Effects of Purified β-Glucosidases from Issatchenkia terricola, Pichia kudriavzevii, Metschnikowia pulcherrima on the Flavor Complexity and Typicality of Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media
2.2. Laboratory-Scale Fermentation of Wine
2.3. Growth and Sugar Consumption Kinetics of S. cerevisiae
2.4. Analysis of Physicochemical Characteristics and Volatile Compounds in Wines
2.5. Sensory Evaluations of Wine
2.6. Data Analyses
3. Results and Discussions
3.1. Growth and Sugar Consumption Kinetics of S. cerevisiae during Wine Fermentation
3.2. The Physicochemical Characteristics and the Volatile Aroma Compounds of Wines
3.3. Varietal Aroma Compounds
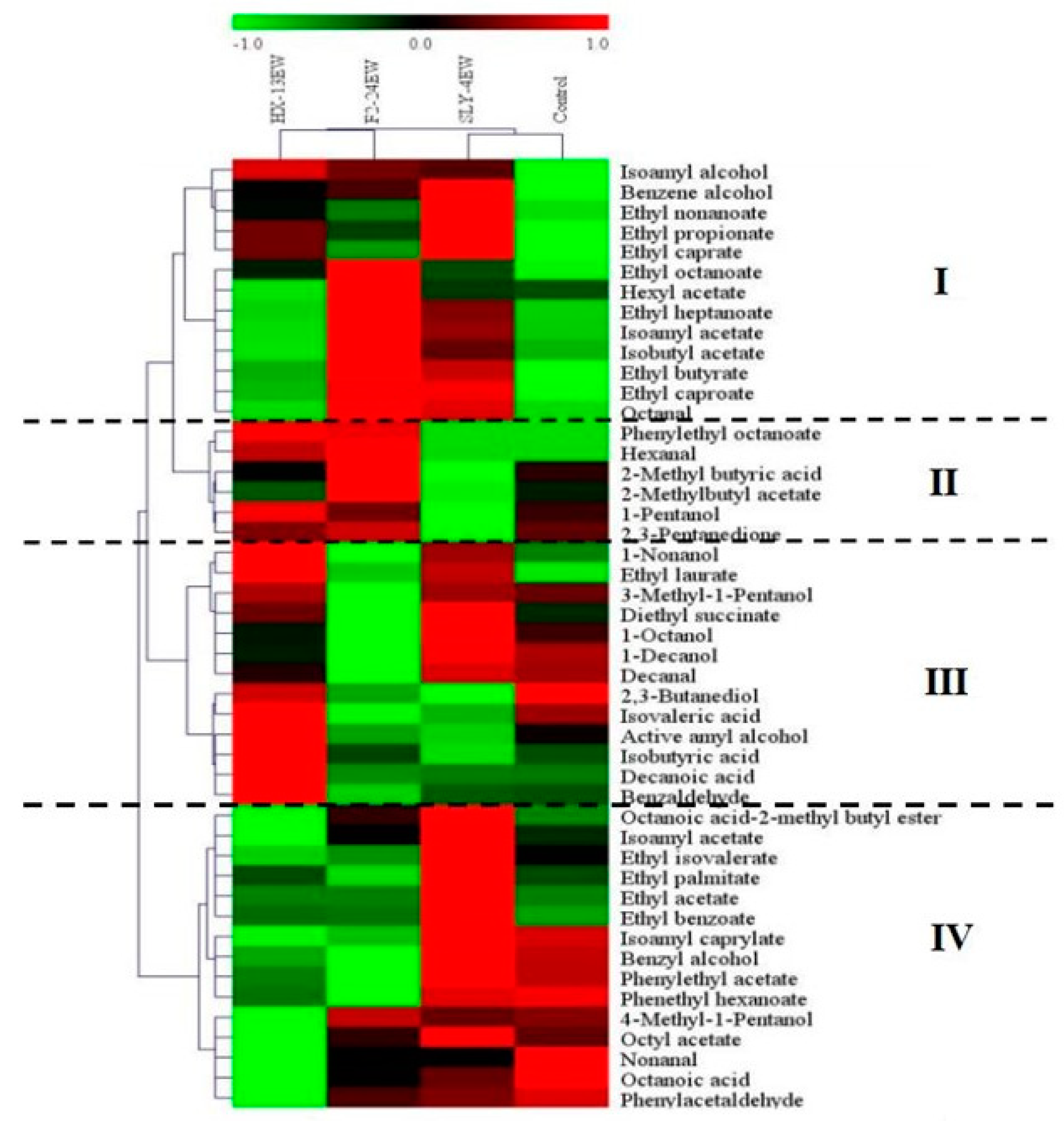
3.4. Fermentative Aroma Compounds
3.5. Sensory Evaluation of Wines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef] [PubMed]
- de Ovalle, S.; Brena, B.; Gonzalez-Pombo, P. Influence of β glucosidases from native yeast on the aroma of Muscat and Tannat wines. Food Chem. 2021, 346, 128899. [Google Scholar] [CrossRef]
- Schmidt, S.; Rainieri, S.; Witte, S.; Matern, U.; Martens, S. Identification of a Saccharomyces cerevisiae glucosidase that hydrolyzes flavonoid glucosides. Appl. Environ. Microbiol. 2011, 77, 1751–1757. [Google Scholar] [CrossRef]
- Tufariello, M.; Capozzi, V.; Spano, G.; Cantele, G.; Venerito, P.; Mita, G.; Grieco, F. Effect of co-inoculation of Candida zemplinina, Saccharomyces cerevisiae and Lactobacillus plantarum for the industrial production of Negroamaro wine in Apulia (southern Italy). Microogranisms 2020, 8, 726. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Viana, R.; Gonzalez-Arenzana, L.; Portu, J.; Garijo, P.; Lopez-Alfaro, I.; Lopez, R.; Santamaria, P.; Gutierrez, A.R. Wine aroma evolution throughout alcoholic fermentation sequentially inoculated with non-Saccharomyces/Saccharomyces yeasts. Food Res. Int. 2018, 112, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, J.; Chen, F.; Zhang, X. Effect of Issatchenkia terricola and Pichia kudriavzevii on wine flavor and quality through simultaneous and sequential co-fermentation with Saccharomyces cerevisiae. LWT 2019, 116, 108477. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Luan, Y.; Duan, C.Q.; Yan, G.L. Use of Torulaspora delbrueckii co-fermentation with two Saccharomyces cerevisiae strains with different aromatic characteristic to improve the diversity of red wine aroma profile. Front. Microbiol. 2018, 9, 606. [Google Scholar] [CrossRef] [PubMed]
- Mehlomakulu, N.N.; Setati, M.E.; Divol, B. Characterization of novel killer toxins secreted by wine-related non-Saccharomyces yeasts and their action on Brettanomyces spp. Int. J. Food Microbiol. 2014, 188, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Villalba, M.L.; Saez, J.S.; Del Monaco, S.; Lopes, C.A.; Sangorrin, M.P. Tdkt, a new killer toxin produced by Torulaspora delbrueckii effective against wine spoilage yeasts. Int. J. Food Microbiol. 2016, 217, 94–100. [Google Scholar] [CrossRef]
- Curiel, J.A.; Morales, P.; Gonzalez, R.; Tronchoni, J. Different non-Saccharomyces yeast species stimulate nutrient consumption in S.cerevisiae mixed cultures. Front. Microbiol. 2017, 8, 2121. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Qin, Y.; Tao, Y.S.; Zhu, X.L.; Peng, C.T.; Ullah, N. Potential of glycosidase from non-Saccharomyces isolates for enhancement of wine aroma. J. Food Sci. 2016, 81, M935–M943. [Google Scholar] [CrossRef] [PubMed]
- Thongekkaew, J.; Fujii, T.; Masaki, K.; Koyama, K. Evaluation of Candida easanensis Jk8 β-glucosidase with potentially hydrolyse non-volatile glycosides of wine aroma precursors. Nat. Prod. Res. 2019, 33, 3563–3567. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Hu, K.; Zhang, J.; Zhu, X.; Tao, Y. Aroma modulation of Cabernet Gernischt dry red wine by optimal enzyme treatment strategy in winemaking. Food Chem. 2018, 245, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhuo, X.; Hu, L.; Zhang, X. Effects of crude β-glucosidases from Issatchenkia terricola, Pichia kudriavzevii, Metschnikowia pulcherrima on the flavor complexity and characteristics of wines. Microorganisms 2020, 8, 953. [Google Scholar] [CrossRef] [PubMed]
- de Ovalle, S.; Brena, B.; Fariña, L.; Gonzalez-Pombo, P. Novel β-glucosidase from Issatchenkia orientalis: Characterization and assessment for hydrolysis of Muscat wine glycosides. J. Biochem. Biotechnol. 2016, 4, 174–183. [Google Scholar]
- González-Pombo, P.; Fariña, L.; Carrau, F.; Batista-Viera, F.; Brena, B.M. A novel extracellular β-glucosidase from Issatchenkia terricola: Isolation, immobilization and application for aroma enhancement of white Muscat wine. Process Biochem. 2011, 46, 385–389. [Google Scholar] [CrossRef]
- González-Pombo, P.; Pérez, G.; Carrau, F.; Guisán, J.M.; Batista-Viera, F.; Brena, B.M. One-step purification and characterization of an intracellular β-glucosidase from Metschnikowia pulcherrima. Biotechnol. Lett. 2008, 30, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Navascues, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic analysis of physiological properties of Torulaspora delbrueckii in wine fermentations and its incidence on wine quality. Appl. Microbiol. Biotechnol. 2015, 99, 1911–1922. [Google Scholar] [CrossRef]
- Hu, L.; Liu, R.; Wang, X.; Zhang, X. The sensory quality improvement of citrus wine through co-fermentations with selected non-Saccharomyces yeast strains and Saccharomyces cerevisiae. Microorganisms 2020, 8, 323. [Google Scholar] [CrossRef] [PubMed]
- López, M.C.; Mateo, J.J.; Maicas, S. Screening of β-glucosidase and β-xylosidase activities in four non-Saccharomyces yeast isolates. J. Food Sci. 2015, 80, C1696–C1704. [Google Scholar] [CrossRef]
- Peng, C.T.; Wen, Y.; Tao, Y.S.; Lan, Y.Y. Modulating the formation of Meili wine aroma by prefermentative freezing process. J. Agric. Food Chem. 2013, 61, 1542–1553. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Peinado, R.A.; Mauricio, J.C.; Moreno, J. Aromatic series in sherry wines with gluconic acid subjected to different biological aging conditions by Saccharomyces cerevisiae var. capensis. Food Chem. 2006, 94, 232–239. [Google Scholar] [CrossRef]
- Lasekan, O.; See, N.S. Key volatile aroma compounds of three black velvet tamarind (Dialium) fruit species. Food Chem. 2015, 168, 561–565. [Google Scholar] [CrossRef]
- López, R.; Ortin, N.; Perez-Trujillo, J.P.; Cacho, J.; Ferreira, V. Impact odorants of different young white wines from the Canary Islands. J. Agric. Food Chem. 2003, 51, 3419–3425. [Google Scholar] [CrossRef]
- Riemer, D.D.; Milne, P.J.; Farmer, C.T.; Zika, R.G. Determination of terpene and related compounds in semi-urban air by GC-MSD. Chemosphere 1994, 28, 837–850. [Google Scholar] [CrossRef]
- Culleré, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas chromatography-olfactometry and chemical quantitative study of the aroma of six premium quality Spanish aged red wines. J. Agric. Food Chem. 2004, 52, 1653–1660. [Google Scholar] [CrossRef]
- Tao, Y.S.; Li, H. Active volatiles of cabernet sauvignon wine from Changli County. Health 2009, 1, 176–182. [Google Scholar] [CrossRef]
- Palassarou, M.; Melliou, E.; Liouni, M.; Michaelakis, A.; Balayiannis, G.; Magiatis, P. Volatile profile of Greek dried white figs (Ficus carica L.) and investigation of the role of beta-damascenone in aroma formation in fig liquors. J. Sci. Food Agric. 2017, 97, 5254–5270. [Google Scholar] [CrossRef]
- Lyumugabe, F.; Iyamarere, I.; Kayitare, M.; Museveni, J.R.; Songa, E.B. Volatile aroma compounds and sensory characteristics of traditional banana wine “Urwagwa” of Rwanda. Rwanda J. 2018, 2. [Google Scholar] [CrossRef]
- Lopez, R.; Aznar, M.; Cacho, J.; Ferreira, V. Determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J. Chromatogr. A 2002, 966, 167–177. [Google Scholar] [CrossRef]
- Mayr, C.M.; Geue, J.P.; Holt, H.E.; Pearson, W.P.; Jeffery, D.W.; Francis, I.L. Characterization of the key aroma compounds in Shiraz wine by quantitation, aroma reconstitution, and omission studies. J. Agric. Food Chem. 2014, 62, 4528–4536. [Google Scholar] [CrossRef] [PubMed]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J.C. Olfactory impact of higher alcohols on red wine fruity ester aroma expression in model solution. J. Agric. Food Chem. 2015, 63, 9777–9788. [Google Scholar] [CrossRef] [PubMed]
- Noguerol-Pato, R.; Gonzalez-Barreiro, C.; Cancho-Grande, B.; Simal-Gandara, J. Quantitative determination and characterisation of the main odourants of encia monovarietal red wines. Food Chem. 2009, 117, 473–484. [Google Scholar] [CrossRef]
- Qin, T.; Liao, J.; Zheng, Y.; Zhang, W.; Zhang, X. Oenological characteristics of four non-Saccharomyces yeast strains with β-glycosidase activity. Front. Microbiol. 2021, 12, 626920. [Google Scholar] [CrossRef]
- Hu, K.; Zhu, X.L.; Mu, H.; Ma, Y.; Ullah, N.; Tao, Y.S. A novel extracellular glycosidase activity from Rhodotorula mucilaginosa: Its application potential in wine aroma enhancement. Lett. Appl. Microbiol. 2016, 62, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Yan, X.; Wang, Q.; Zhang, Y.; Tao, Y. Performance of selected P. fermentans and its excellular enzyme in co-inoculation with S. cerevisiae for wine aroma enhancement. LWT 2017, 86, 361–370. [Google Scholar] [CrossRef]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its importance to wine aroma—A review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef]
- Mendez-Costabel, M.P.; Wilkinson, K.L.; Bastian, S.E.; Jordans, C.; McCarthy, M.; Ford, C.M.; Dokoozlian, N.K. Effect of increased irrigation and additional nitrogen fertilisation on the concentration of green aroma compounds in Vitis vinifera L. M erlot fruit and wine. Aust. J. Grape Wine Res. 2014, 20, 80–90. [Google Scholar] [CrossRef]
- Vilanova, M.; Genisheva, Z.; Grana, M.; Oliveira, J.M. Determination of odorants in varietal wines from international grape cultivars (Vitis vinifera) grown in NW spain. S. Afr. J. Enol. Vitic. 2013, 34, 212–222. [Google Scholar] [CrossRef]
- Swangkeaw, J.; Vichitphan, S.; Butzke, C.E.; Vichitphan, K. The characterisation of a novel Pichia anomala β-glucosidase with potentially aroma-enhancing capabilities in wine. Ann. Microbiol. 2009, 59, 335–343. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, W.; Xu, Y.; Li, J. Effect of different indigenous yeast β-glucosidases on the liberation of bound aroma compounds. J. Inst. Brew. 2011, 117, 230–237. [Google Scholar] [CrossRef]
- Dutraive, O.; Benito, S.; Fritsch, S.; Beisert, B.; Patz, C.D.; Rauhut, D. Effect of sequential inoculation with non-Saccharomyces and Saccharomyces yeasts on riesling wine chemical composition. Fermentation 2019, 5, 79. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Yeast modulation of wine flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar] [PubMed]
- Andorrà, I.; Berradre, M.; Rozès, N.; Mas, A.; Guillamón, J.M.; Esteve-Zarzoso, B. Effect of pure and mixed cultures of the main wine yeast species on grape must fermentations. Eur. Food Res. Technol. 2010, 231, 215–224. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Cacho, J.F.; Ferreira, V. Relationship between varietal amino acid profile of grapes and wine aromatic composition. Experiments with model solutions and chemometric study. J. Agric. Food Chem. 2002, 50, 2891–2899. [Google Scholar] [CrossRef] [PubMed]
- Lilly, M.; Bauer, F.F.; Styger, G.; Lambrechts, M.G.; Pretorius, I.S. The effect of increased branched-chain amino acid transaminase activity in yeast on the production of higher alcohols and on the flavour profiles of wine and distillates. FEMS Yeast Res. 2006, 6, 726–743. [Google Scholar] [CrossRef]
- Lorensini, F.; Ceretta, C.A.; Lourenzi, C.R.; De Conti, L.; Tiecher, T.L.; Trentin, G.; Brunetto, G. Nitrogen fertilization of Cabernet Sauvignon grapevines: Yield, total nitrogen content in the leaves and must composition. Acta Sci. Agron. 2015, 37, 321–329. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Li, J.; Xu, Y. Different influences of beta-glucosidases on volatile compounds and anthocyanins of Cabernet Gernischt and possible reason. Food Chem. 2013, 140, 245–254. [Google Scholar] [CrossRef]
- Barbagallo, R.N.; Palmeri, R.; Fabiano, S.; Rapisarda, P.; Spagna, G. Characteristic of β-glucosidase from Sicilian blood oranges in relation to anthocyanin degradation. Enzym. Microb. Technol. 2007, 41, 570–575. [Google Scholar] [CrossRef]
- Corduas, M.; Cinquanta, L.; Ievoli, C. The importance of wine attributes for purchase decisions: A study of Italian consumers’ perception. Food Qual. Prefer. 2013, 28, 407–418. [Google Scholar] [CrossRef]
- Yang, H.; Cai, G.L.; Lu, J.; Plaza, E.G. The production and application of enzymes related to the quality of fruit wine. Crit. Rev. Food Sci. 2021, 61, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Y.; Voon, M.; Chua, J.Y.; Huang, D.J.; Lee, P.R.; Liu, S.Q. The effects of co- and sequential inoculation of Torulaspora delbrueckii and Pichia kluyveri on chemical compositions of durian wine. Appl. Microbiol. Biotechnol. 2017, 101, 7853–7863. [Google Scholar] [CrossRef] [PubMed]
- Siebert, T.E.; Barker, A.; Pearson, W.; Barter, S.R.; de Barros, L.M.; Darriet, P.; Herderich, M.J.; Francis, I.L. Volatile compounds related to ‘stone fruit’ aroma attributes in Viognier and Chardonnay wines. J. Agric. Food Chem. 2018, 66, 2838–2850. [Google Scholar] [CrossRef] [PubMed]
- Puertas, B.; Jimenez, M.J.; Cantos-Villar, E.; Cantoral, J.M.; Rodriguez, M.E. Use of Torulaspora delbrueckii and Saccharomyces cerevisiae in semi-industrial sequential inoculation to improve quality of Palomino and Chardonnay wines in warm climates. J. Appl. Microbiol. 2017, 122, 733–746. [Google Scholar] [CrossRef] [PubMed]
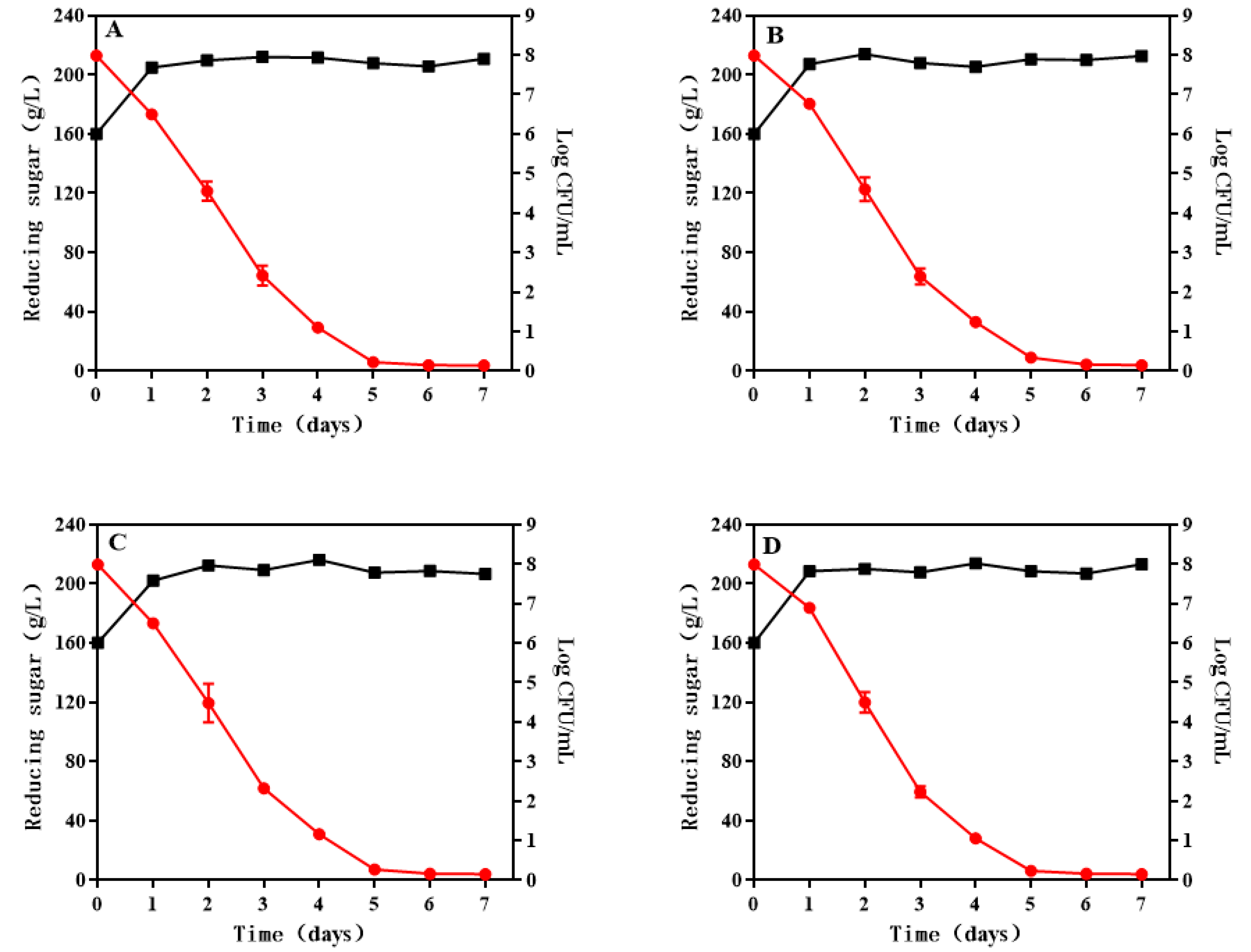
 Wines;
Wines;  Varietal aroma compounds.
Varietal aroma compounds.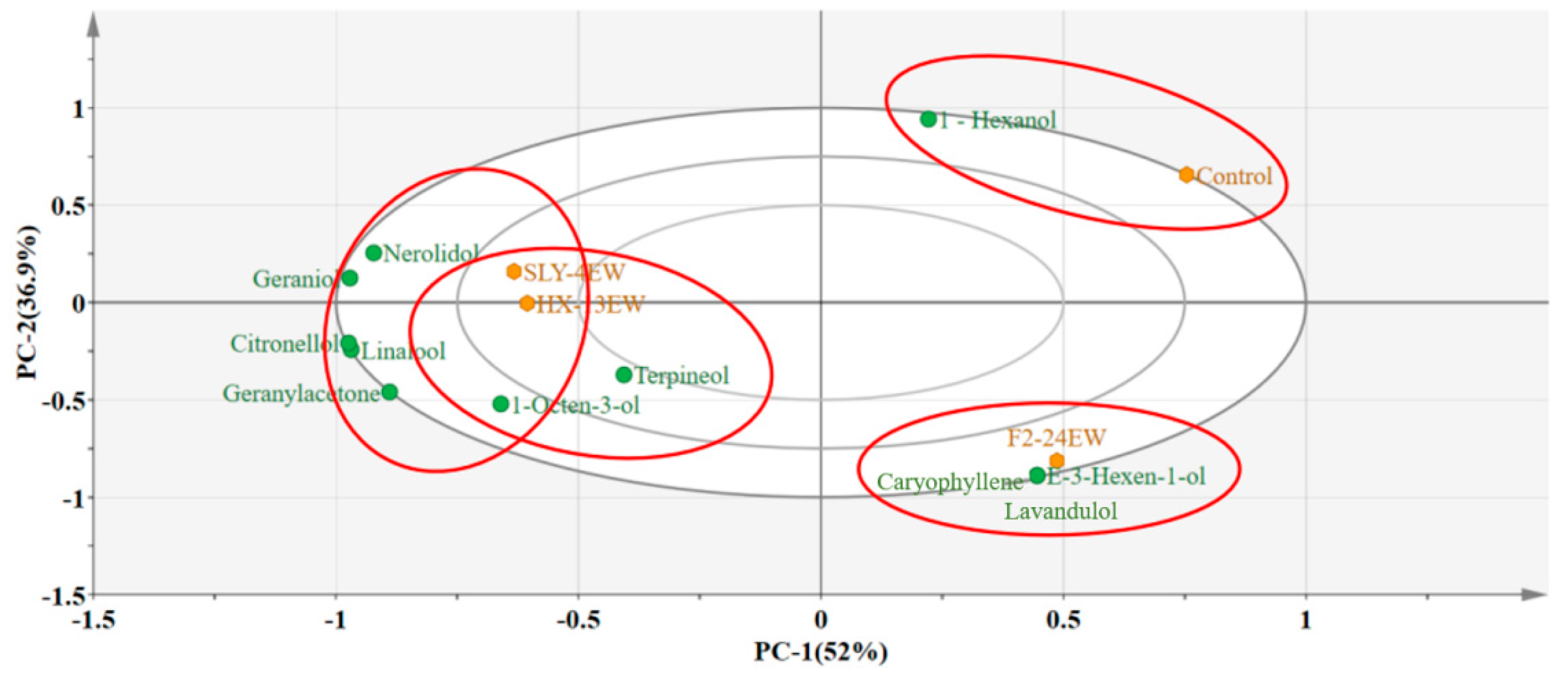
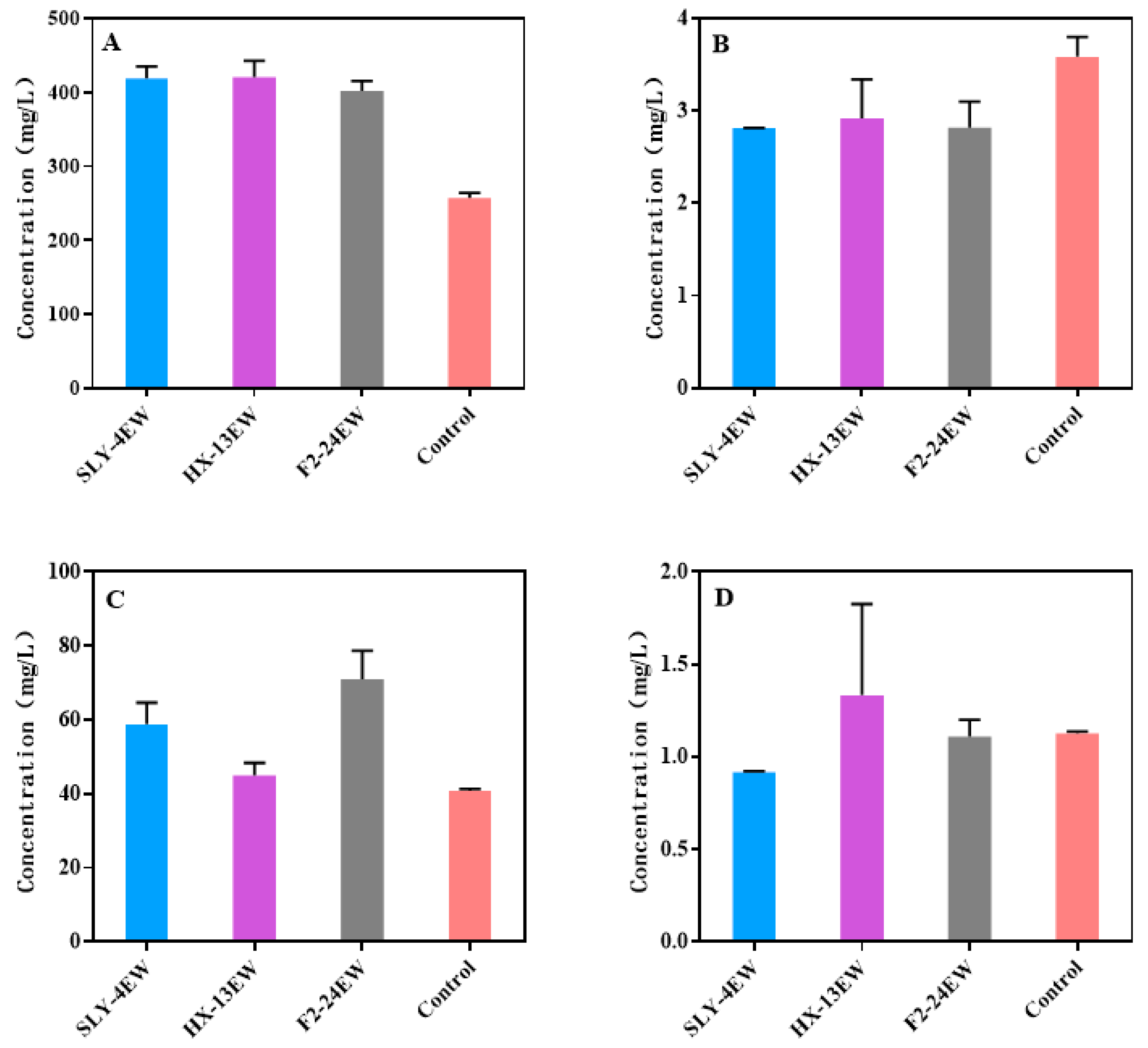
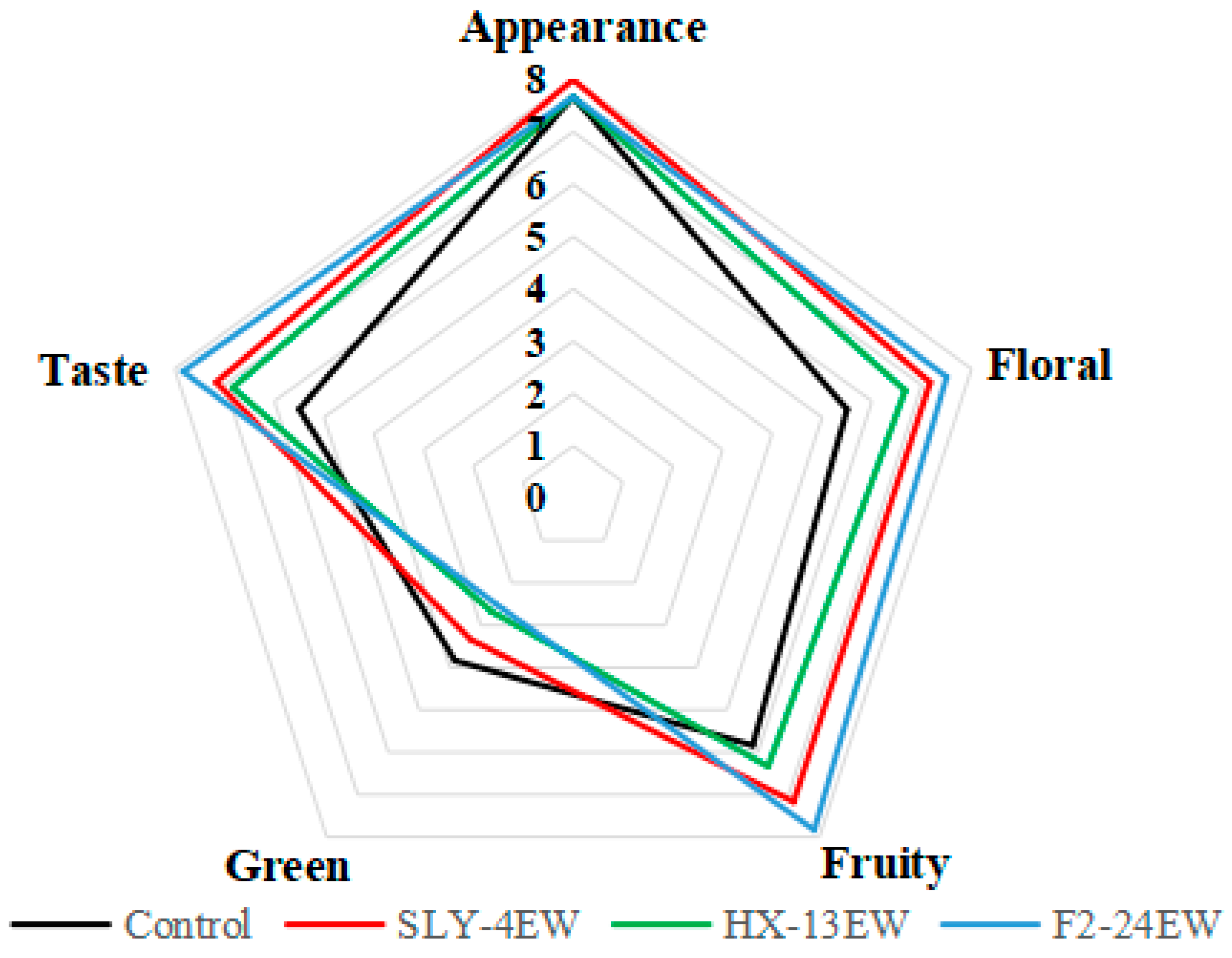
| Wine | Fermentation Period (d) | Residual Sugar (g/L) | Alcohol (%, v/v) | Total Acid (g/L) | Volatile Acid (g/L) |
|---|---|---|---|---|---|
| Control | 7 | 3.83 ± 0.02 a | 11.62 ± 0.15 a | 6.66 ± 0.13 a | 0.29 ± 0.02 a |
| SLY-4EW | 7 | 4.00 ± 0.08 a | 11.99 ± 1.02 a | 6.75 ± 0.27 a | 0.28 ± 0.01 a |
| HX-13EW | 7 | 3.93 ± 0.05 a | 11.95 ± 0.15 a | 6.56 ± 0.27 a | 0.27 ± 0.01 a |
| F2-24EW | 7 | 3.98 ± 0.16 a | 11.63 ± 0.59 a | 6.66 ± 0.40 a | 0.27 ± 0.01 a |
| Compounds | Wines | Odor Threshold | OAV | Sensory Description | |||
|---|---|---|---|---|---|---|---|
| SLY-4EW | HX-13EW | F2-24EW | Control | ||||
| 1-Hexanol | 0.91 ± 0.08 ab | 0.84 ± 0.04 b | 0.79 ± 0.04 b | 0.98 ± 0.07 a | 8 [21] | 0.1–1 | Herbaceous, grass [21] |
| E-3-Hexen-1-ol | - | - | 0.03 ± 0.00 a | - | 0.4 [22] | <0.1 | Herbaceous, grass [21] |
| C6 compounds | 0.91 ± 0.08 ab | 0.84 ± 0.04 b | 0.82 ± 0.04 b | 0.98 ± 0.07 a | |||
| Linalool | 0.40 ± 0.03 a | 0.39 ± 0.01 a | 0.30 ± 0.02 b | 0.21 ± 0.05 c | 0.1 [22] | >1 | Muscat, flowery, fruity [23] |
| Citronellol | 0.24 ± 0.00 a | 0.23 ± 0.03 a | 0.14 ± 0.03 b | 0.08 ± 0.01 c | 0.1 [23] | >1 | Green lemon [21] |
| 1-Octen-3-ol | 0.20 ± 0.03 a | 0.14 ± 0.04 b | 0.16 ± 0.04 ab | 0.08 ± 0.01 c | 0.02 [23] | >1 | Mushroom [23] |
| Geranylacetone | 0.05 ± 0.02 a | 0.06 ± 0.03 a | 0.04 ± 0.00 a | - | 0.06 [24] | 0.1–1 | Flowery [24] |
| Nerolidol | 0.12 ± 0.01 a | 0.10 ± 0.01 b | 0.06 ± 0.00 c | 0.07 ± 0.00 c | 0.7 [23] | 0.1–1 | Roses, apple, orange [23] |
| Terpineol | - | 0.06 ± 0.01 a | 0.02 ± 0.02 b | - | 0.25 [25] | 0.1–1 | Flowery, piny [26] |
| Geraniol | 0.06 ± 0.01 b | 0.07 ± 0.00 a | - | - | 0.03 [25] | >1 | Roses [25] |
| Caryophyllene | - | - | 0.08 ± 0.01 a | - | 0.064 | >1 | Spicy, woody, orange |
| Lavandulol | - | - | 0.02 ± 0.000 a | - | - | ||
| Terpenes | 1.07 ± 0.11 a | 1.04 ± 0.13 a | 0.82 ± 0.08 b | 0.43 ± 0.07 c | |||
| Varietal aroma compounds | 1.98 ± 0.19 a | 1.88 ± 0.17 a | 1.64 ± 0.12 a | 1.41 ± 0.13 b | |||
| Isoamyl alcohol | 211.78 ± 15.74 b | 238.09 ± 1.14 a | 217.26 ± 6.67 b | 114.37 ± 1.24 c | 30 [27] | >1 | Whiskey, malt, burnt [27] |
| 2,3-Butanediol | 0.93 ± 0.31 a | 1.15 ± 0.35 a | 0.98 ± 0.04 a | 1.19 ± 0.12 a | 120 [28] | <0.1 | Butter, creamy [28] |
| 1-Pentanol | 0.02 ± 0.00 d | 0.10 ± 0.00 a | 0.09 ± 0.01 b | 0.08 ± 0.01 c | 80 | <0.1 | Mellow, astringency |
| 1-Octanol | 0.85 ± 0.06 a | 0.70 ± 0.21 ab | 0.56 ± 0.06 b | 0.74 ± 0.15 ab | 0.9 [29] | 0.1–1 | Flesh orange, rose, sweetherb [29] |
| 1-Nonanol | 0.71 ± 0.36 ab | 0.82 ± 0.04 a | 0.39 ± 0.06 b | 0.51 ± 0.02 b | 0.015 [30] | >1 | Orange [30] |
| 1-Decanol | 0.19 ± 0.07 a | 0.16 ± 0.07 a | 0.13 ± 0.00 a | 0.18 ± 0.01 a | 0.4 [27] | 0.1–1 | Flowery [27] |
| 4-Methyl-1-Pentanol | 0.47 ± 0.01 a | 0.41 ± 0.03 b | 0.48 ± 0.01 a | 0.47 ± 0.08 a | 50 [31] | <0.1 | Almonds, toast [31] |
| 3-Methyl-1-Pentanol | 0.98 ± 0.17 a | 0.98 ± 0.07 a | 0.85 ± 0.02 a | 0.96 ± 0.14 a | 0.5 [31] | >1 | Soil, mushroom [31] |
| Active amyl alcohol | 48.44 ± 3.11b | 52.32 ± 0.77 a | 48.76 ± 1.32 b | 50.01 ± 0.44 ab | 65 [32] | 0.1–1 | Hetero alcohol, almond [32] |
| Benzyl alcohol | 0.24 ± 0.01 a | 0.17 ± 0.00 b | 0.16 ± 0.03 b | 0.23 ± 0.05 a | 200 [29] | <0.1 | Almond [29] |
| Benzene alcohol | 154.28 ± 2.76 a | 125.79 ± 23.35 b | 132.20 ± 5.58 b | 88.23 ± 9.09 c | 7.5 [29] | >1 | Soil, mushroom [29] |
| Higher alcohols | 418.89 ± 22.60 a | 420.71 ± 26.03 a | 401.83 ± 13.79 a | 256.97 ± 11.36 b | |||
| Isobutyric acid | 0.04 ± 0.00 c | 0.13 ± 0.04 b | 0.28 ± 0.01 a | 0.27 ± 0.04 a | 2.3 [25] | 0.1–1 | Cheese, rancid [25] |
| Isovaleric acid | 0.77 ± 0.04 a | 0.97 ± 0.30 a | 0.74 ± 0.08 a | 0.90 ± 0.04 a | 0.03 [25] | >1 | Fatty [25] |
| 2-Methyl butyric acid | 0.66 ± 0.01 b | 0.97 ± 0.08 a | 0.71 ± 0.28 b | 0.69 ± 0.06 b | 0.033 [21] | >1 | Cheese [21] |
| Octanoic acid | 1.10 ± 0.04 b | 0.69 ± 0.04 d | 0.93 ± 0.05 c | 1.46 ± 0.23 a | 0.5 [22] | >1 | Cheese, rancid [22] |
| Decanoic acid | 0.25 ± 0.00 a | 0.16 ± 0.03 b | 0.16 ± 0.02 b | 0.27 ± 0.01 a | 1 [25] | 0.1–1 | Fatty, unpleasant [25] |
| Fatty acids | 2.81 ± 0.09b | 2.92 ± 0.50 b | 2.82 ± 0.44 b | 3.58 ± 0.38 a | |||
| Ethyl acetate | 0.10 ± 0.02 a | - | - | - | 7.5 [29] | <0.1 | Fruity, sweet taste [29] |
| Ethyl propionate | 0.36 ± 0.11 a | 0.30 ± 0.03 a | 0.28 ± 0.03 a | 0.25 ± 0.08 a | 1.8 [23] | 0.1–1 | Pineapples, bananas, apples [23] |
| Ethyl butyrate | 0.77 ± 0.43 a | 0.48 ± 0.05 a | 0.83 ± 0.54 a | 0.41 ± 0.14 a | 0.02 [27] | >1 | Strawberries, apples, bananas [27] |
| Ethyl isovalerate | 1.05 ± 0.42 a | - | 0.11 ± 0.01 bc | 0.38 ± 0.02 b | 0.003 | >1 | Bananas, fruity |
| Ethyl caproate | 21.46 ± 2.68 a | 9.26 ± 0.80 b | 21.58 ± 4.69 a | 7.27 ± 1.28 b | 0.014 [27] | >1 | Green apples, fennel [27] |
| Ethyl heptanoate | 0.11 ± 0.01 a | 0.07 ± 0.01 b | 0.13 ± 0.05 a | 0.07 ± 0.01 b | 0.002 [27] | >1 | Sweet, strawberries, bananas [27] |
| Diethyl succinate | 0.31 ± 0.10 a | 0.29 ± 0.01 ab | 0.21 ± 0.01 b | 0.26 ± 0.09 ab | 200 [27] | <0.1 | Fruity, melons [27] |
| Ethyl octanoate | 9.80 ± 0.27 b | 10.48 ± 1.19 b | 16.60 ± 0.61 a | 7.10 ± 0.19 c | 0.25 [27] | >1 | Fruity [27] |
| Ethyl nonanoate | 0.44 ± 0.24 a | 0.29 ± 0.03 ab | 0.25 ± 0.03 b | 0.21 ± 0.06 b | 1.3 [27] | 0.1–1 | Flowery, fruity [27] |
| Ethyl caprate | 7.29 ± 0.28 a | 5.79 ± 0.60 b | 4.01 ± 0.61 c | 3.27 ± 0.57 d | 0.2 [33] | >1 | Apples, flowery [29] |
| Ethyl laurate | 0.53 ± 0.15 a | 0.57 ± 0.05 a | 0.35 ± 0.04 b | 0.34 ± 0.07 b | 1.5 [34] | 0.1–1 | Fruity, fatty [34] |
| Ethyl palmitate | 0.15 ± 0.01 a | 0.08 ± 0.05 b | 0.06 ± 0.01 c | 0.08 ± 0.00 b | 1.5 [34] | <0.1 | Fatty, fruity, sweet [34] |
| Fatty acid ethyl esters | 42.32 ± 4.72 a | 27.60 ± 2.82 b | 44.40 ± 6.62 a | 19.63 ± 2.52 c | |||
| Isoamyl acetate | 6.82 ± 0.44 b | 3.44 ± 0.44 d | 8.43 ± 0.32 a | 3.64 ± 0.08 c | 0.2 [27] | >1 | Green apples, bananas [27] |
| 2-Methylbutyl acetate | - | 1.61 ± 0.48 b | 6.33 ± 0.38 a | 2.16 ± 0.35 b | 0.16 [27] | >1 | Bananas, fruity [27] |
| Isobutyl acetate | 0.29 ± 0.14 a | 0.19 ± 0.07 a | 0.36 ± 0.23 a | 0.21 ± 0.02 a | 1.6 [27] | 0.1–1 | Bananas [29] |
| Hexyl acetate | 0.44 ± 0.00 b | 0.32 ± 0.09 b | 0.73 ± 0.47 a | 0.42 ± 0.07 ab | 1.5 [27] | 0.1–1 | Fruity, pear, cherry [27] |
| Octyl acetate | 0.20 ± 0.09 a | - | 0.13 ± 0.04 a | 0.15 ± 0.01 a | - | ||
| Phenylethyl acetate | 13.89 ± 0.19 a | 10.761 ± 0.241 c | 9.39 ± 0.21 d | 13.17 ± 0.42 b | 0.65 [34] | >1 | Fruity, flowery [34] |
| Acetic esters | 21.63 ± 0.86 ab | 16.33 ± 1.31 b | 25.36 ± 1.65 a | 19.74 ± 0.94 b | |||
| Octanoic acid-2-methyl butyl ester | 0.30 ± 0.00 a | 0.12 ± 0.00 d | 0.21 ± 0.01 b | 0.16 ± 0.02 c | |||
| Isoamyl acetate | 0.35 ± 0.08 a | 0.13 ± 0.03 c | 0.23 ± 0.11 b | 0.21 ± 0.02 bc | 1 [25] | 0.1–1 | Apples, pineapples [25] |
| Isoamyl caprylate | 0.55 ± 0.14 a | 0.32 ± 0.07 b | 0.34 ± 0.06 b | 0.52 ± 0.04 a | 0.125 [25] | 0.1–1 | Fruity, cheese [25] |
| Ethyl benzoate | 0.34 ± 0.02 a | 0.21 ± 0.01 b | 0.21 ± 0.00 b | 0.20 ± 0.00 b | |||
| Phenethyl hexanoate | 0.34 ± 0.03 a | 0.23 ± 0.02 b | 0.17 ± 0.01 c | 0.35 ± 0.06 a | |||
| Phenethyl octanoate | - | 0.03 ± 0.01 a | 0.03 ± 0.01 a | - | |||
| Other esters | 1.88 ± 0.27 a | 1.03 ± 0.14 c | 1.18 ± 0.27 c | 1.43 ± 0.15 b | |||
| Esters | 58.82 ± 5.81 b | 44.96 ± 1.68 c | 70.95 ± 7.77 a | 40.80 ± 4.17 c | |||
| Nonanal | 0.11 ± 0.01 b | 0.08 ± 0.02 c | 0.11 ± 0.02 b | 0.14 ± 0.02 a | 0.015 [27] | >1 | Green, spicy [27] |
| Decanal | 0.07 ± 0.01 a | 0.06 ± 0.00 b | 0.03 ± 0.00 c | 0.06 ± 0.00 a | 0.001 | >1 | Flowery |
| Octanal | 0.06 ± 0.01 b | - | 0.06 ± 0.01 a | - | 0.001 | >1 | Bitter, lemons |
| Hexanal | - | 0.01 ± 0.01 a | 0.01 ± 0.01 a | - | |||
| 2,3-Pentanedione | 0.16 ± 0.08 b | 0.41 ± 0.02 a | 0.44 ± 0.07 a | 0.40 ± 0.05 a | 2 [21] | 0.1–1 | Pecans [21] |
| Benzaldehyde | 0.40 ± 0.10 a | 0.68 ± 0.48 a | 0.327 ± 0.024 b | 0.40 ± 0.02 a | 2 [21] | 0.1–1 | Toasted almonds [21] |
| Phenylacetaldehyde | 0.12 ± 0.00 a | 0.10 ± 0.01 a | 0.12 ± 0.04 a | 0.12 ± 0.02 a | 0.005 [25] | >1 | Flowers, roses, honey [25] |
| Carbonyl compounds | 0.92 ± 0.19 b | 1.33 ± 0.53 a | 1.11 ± 0.16 ab | 1.13 ± 0.10 ab | |||
| Fermentation aroma | 488.45 ± 28.73 a | 469.92 ± 31.32 a | 476.71 ± 22.85 a | 302.48 ± 15.44 b | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Zhang, W.; Qin, T.; Liao, J.; Zhang, X. Effects of Purified β-Glucosidases from Issatchenkia terricola, Pichia kudriavzevii, Metschnikowia pulcherrima on the Flavor Complexity and Typicality of Wines. J. Fungi 2022, 8, 1057. https://doi.org/10.3390/jof8101057
Zhu W, Zhang W, Qin T, Liao J, Zhang X. Effects of Purified β-Glucosidases from Issatchenkia terricola, Pichia kudriavzevii, Metschnikowia pulcherrima on the Flavor Complexity and Typicality of Wines. Journal of Fungi. 2022; 8(10):1057. https://doi.org/10.3390/jof8101057
Chicago/Turabian StyleZhu, Wanying, Wenxia Zhang, Tao Qin, Jing Liao, and Xiuyan Zhang. 2022. "Effects of Purified β-Glucosidases from Issatchenkia terricola, Pichia kudriavzevii, Metschnikowia pulcherrima on the Flavor Complexity and Typicality of Wines" Journal of Fungi 8, no. 10: 1057. https://doi.org/10.3390/jof8101057
APA StyleZhu, W., Zhang, W., Qin, T., Liao, J., & Zhang, X. (2022). Effects of Purified β-Glucosidases from Issatchenkia terricola, Pichia kudriavzevii, Metschnikowia pulcherrima on the Flavor Complexity and Typicality of Wines. Journal of Fungi, 8(10), 1057. https://doi.org/10.3390/jof8101057






