Novel and Investigational Treatments for Onychomycosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Novel Topical Antifungal Agents
2.2. Antifungal Drugs under Investigation
3. New Topical Agents for Onychomycosis
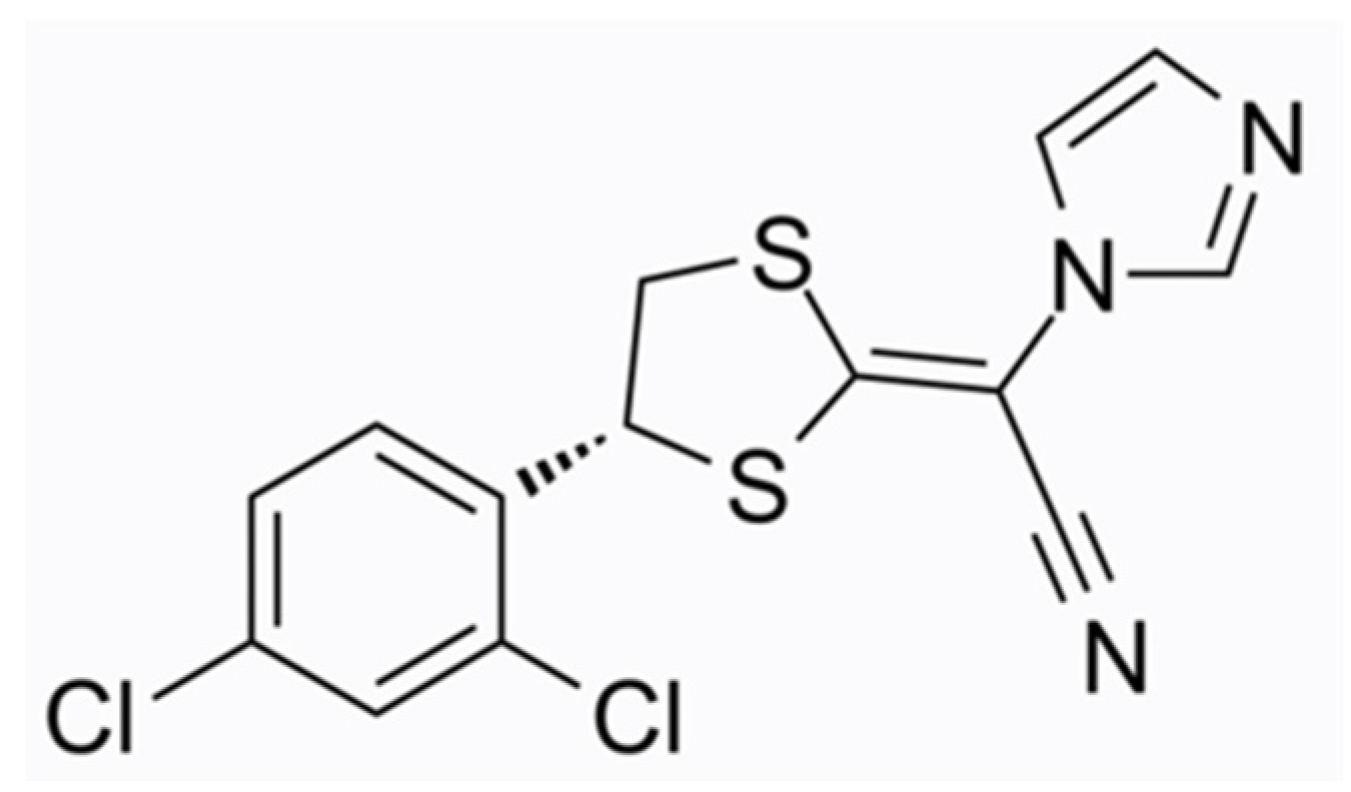
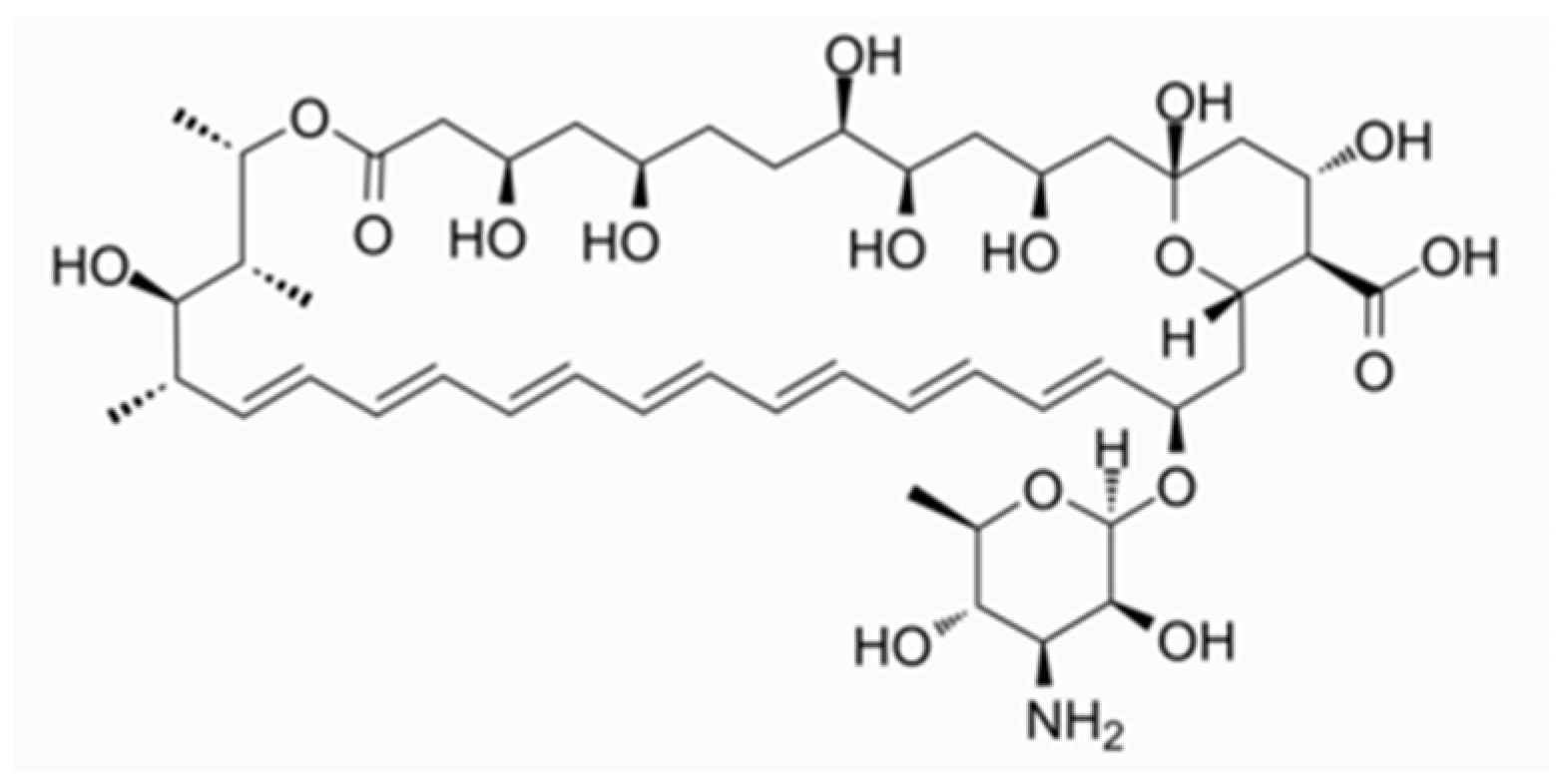
3.1. Efinaconazole
3.2. Tavaborole
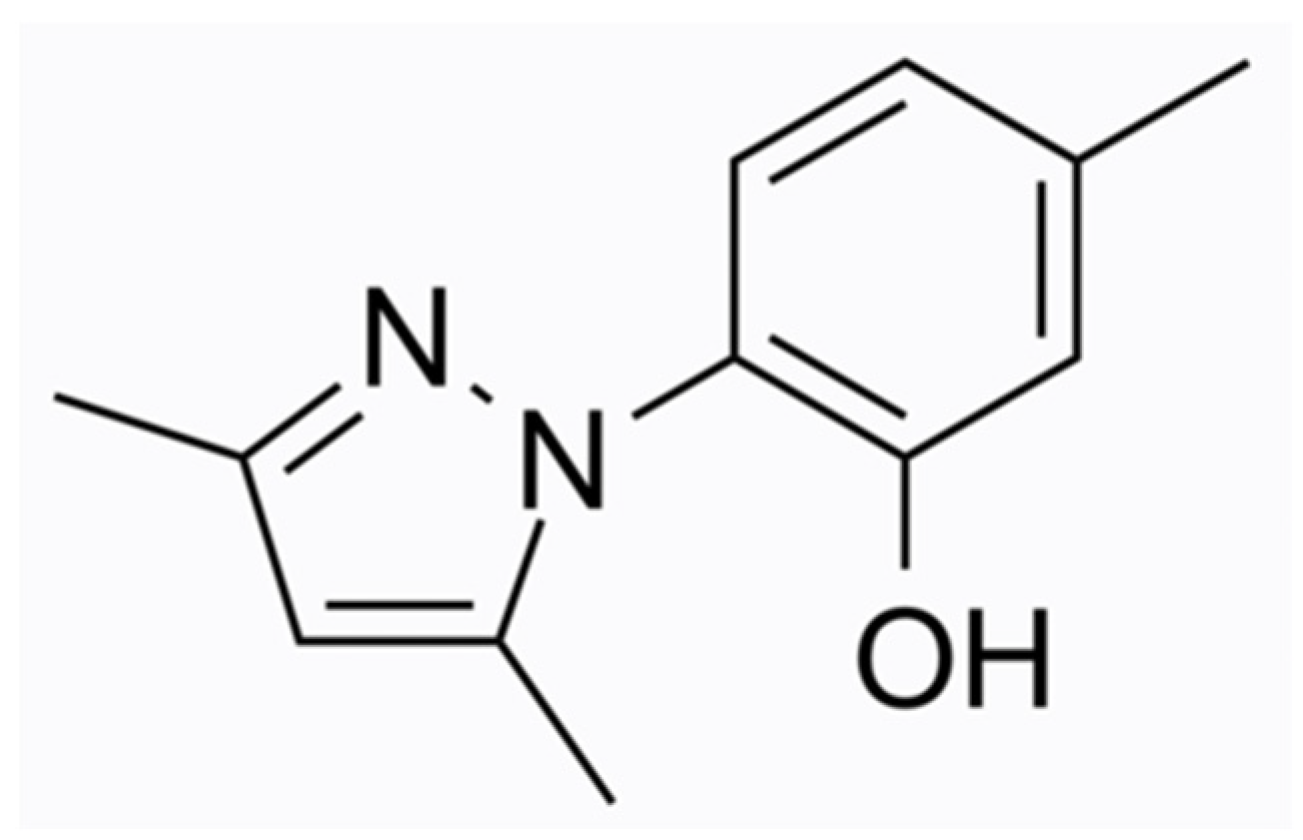
3.3. Luliconazole
4. Topical Antifungal Nail Therapies under Development and Investigation
4.1. ME-1111
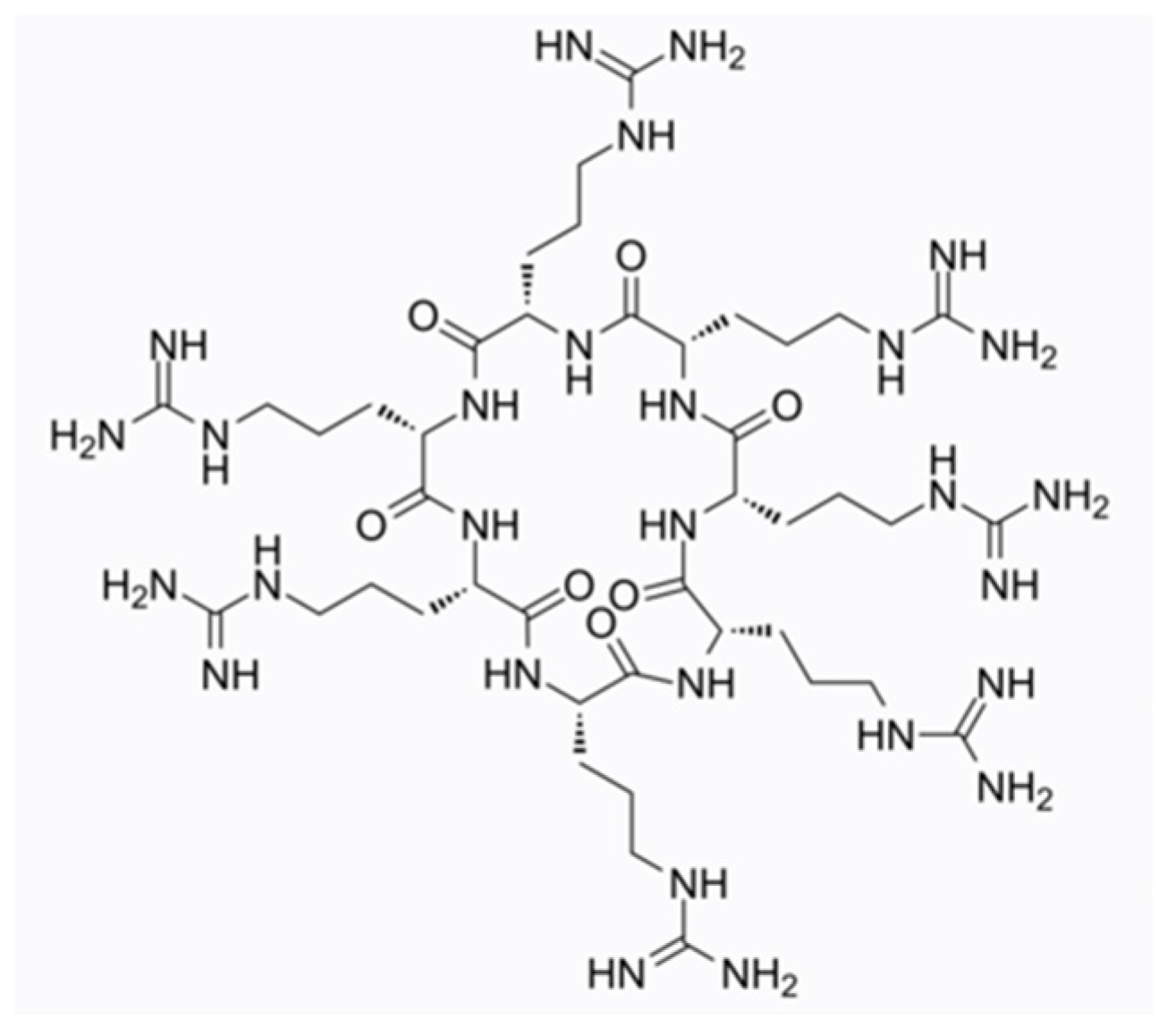
4.2. NP213
4.3. Topical Terbinafine
4.4. Topical Amphotericin B
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piraccini, B.M.; Starace, M.; Rubin, A.I.; Di Chiacchio, N.G.; Iorizzo, M.; Rigopoulos, D. Onychomycosis: Recommendations for Diagnosis, Assessment of Treatment Efficacy, and Specialist Referral. The CONSONANCE Consensus Project. Dermatol. Ther. 2022, 12, 885–898. [Google Scholar] [CrossRef]
- Siguregeirsson, B.; Baran, R. The prevalence of onychomycosis in the global population: A literature study. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Mays, R.R.; Versteeg, S.G.; Piraccini, B.M.; Takwale, A.; Shemer, A.; Babaev, M.; Grover, C.; di Chiacchio, N.G.; Taborda, P.R.O.; et al. Global perspectives for the management of onychomycosis. Int. J. Dermatol. 2019, 58, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Razavyoon, T.; Hashemi, S.J.; Mansouri, P.; Rafat, Z.; Saboor-Yaraghi, A.A.; Sarvestani, H.K.; Ghasemi, Z. The epidemiology and etiology of onychomycosis in 2 laboratory centers affiliated to Tehran university of medical sciences during 2019–2020. Iran. J. Microbiol. 2022, 14, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Zaias, N.; Tosti, A.; Rebel, G.; Morelli, R.; Bardazzi, F.; Bieley, H.; Zaiac, M.; Glick, B.; Paley, B.; Allevato, M.; et al. Autosomal dominant pattern of distal subungual onychomycosis caused by Trichophyton rubrum. J. Am. Acad. Dermatol. 1996, 34 Pt 1, 302–304. [Google Scholar] [CrossRef]
- Gupta, A.K.; Summerbell, R.C.; Venkataraman, M.; Quinlan, E.M. Nondermatophyte mold onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1628–1641. [Google Scholar] [CrossRef]
- Zaias, N.; Rebell, G.; Casal, G.; Appel, J. The asymmetric gait toenail unit sign. Skinmed 2012, 10, 213–217. [Google Scholar]
- Zaias, N.; Escovar, S.X.; Rebell, G. Opportunistic toenail onychomycosis. The fungal colonization of an available nail unit space by non-dermatophytes is produced by the trauma of the closed shoe by an asymmetric gait or other trauma. A plausible theory. Skinmed 2012, 10, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Vilhais-Neto, G.C.; Maruhashi, M.; Smith, K.T.; Vasseur-Cognet, M.; Peterson, A.S.; Workman, J.L.; Pourquie, O. Rere controls retinoic acid signalling and somite bilateral symmetry. Nature 2010, 463, 953–957. [Google Scholar] [CrossRef]
- Haghani, I.; Shams-Ghahfarokhi, M.; Asl, A.D.; Shokohi, T.; Hedayati, M.T. Prevalence, genetic diversity and antifungal susceptibility profiles of F. fujikuroi, F. solani and Fusarium incarnatum-equiseti species complexes from onychomycosis in north of Iran. Mycoses 2022, 65, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Piraccini, B.M.; Sisti, A.; Tosti, A. Long-term follow-up of toenail onychomycosis caused by dermatophytes after successful treatment with systemic antifungal agents. J. Am. Acad. Dermatol. 2010, 62, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Sigurgeirsson, B.; Olafsson, J.H.; Steinsson, J.R.; Paul, C.; Billstein, S.; Evans, E.G.V. Long-term effectiveness of treatment with terbinafine vs. itraconazole in onychomycosis: A 5-year blinded prospective follow-up study. Arch. Dermatol. 2002, 138, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Tosti, A.; Piraccini, B.M.; Stinchi, C.; Colombo, M.D. Relapses of onychomycosis after successful treatment with systemic antifungals: A three-year follow-up. Dermatology 1998, 197, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Tschen, E.H.; Bucko, A.D.; Oizumi, N.; Kawabata, H.; Olin, J.T.; Pillai, R. Efinaconazole solution in the treatment of toenail onychomycosis: A phase 2, multicenter, randomized, double-blind study. J. Drugs Dermatol. 2013, 12, 186–192. [Google Scholar] [PubMed]
- Elewski, B.E.; Rich, P.; Pollak, R.; Pariser, D.M.; Watanabe, S.; Senda, H.; Ieda, C.; Smith, K.; Pillai, R.; Ramakrishna, T.; et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: Two phase III multicenter, randomized, double-blind studies. J. Am. Acad. Dermatol. 2013, 68, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Cooper, E.A. Long-term Efficacy and Safety of Once-daily Efinaconazole 10% Topical Solution (Jublia) for Dermatophyte Toenail Onychomycosis: An Interim Analysis. Ski. Ther. Lett. 2021, 26, 5–10. [Google Scholar] [CrossRef]
- Toledo-Bahena, M.E.; Bucko, A.; Ocampo-Candiani, J.; Herz-Ruelas, M.E.; Jones, T.M.; Jarratt, M.T.; Pollak, R.A.; Zane, L.T. The efficacy and safety of tavaborole, a novel, boron-based pharmaceutical agent: Phase 2 studies conducted for the topical treatment of toenail onychomycosis. J. Drugs Dermatol. 2014, 13, 1124–1132. [Google Scholar] [PubMed]
- Elewski, B.E.; Aly, R.; Baldwin, S.L.; Soto, R.F.G.; Rich, P.; Wiltz, M.W.H.; Zane, L.T.; Pollak, R. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: Results from 2 randomized phase-III studies. J. Am. Acad. Dermatol. 2015, 73, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Hall, S.; Zane, L.T.; Lipner, S.R.; Rich, P. Evaluation of the efficacy and safety of tavaborole topical solution, 5%, in the treatment of onychomycosis of the toenail in adults: A pooled analysis of an 8-week, post-study follow-up from two randomized phase 3 studies. J. Dermatol. Treat. 2018, 29, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Kishida, H.; Okubo, A. Efficacy and safety of luliconazole 5% nail solution for the treatment of onychomycosis: A multicenter, double-blind, randomized phase III study. J. Dermatol. 2017, 44, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.K.; Robertson, J.C.; Miller, L.; Stewart, C.S.; O’Neil, D.A. NP213 (Novexatin®): A unique therapy candidate for onychomycosis with a differentiated safety and efficacy profile. Med. Mycol. 2020, 58, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Surprenant, M.S.; Kempers, S.E.; Pariser, D.M.; Rensfeldt, K.; Tavakkol, A. Efficacy and safety of topical terbinafine 10% solution (MOB-015) in the treatment of mild to moderate distal subungual onychomycosis: A randomized, multicenter, double-blind, vehicle-controlled phase 3 study. J. Am. Acad. Dermatol. 2021, 85, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Baran, R.; Mailland, F.; Friscenda, L.; Caserini, M. An innovative terbinafine transungual solution (P-3058): A phase 2b dose finding study in patients with mild to moderate onychomycosis. J. Am. Acad. Dermatol. 2014, 70, AB88. [Google Scholar]
- Leeyaphan, C.; Suiwongsa, B.; Komesmuneeborirak, P.; Kiratiwongwan, R.; Wongdama, S.; Prasong, W.; Supcharoenkul, S.; Bunyaratavej, S. Effectiveness and safety of topical amphotericin B in 30% dimethyl sulfoxide cream versus 30% dimethyl sulfoxide cream for nondermatophyte onychomycosis treatment: A pilot study. Indian J. Dermatol. Venereol. Leprol. 2022, 88, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Talukder, M. Efinaconazole in onychomycosis. Am. J. Clin. Dermatol. 2022, 23, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Mazzantini, D.; Tampucci, S.; Vecchione, A.; Celandroni, F.; Burgalassi, S.; Ghelardi, E. Ciclopirox and Efinaconazole Transungual Permeation, Antifungal Activity, and Proficiency To Induce Resistance in Trichophyton rubrum. Antimicrob. Agents Chemother. 2019, 63, e00442-19. [Google Scholar] [CrossRef]
- Shimoyama, H.; Kuwano, Y.; Sei, Y. Retrospective survey of treatment outcomes of eficonmazole 10% solution and luliconazole 5% solution for onychomycosis in our facility. Med. Mycol. J. 2019, 60, 95–100. [Google Scholar] [CrossRef]
- Hui, X.; Baker, S.J.; Wester, R.C.; Barbadillo, S.; Cashmore, A.K.; Sanders, V.; Hold, K.M.; Akama, T.; Zhang, Y.K.; Plattner, J.J.; et al. In vitro penetration of a novel oxaborole antifungal (AN2690) into the human nail plate. J. Pharm. Sci. 2007, 96, 2622–2631. [Google Scholar] [CrossRef]
- Gupta, A.K.; Versteeg, S. Tavaborole—A treatment for onychomycosis of the toenails. Expert Rev. Clin. Pharmacol. 2016, 9, 1145–1152. [Google Scholar] [CrossRef]
- Mazzantini, D.; Celandroni, F.; Calvigioni, M.; Lupetti, A.; Ghelardi, E. In Vitro Resistance and Evolution of Resistance to Tavaborole in Trichophyton rubrum. Antimicrob. Agents Chemother. 2021, 65, e02324-20. [Google Scholar] [CrossRef]
- Abastabar, M.; Haghani, I.; Shokohi, T.; Hedayati, M.T.; Aghili, S.R.; Jedi, A.; Dadashi, S.; Shabanzadeh, S.; Hosseini, T.; Aslani, N.; et al. Low in vitro antifungal activity of tavaborole against yeasts and moulds from onychomycosis. Antimicrob. Agents Chemother. 2018, 62, e01632-18. [Google Scholar] [CrossRef]
- Gupta, A.K.; Cvetkovic, D.; Abramovits, W.; Vincent, K.D. LUZU (luliconazole) 1% cream. Skinmed 2014, 12, 90–93. [Google Scholar] [PubMed]
- Shimamura, T.; Miyamae, A.; Arai, M.; Minemura, A.; Nozawa, A.; Kubota, N. Distribution of luliconazole in nail plate by in vitro permeation and efficacy by zone of inhibition test after treatment of luliconazole nail solution. Med. Mycol. J. 2016, 57, J19–J25. [Google Scholar] [CrossRef] [PubMed]
- Takahata, S.; Kubota, N.; Takei-Masuda, N.; Yamada, T.; Maeda, M.; Alshahni, M.M.; Abe, S.; Tabata, Y.; Maebashi, K. Mechanism of Action of ME1111, a Novel Antifungal Agent for Topical Treatment of Onychomycosis. Antimicrob. Agents Chemother. 2015, 60, 873–880. [Google Scholar] [CrossRef]
- Kubota-Ishida, N.; Takei-Masuda, N.; Kaneda, K.; Nagira, Y.; Chikada, T.; Nomoto, M.; Tabata, Y.; Takahata, S.; Maebashi, K.; Hui, X.; et al. In Vitro Human Onychopharmacokinetic and Pharmacodynamic Analyses of ME1111, a New Topical Agent for Onychomycosis. Antimicrob. Agents Chemother. 2018, 62, e00779-17. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Jung, E.C.; Zhu, H.; Maibach, H.I. Antifungal ME1111 In Vitro Human Onychopharmacokinetics. Drug Dev. Ind. Pharm. 2017, 43, 22–29. [Google Scholar] [CrossRef]
- Mercer, D.K.; Stewart, C.S.; Miller, L.; Robertson, J.C.; Duncan, V.M.S.; O’Neil, D.A. Improved Methods for Assessing Therapeutic Potential of Antifungal Agents against Dermatophytes and Their Application in the Development of NP213, a Novel Onychomycosis Therapy Candidate. Antimicrob. Agents Chemother. 2019, 63, e02117-18. [Google Scholar] [CrossRef]
- Drake, L.A.; Shear, N.H.; Arlette, J.R.; Cloutier, R.; Danby, F.W.; Elewski, B.E.; Garnis-Jones, S.; Giroux, J.M.; Gratton, D.; Gulliver, W.; et al. Oral terbinafine in the treatment of toenail onychomycosis: North American multicenter trial. J. Am. Acad. Dermatol. 1997, 37 Pt 1, 740–745. [Google Scholar] [CrossRef]
- Faergemann, J. An open, single center pilot study of efficacy and safety of topical MOB015B in the treatment of distal subungual onychomycosis. J. Am. Acad. Dermatol. 2015, 72, AB132. [Google Scholar]
- Fuhr, R.; Cook, D.; Ridden, J.; Nield, K.; Leigh, E.; Cook, J.; Davies-Strickleton, H.; Dobmeyer, J. Results from a Phase 1/2 trial of BB2603, a terbinafine-based topical nano-formulation, in onychomycosis and tinea pedis. Mycoses 2022, 65, 661–669. [Google Scholar] [CrossRef]
- Krawczyk-Santos, A.P.; Marreto, R.N.; Concheiro, A.; Alvarez-Lorenzo, C.; Taveira, S.F. Poly(pseudo)rotaxanes formed by mixed micelles and α-cyclodextrin enhance terbinafine nail permeation to deeper layers. Int. J. Pharm. X 2022, 4, 100118. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, K.; Capriotti, J.A. Dimethyl sulfoxide: History, chemistry, and clinical utility in dermatology. J. Clin. Aesthetic Dermatol. 2012, 5, 24–26. [Google Scholar]
- Randhawa, M.A. Dimethyl sulfoxide (DMSO) inhibits the germination of Candida albicans and the arthrospores of Trichophyton mentagrophytes. Nippon Ishinkin Gakkai Zasshi 2008, 49, 125–128. [Google Scholar] [CrossRef]
- Souza, A.M.S.; Ribeiro, R.C.A.; Pinheiro, G.K.L.O.; Pinheiro, F.I.; Oliveira, W.N.; Souza, L.B.F.C.; Silva, A.L.; Amaral-Machado, L.; Alencar, É.; Chaves, G.M.; et al. Polishing the Therapy of Onychomycosis Induced by Candida spp.: Amphotericin B-Loaded Nail Lacquer. Pharmaceutics 2021, 13, 784. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahi, S.A.; Fattahi, A.; Naeimifar, A.; Lotfali, E.; Firooz, A.; Khamesipoor, A.; Skandari, S.E.; Mohammadi, A.M. The in vitro effect of nanoliposomal amphotericin B against two clinically important dermatophytes. Int. J. Dermatol. 2022, 61, 383–389. [Google Scholar] [CrossRef]
- Rigopoulos, D.; Papanagiotou, V.R.D., 3rd; Piraccini, B.M. Onychomycosis in patients with nail psoriasis: A point to point discussion. Mycoses 2017, 60, 6–10. [Google Scholar] [CrossRef]
- Rosen, T.; Friedlander, F.S.; Kircik, L.; Zirwas, M.J.; Gold, L.S.; Bhatia, N.; Gupta, A.K. Onychomycosis: Epidemiology, diagnosis, and treatment in a changing landscape. J. Drugs Dermatol. 2015, 14, 223–228. [Google Scholar]
- Baran, R. The nail in the elderly. Clin. Dermatol. 2011, 29, 54–60. [Google Scholar] [CrossRef]
- Fukunaga, A.; Washio, K.; Ogura, K.; Taguchi, K.; Chiyomaru, K.; Ohno, Y.; Masaki, T.; Nagai, H.; Nagano, T.; Oka, M.; et al. Onychomycosis as a warning sign for peripheral arterial disease. Acta Derm. Venereol. 2013, 93, 747–748. [Google Scholar] [CrossRef]
- Ameen, M.; Lear, J.T.; Madan, V.; Mustapa, M.F.M.; Richardson, M. British Association of Dermatologists’ guidelines for the management of onychomycosis 2014. Br. J. Dermatol. 2014, 171, 937–958. [Google Scholar] [CrossRef]
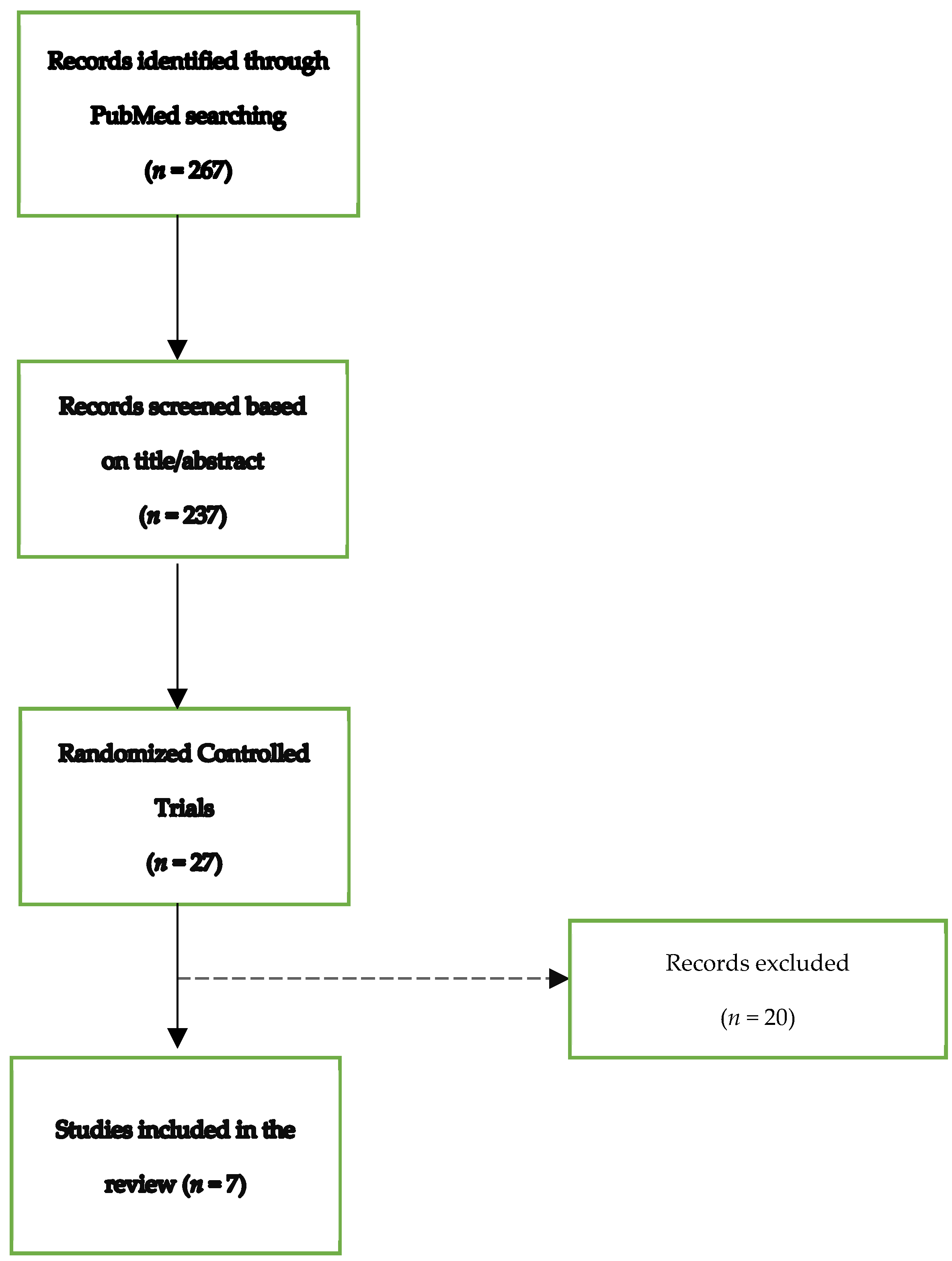



| Search Terms (PubMed) | Results * |
|---|---|
| Onychomycosis | 4101 |
| Efinaconazole | 156 |
| Efinaconazole [Title/Abstract] AND onychomycosis [Title/Abstract] | 121 |
| Tavaborole | 83 |
| Tavaborole [Title/Abstract] AND onychomycosis [Title/Abstract] | 67 |
| Luliconazole | 102 |
| Luliconazole [Title/Abstract] AND onychomycosis [Title/Abstract] | 29 |
| Onychomycosis [Title/Abstract] AND topical [Title/Abstract] AND antifungal [Title/Abstract] | 333 |
| Onychomycosis [Title/Abstract] AND Efinaconazole [Title/Abstract] OR Tavaborole [Title/Abstract] OR Luliconazole[Title/Abstract] | 267 |
| Onychomycosis [Title/Abstract] AND Efinaconazole [Title/Abstract] | 237 |
| OR Tavaborole [Title/Abstract] OR Luliconazole [Title/Abstract] |
| Study (Recruitment Status) | Phase | Study Group | Intervention Study Arms (Application) | Primary Endpoint (Timeframe or Timepoint) |
|---|---|---|---|---|
| Efinaconazole | ||||
| EH Tche, et al. (2013) [14] NCT00777868 (Completed) | II | Adults mild to moderate toenail DLSO (n = 135) | • IDP-108 10% sol * • IDP-108 10% sol • IDP-108 5% sol • Vehicle (one daily application for 36 weeks) | Change from baseline in area of infected nail (time frame: 9 months) |
| BE Elewski et al. (2013) [15] NCT01007708 NCT01008033 (Completed) | III (two identical multicenter parallel-group studies) | Adults with DLSO (20–50% clinical incolvement), without dermatophytomas or lunula involvement (n = 870, n = 785) | • Efinaconazole 10% sol • Vehicle (one daily application for 48 weeks) | Percentage of patients who achieve clinical cure (time frame: 52 weeks) |
| AK Gupta et al. (2021) [16] (Unknown) | IV (single-site) | Adults with mild to moderate DLSO (20–50% involvement) in a TGT (n = 101, interim analysis of 47 patients at mo 24) | • Efinaconazole 10% (24 months) • Vehicle (6 mo) followed by Efinaconazole 10% sol (18 months) (one daily application) | • Mycological cure • Effective cure i.e., mycological cure and ≤10% clinical involvement (at week 24) |
| Tavaborole (AΝ2690) | ||||
| ME Toledo-Bahena et al. (2014) [17] NCT00679965 (Completed) | II | Adults with mild to moderate (20–60% involvement) onychomycosis of at least one TGT (n = 187) | • AN2690 2.5% sol • AN2690 5% sol • AN2690 7.5% sol • Vehicle (one daily application for 3 months; then 3 times a week for 3 months) | Clinical evidence of complete great toenail clearance or at least fungal-clear great toenail growth (“complete” = 5 mm; “partial” = 2 mm), plus a negative culture from the treatment-TGT (time frame: 180 days) |
| BE Elewski et al. (2015) [18] NCT01270971 NCT01302119 (Completed) | III (two identical multicenter parallel-group trials) | Adults with mild to moderate (20–60%) DLSO of the TGT (n = 594 and n = 604) | • Tavaborole 5% sol • Vehicle (one daily application for 48 weeks) | Completely clear nail and negative mycology of the TGT (at week 52) |
| AK Gupta et al. (2018) [19] | PSFU (III) | Study group described in Elewski et al. (2015). Analysis of FU at week 60 (post week 52) | • Complete cure of the TGT at week 52 vs. week 60 (completely clear nail and negative mycology) • ASRs and AEs | |
| Luliconazole (SKX-16) | ||||
| S Watanabe et al. (2017) [20] (Completed) | III | Adults (20–80 years old) with DLSO (20–50% clinical involvement) of the TGT | • Luliconazole 5% solution • Vehicle (one daily application for 48 weeks) | Complete cure rate i.e., 0% clinical involvement plus negative KOH direct microscopy |
| NCT01431820 ** (Completed) | IIB/III | Adults with mild to moderate DSO (n = 334) | • Luliconazole 10% sol; (two dosing regimens) • Vehicle; two control arms (one daily application for 52 weeks) | The proportion of subjects who achieve complete cure of the TGT (time frame: week 52) |
| Study (Recruitment Status) | Phase | Study Group | Intervention Study Arms (Application) | Primary Endpoint |
|---|---|---|---|---|
| ME-1111 | ||||
| NCT01841996 * (Completed) | I | Adults with moderate to severe DSO of the TGT (n = 24) | • ME-1111 solution • Vehicle (one daily application for 28 days) | AEs, application site, plasma concentration, urinary excretion (time frame: baseline to day 57) |
| NCT02022215 * (Completed) | II | Adults with mild to moderate DLSO (n = 304) | • ME-1111 solution, low strength • ME-1111 solution, high strength • Vehicle (one daily application for 48 weeks) | Complete cure (0% clinical involvement of the TGT and mycological cure) at week 52 |
| NP213 or Novexatin or NVXT | ||||
| NCT02343627 DK Mercer et al. (2020) [21] (Completed) | II | Adults with mild to moderate onychomycosis ** of at least one toe involving 10–50% of the nail (n = 47) | • NVXT solution • Vehicle (one daily application on the infected nail and 0.5 mm of adjacent skin for 60 days) | Negative culture after application for 28 days |
| NCT02933879 * (Completed) | II | Adults with onychomycosis of the TGT (n = 184) | • NVXT solution Group A once daily application for 8 weeks; Group B, two 8-week treatment periods separated by a 32-week rest interval • Vehicle Group C, application as per Group B) | Complete cure (clinical and mycological cure) at day 365 |
| Terbinafine | ||||
| NCT02859519 AK Gupta et al. [22] (Completed) | III | Subjects (12–75 years old) with DSO affecting 20–60% of the TGT (n = 365) | • MOB015B 10% sol (Terbinafine HCl) • Vehicle (one daily application for 48 weeks at bedtime) | Complete cure (0% clinical disease and negative mycology) at week 52 |
| NCT02866032 * (Completed) | III | Adults with DSO affecting 20–60% of the TGT (n = 452) | • MOB015B solution • Ciclopirox 80 mg/g (one daily application for 48 weeks) | Complete cure (0% clinical disease and mycological cure) |
| NCT05279846 * (Recruiting) | III | Subjects (12–75 y old) with DSO affecting 20–60% of the TGT | • MOB015B solution • Placebo (one daily application at bedtime for 8 weeks and then once weekly for 40 weeks) | • Complete cure of the TGT at week 52 • AEs (time frame: 52 weeks) |
| EudraCT No. 2008-002707-10 [23] (Completed) | IIB | Subjects (12–80 years old) with DSO affecting 25–60% of the distal nail plate *** | • P-3058 10% once daily • P-3058 5% once daily • P-3058 10% once weekly • Vehicle once daily • Vehicle once weekly (treatment for 52 weeks) | Responder Rate at the end of FU at week 76 (nail disease area ≤10% of the total plus negative mycology) |
| NCT02549001 * (EudraCT No. 2015-000561-31) (Completed) | III | Subjects (≥12 years old) with onychomycosis involving 20-50% of the TGT (n = 953) | • P-3058 10% solution • Vehicle of P-3058 • Loceryl® nail lacquer • (Amorolfine 5%) | Complete cure of the TGT (negative mycology and totally clear nail) |
| NCT04188574 * (Active, not recruiting) [EudraCT No. 2019-002098-68] (Ongoing) | II | Adults with DSO affecting 25–60% of the TGT | • BB2603-10 topical spray • (0.1% Terbinafine) • BB2603-3 topical spray • (0.03% Terbinafine) • BB2603-1 topical spray • (0.01% Terbinafine) • Vehicle • (BID for 12 weeks) | Early response for BB2603-10 vs. vehicle at week 16 (clear nail and negative culture) |
| Amphotericin B | ||||
| NCT03141840 * (Completed) | N/A | Adults with DSO of the TGT (≤50%) (n = 69) | • ABL01 • Vehicle • (once weekly for 6 months) | Clinical improvement (40% reduction in infected area) at 6 months) |
| NCT03814343 (Completed) C Leeyaphan et al. (2021) [24] | IV | Adults with non-dermatophyte onychomycosis (n = 19) | • Amphotericin B • in DMSO 30% • DMSO 30% (application of a pea-sized amount at bedtime and overnight wrapping for 36 weeks) | • Time to mycological cure • Evaluation by the patients with negative mycology |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregoriou, S.; Kyriazopoulou, M.; Tsiogka, A.; Rigopoulos, D. Novel and Investigational Treatments for Onychomycosis. J. Fungi 2022, 8, 1079. https://doi.org/10.3390/jof8101079
Gregoriou S, Kyriazopoulou M, Tsiogka A, Rigopoulos D. Novel and Investigational Treatments for Onychomycosis. Journal of Fungi. 2022; 8(10):1079. https://doi.org/10.3390/jof8101079
Chicago/Turabian StyleGregoriou, Stamatios, Maria Kyriazopoulou, Aikaterini Tsiogka, and Dimitrios Rigopoulos. 2022. "Novel and Investigational Treatments for Onychomycosis" Journal of Fungi 8, no. 10: 1079. https://doi.org/10.3390/jof8101079
APA StyleGregoriou, S., Kyriazopoulou, M., Tsiogka, A., & Rigopoulos, D. (2022). Novel and Investigational Treatments for Onychomycosis. Journal of Fungi, 8(10), 1079. https://doi.org/10.3390/jof8101079







