelium Quantified: Advantages and Limitations of a QPCR Approach. Mycorrhiza 2013, 23, 341–348. [Google Scholar] [CrossRef]
- Todesco, F.; Belmondo, S.; Guignet, Y.; Laurent, L.; Fizzala, S.; Le Tacon, F.; Murat, C. Soil Temperature and Hydric Potential Influences the Monthly Variations of Soil Tuber aestivum DNA in a Highly Productive Orchard. Sci. Rep. 2019, 9, 12964. [Google Scholar] [CrossRef]
- Parladé, J.; Hortal, S.; Pera, J.; Galipienso, L. Quantitative Detection of Lactarius deliciosus Extraradical Soil Mycelium by Real-Time PCR and Its Application in the Study of Fungal Persistence and Interspecific Competition. J. Biotechnol. 2007, 128, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Hortal, S.; Pera, J.; Parladé, J. Tracking Mycorrhizas and Extraradical Mycelium of the Edible Fungus Lactarius deliciosus under Field Competition with Rhizopogon spp. Mycorrhiza 2008, 18, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Hortal, S.; Pera, J.; Parladé, J. Field Persistence of the Edible Ectomycorrhizal Fungus Lactarius deliciosus: Effects of Inoculation Strain, Initial Colonization Level, and Site Characteristics. Mycorrhiza 2009, 19, 167–177. [Google Scholar] [CrossRef] [PubMed]
- De la Varga, H.; Águeda, B.; Ágreda, T.; Martínez-Peña, F.; Parladé, J.; Pera, J. Seasonal Dynamics of Boletus edulis and Lactarius deliciosus Extraradical Mycelium in Pine Forests of Central Spain. Mycorrhiza 2013, 23, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Narimatsu, M.; Fujita, T.; Kawai, M.; Kobayashi, H.; Ohta, A.; Yamada, A.; Matsushita, N.; Neda, H.; Shimokawa, T.; et al. A QPCR Assay That Specifically Quantifies Tricholoma matsutake Biomass in Natural Soil. Mycorrhiza 2016, 26, 847–861. [Google Scholar] [CrossRef]
- De la Varga, H.; Águeda, B.; Martínez-Peña, F.; Parladé, J.; Pera, J. Quantification of Extraradical Soil Mycelium and Ectomycorrhizas of Boletus edulis in a Scots Pine Forest with Variable Sporocarp Productivity. Mycorrhiza 2011, 22, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Parladé, J.; Martínez-Peña, F.; Pera, J. Effects of Forest Management and Climatic Variables on the Mycelium Dynamics and Sporocarp Production of the Ectomycorrhizal Fungus Boletus edulis. For. Ecol. Manag. 2017, 390, 73–79. [Google Scholar] [CrossRef]
- Aviram, S.; Roth-Bejerano, N.; Kagan-Zur, V. Two ITS Forms Co-Inhabiting a Single Genet of an Isolate of Terfezia boudieri (Ascomycotina), a Desert Truffle. Antonie Van Leeuwenhoek Int. 2004, 85, 169–174. [Google Scholar] [CrossRef]
- Bordallo, J.-J.; Rodríguez, A.; Kaounas, V.; Camello, F.; Honrubia, M.; Morte, A. Two New Terfezia Species from Southern Europe. Phytotaxa 2015, 230, 239–249. [Google Scholar] [CrossRef]
- Bordallo, J.J.; Rodríguez, A.; Santos-Silva, C.; Louro, R.; Muñoz-Mohedano, J.; Morte, A. Terfezia lusitanica, a New Mycorrhizal Species Associated to Tuberaria guttata (Cistaceae). Phytotaxa 2018, 357, 141–147. [Google Scholar] [CrossRef]
- Zitouni-Haouar, F.E.H.; Carlavilla, J.R.; Moreno, G.; Manjón, J.L.; Fortas, Z. Genetic Diversity of the Genus Terfezia (Pezizaceae, Pezizales): New Species and New Record from North Africa. Phytotaxa 2018, 334, 183–194. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.E.S.J.; Gené, J.; Guarro, J.; Baseia, I.G.; García, D.; Gusmão, L.F.P.; Souza-Motta, C.M.; et al. Fungal Planet Description Sheets: 716–784. Pers. Mol. Phylogeny Evol. Fungi 2018, 40, 240–393. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Lombard, L.; Roets, F.; Swart, W.J.; Alvarado, P.; Carnegie, A.J.; Moreno, G.; Luangsa-Ard, J.; Thangavel, R.; et al. Fungal Planet Description Sheets: 951–1041. Pers. Mol. Phylogeny Evol. Fungi 2019, 43, 223–425. [Google Scholar] [CrossRef]
- Moreno, G.; Manjón, J.L.; Alvarado, P. A New Terfezia from Spain. Bol. Soc. Micol. Madr. 2019, 43, 55–60. [Google Scholar]
- Rodríguez, A.; Navarro-Ródenas, A.; Arenas, F.; Muñoz-Mohedano, J.M.; Morte, A. Solving the Identity of Terfezia trappei (Pezizaceae, Ascomycota). Phytotaxa 2019, 411, 230–236. [Google Scholar] [CrossRef]
- Vizzini, A.; Arenas, F.; Rodríguez, A.; Mello, A.; Lainé, P.; Muñoz-Mohedano, J.M.; Morte, A. Typification of Terfezia fanfani (Ascomycota, Pezizaceae). Phytotaxa 2019, 387, 73–76. [Google Scholar] [CrossRef]
- Kovács, G.M.; Balázs, T.K.; Calonge, F.D.; Martín, M.P. The Diversity of Terfezia Desert Truffles: New Species and a Highly Variable Species Complex with Intrasporocarpic NrDNA ITS Heterogeneity. Mycologia 2011, 103, 841–853. [Google Scholar] [CrossRef]
- Bordallo, J.-J.; Rodríguez, A. Cryptic and New Species. In Desert Truffles. Soil Biology; Kagan-Zur, V., Roth-Bejerano, N., Sitrit, Y., Morte, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 39–53. ISBN 978-3-642-40095-7. [Google Scholar]
- Louro, R.; Santos-Silva, C.; Nobre, T. What Is in a Name? Terfezia Classification Revisited. Fungal. Biol. 2019, 123, 267–273. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Consortium, F.B. Nuclear Ribosomal Internal Transcribed Spacer (ITS) Region as a Universal DNA Barcode Marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Oliach, D.; Morte, A.; Sánchez, S.; Navarro-Ródenas, A.; Marco, P.; Gutiérrez, A.; Martín- Santafé, M.; Fischer, C.; Albisu, L.M.; García-Barreda, S.; et al. Las Trufas y Las Turmas. In Los Productos Forestales No Madereros en España: Del Monte a La Industria; Sánchez-González, M., Calama, R., Bonet, J.A., Eds.; INIA, Ministerio de Economía Industria y Competitividad: Madrid, Spain, 2020; pp. 283–324. ISBN 9788474985856. [Google Scholar]
- Arenas, F.; Navarro-Ródenas, A.; Chávez, D.; Gutiérrez, A.; Pérez-Gilabert, M.; Morte, A. Mycelium of Terfezia claveryi as Inoculum Source to Produce Desert Truffle Mycorrhizal Plants. Mycorrhiza 2018, 28, 691–701. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Thornton, B.; Basu, C. Rapid and Simple Method of QPCR Primer Design. In PCR Primer Design. Methods in Molecular Biology; Basu, C., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2015; Volume 1275, pp. 173–179. [Google Scholar]
- Rodríguez, A.; Rodríguez, M.; Córdoba, J.J.; Andrade, M.J. Design of Primers and Probes for Quantitative Real-Time PCR Methods. In PCR Primer Design. Methods in Molecular Biology; Basu, C., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2015; Volume 1275, pp. 31–56. [Google Scholar]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Morgulis, A.; Coulouris, G.; Raytselis, Y.; Madden, T.L.; Agarwala, R.; Schäffer, A.A. Database Indexing for Production MegaBLAST Searches. Bioinformatics 2008, 24, 1757–1764. [Google Scholar] [CrossRef]
- Bonito, G. Fast DNA-Based Identification of the Black Truffle Tuber melanosporum with Direct PCR and Species-Specific Primers. FEMS Microbiol. Lett. 2009, 301, 171–175. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications.; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS Primers with Enhanced Specificity for Basidiomycetes-Application to the Identification of Mycorrhizae and Rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Kralik, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2021. Available online: https://Www.R-Project.Org/ (accessed on 20 May 2022).
- Ogle, D.H.; Doll, J.C.; Wheeler, P.; Dinno, A.; FSA: Fisheries Stock Analysis. R Package Version 0.9.3. 2022. Available online: https://Github.Com/FishR-Core-Team/FSA (accessed on 20 May 2022).
- Nilsson, R.H.; Kristiansson, E.; Ryberg, M.; Hallenberg, N.; Larsson, K.H. Intraspecific ITS Variability in the Kingdom Fungi as Expressed in the International Sequence Databases and Its Implications for Molecular Species Identification. Evol. Bioinform. 2008, 4, EBO-S653. [Google Scholar] [CrossRef]
- Chemidlin Prévost-Bouré, N.; Christen, R.; Dequiedt, S.; Mougel, C.; Lelièvre, M.; Jolivet, C.; Shahbazkia, H.R.; Guillou, L.; Arrouays, D.; Ranjard, L. Validation and Application of a PCR Primer Set to Quantify Fungal Communities in the Soil Environment by Real-Time Quantitative PCR. PLoS ONE 2011, 6, e24166. [Google Scholar] [CrossRef]
- Singh, A.; Pandey, G.K. Primer Design Using Primer Express ® for SYBR Green- Based Quantitative PCR. In PCR Primer Design. Methods in Molecular Biology; Basu, C., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2015; Volume 1275, pp. 153–164. [Google Scholar]
- Bordallo, J.J.; Rodríguez, A.; Muñoz-Mohedano, J.M.; Suz, L.M.; Honrubia, M.; Morte, A. Five New Terfezia Species from the Iberian Peninsula. Mycotaxon 2013, 124, 189–208. [Google Scholar] [CrossRef]
- Hall, I.R.; Zambonelli, A.; Wang, Y. The Cultivation of Mycorrhizal Mushrooms-Success and Failure. In Proceedings of the Internation Conference on Mushroom Biology and Mushroom Products, Nantong, China, 18–21 June 2009. [Google Scholar]
- Landeweert, R.; Veenman, C.; Kuyper, T.W.; Fritze, H.; Wernars, K.; Smit, E. Quantification of Ectomycorrhizal Mycelium in Soil by Real-Time PCR Compared to Conventional Quantification Techniques. FEMS Microbiol. Ecol. 2003, 45, 283–292. [Google Scholar] [CrossRef]
- Johnson, G.; Nolan, T.; Bustin, S.A. Real-Time Quantitative PCR, Pathogen Detection and MIQE. Methods Mol. Biol. 2013, 943, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.; Huggett, J. QPCR Primer Design Revisited. Biomol. Detect. Quantif. 2017, 14, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Tajadini, M.; Panjehpour, M.; Javanmard, S. Comparison of SYBR Green and TaqMan Methods in Quantitative Real-Time Polymerase Chain Reaction Analysis of Four Adenosine Receptor Subtypes. Adv. Biomed. Res. 2014, 3, 85. [Google Scholar] [CrossRef]
- Marqués-Gálvez, J.E.; Miyauchi, S.; Paolocci, F.; Navarro-Ródenas, A.; Arenas, F.; Pérez-Gilabert, M.; Morin, E.; Auer, L.; Barry, K.W.; Kuo, A.; et al. Desert Truffle Genomes Reveal Their Reproductive Modes and New Insights into Plant–Fungal Interaction and Ectendomycorrhizal Lifestyle. New Phytol. 2021, 229, 2917–2932. [Google Scholar] [CrossRef]
- Arenas, F.; López-García, Á.; Berná, L.M.; Morte, A.; Navarro-Ródenas, A. Desert Truffle Mycorrhizosphere Harbors Organic Acid Releasing Plant Growth–Promoting Rhizobacteria, Essentially during the Truffle Fruiting Season. Mycorrhiza 2022, 32, 193–202. [Google Scholar] [CrossRef]
- Selosse, M.A.; Schneider-Maunoury, L.; Taschen, E.; Rousset, F.; Richard, F. Black Truffle, a Hermaphrodite with Forced Unisexual Behaviour. Trends Microbiol. 2017, 25, 784–787. [Google Scholar] [CrossRef]
- Martin, F.; Kohler, A.; Murat, C.; Balestrini, R.; Coutinho, P.M.; Jaillon, O.; Montanini, B.; Morin, E.; Noel, B.; Percudani, R.; et al. Périgord Black Truffle Genome Uncovers Evolutionary Origins and Mechanisms of Symbiosis. Nature 2010, 464, 1033–1038. [Google Scholar] [CrossRef]
- Chen, J.; De la Varga, H.; Todesco, F.; Beacco, P.; Martino, E.; Le Tacon, F.; Murat, C. Frequency of the Two Mating Types in the Soil under Productive and Non-Productive Trees in Five French Orchards of the Périgord Black Truffle (Tuber melanosporum Vittad.). Mycorrhiza 2021, 31, 361–369. [Google Scholar] [CrossRef]
- Zampieri, E.; Rizzello, R.; Bonfante, P.; Mello, A. The Detection of Mating Type Genes of Tuber melanosporum in Productive and Non Productive Soils. Appl. Soil Ecol. 2012, 57, 9–15. [Google Scholar] [CrossRef]
- Splivallo, R.; Vahdatzadeh, M.; MacIá-Vicente, J.G.; Molinier, V.; Peter, M.; Egli, S.; Uroz, S.; Paolocci, F.; Deveau, A. Orchard Conditions and Fruiting Body Characteristics Drive the Microbiome of the Black Truffle Tuber aestivum. Front. Microbiol. 2019, 10, 1437. [Google Scholar] [CrossRef]
- Oliach, D.; Colinas, C.; Castaño, C.; Fischer, C.R.; Bolaño, F.; Bonet, J.A.; Oliva, J. The Influence of Forest Surroundings on the Soil Fungal Community of Black Truffle (Tuber melanosporum) Plantations. For. Ecol. Manag. 2020, 469, 118119. [Google Scholar] [CrossRef]
- Olivera, A.; Fischer, C.R.; Bonet, J.A.; de Aragón, J.M.; Oliach, D.; Colinas, C. Weed Management and Irrigation Are Key Treatments in Emerging Black Truffle (Tuber melanosporum) Cultivation. New For. 2011, 42, 227–239. [Google Scholar] [CrossRef]
- Piñuela, Y.; Alday, J.G.; Oliach, D.; Castaño, C.; Bolaño, F.; Colinas, C.; Bonet, J.A. White Mulch and Irrigation Increase Black Truffle Soil Mycelium When Competing with Summer Truffle in Young Truffle Orchards. Mycorrhiza 2021, 31, 371–382. [Google Scholar] [CrossRef]
- Fischer, C.; Oliach, D.; Bonet, A.; Colinas, C. Best Practices for Cultivation of Truffles; Forest Sciences Centre of Catalonia: Solsona, Spain; Yaşama Dair Vakıf: Antalaya, Turkey, 2017; ISBN 978-84-697-8163-0. [Google Scholar]

| Taxon | Specimen ID 1 | GenBank Accession Number |
|---|---|---|
| Terfezia albida Ant. Rodr., Muñoz-Mohedano & Bordallo | j574 | OP458226 |
| Terfezia eliocrocae Bordallo, Morte & Honrubia | j579 | OP458228 |
| Terfezia olbiensis (Tul. & C. Tul.) Sacc. | j588 | OP458229 |
| Terfezia claveryi Chatin | j592 | OP458224 |
| Terfezia claveryi Chatin | j596 | OP458223 |
| Terfezia claveryi Chatin | j597 | OP458222 |
| Terfezia claveryi Chatin | j216 | OP458220 |
| Terfezia claveryi Chatin | j73 | OP458219 |
| Terfezia crassiverrucosa Zitouni-Haouar, G. Moreno, Manjón, Fortas, & Carlavilla | j53 | OP458218 |
| Terfezia crassiverrucosa Zitouni-Haouar, G. Moreno, Manjón, Fortas, & Carlavilla | j235 | OP458221 |
| Tirmania pinoyi (Maire) Malençon | j601 | MG920185.1 |
| Tirmania nivea (Desf.) Trappe | j590 | OP458225 |
| Terfezia grisea Bordallo, V. Kaounas & Ant. Rodr. | j485 | KP189333 |
| Terfezia fanfani Mattir. | j484 | OP458230 |
| Terfezia pseudoleptoderma Bordallo, Ant. Rodr. & Muñoz-Mohedano | j478 | OP458231 |
| Terfezia arenaria (Moris) Trappe | j466 | OP458227 |
| Terfezia boudieri Chatin | j371 | OP458234 |
| Tirmania honrubiae Morte, Bordallo & Ant. Rodr. | j366 | OP458233 |
| Terfezia fanfani Mattir. | L14 | HM056219 |
| Terfezia extremadurensis Muñoz-Mohedano, Ant. Rodr. & Bordallo | j96 | OP458232 |
| Terfezia pini Bordallo, Ant. Rodr. & Muñoz-Mohedano | j151 | OP458235 |
| Picoa sp. Vittad. | j442 | OP458217 |
| Picoa sp. Vittad. | j17 | OP458215 |
| Picoa sp. Vittad. | j59 | OP458214 |
| Picoa sp. Vittad. | j41 | OP458213 |
| Picoa sp. Vittad. | j45 | OP458212 |
| Picoa sp. Vittad. | j20 | OP458216 |
| Geopora sp. Harkn. | R21b | OP458210 |
| Geopora sp. Harkn. | R23 | OP458209 |
| Geopora sp. Harkn. | j121 | OP458211 |
| Primer Set | Sequence (5′ → 3′) | Length (nt) | Tm (°C) | GC (%) | Amplicon (nt) |
|---|---|---|---|---|---|
| TerclaF1 TerclaR1 | ATAGGGCATGCCTGTCTGAG | 20 | 60.0 | 55 | 106 |
| TGGAGGGCAACTTAATACACAGT | 23 | 59.2 | 43 | ||
| TerclaF2 TerclaR2 | TAACTGTGTATTAAGTTGCCCTCCAG | 26 | 59.0 | 42 | 120 |
| GAGTTGAGGCAAGTACAATCAATCATAC | 28 | 59.2 | 39 | ||
| TerclaF3 TerclaR1 | GCTCCCCCTCACTCAAGTAT | 20 | 59.1 | 55 | 79 |
| TGGAGGGCAACTTAATACACAGT | 23 | 59.2 | 43 |
| Variable | Samples (N) | Mean Fungal Biomass (Mg Mycelium/g Soil) | SD | Significance Level (p-Value < 0.05) |
|---|---|---|---|---|
| Year 2016 | 18 | 0.386 | 0.350 | a |
| Year 2018 | 12 | 0.574 | 0.684 | a |
| Year 2019 | 6 | 0.577 | 0.451 | a |
| SP-1 | 5 | 0.394 | 0.474 | ab |
| SP-2 | 6 | 0.142 | 0.215 | a |
| SP-3 | 6 | 0.588 | 0.792 | ab |
| SP-4 | 6 | 0.358 | 0.214 | ab |
| SP-5 | 6 | 0.700 | 0.592 | b |
| SP-6 | 6 | 0.695 | 0.383 | b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arenas, F.; Morte, A.; Navarro-Ródenas, A. Design and Validation of qPCR-Specific Primers for Quantification of the Marketed Terfezia claveryi and Terfezia crassiverrucosa in Soil. J. Fungi 2022, 8, 1095. https://doi.org/10.3390/jof8101095
Arenas F, Morte A, Navarro-Ródenas A. Design and Validation of qPCR-Specific Primers for Quantification of the Marketed Terfezia claveryi and Terfezia crassiverrucosa in Soil. Journal of Fungi. 2022; 8(10):1095. https://doi.org/10.3390/jof8101095
Chicago/Turabian StyleArenas, Francisco, Asunción Morte, and Alfonso Navarro-Ródenas. 2022. "Design and Validation of qPCR-Specific Primers for Quantification of the Marketed Terfezia claveryi and Terfezia crassiverrucosa in Soil" Journal of Fungi 8, no. 10: 1095. https://doi.org/10.3390/jof8101095
APA StyleArenas, F., Morte, A., & Navarro-Ródenas, A. (2022). Design and Validation of qPCR-Specific Primers for Quantification of the Marketed Terfezia claveryi and Terfezia crassiverrucosa in Soil. Journal of Fungi, 8(10), 1095. https://doi.org/10.3390/jof8101095







