Overexpression of the White Opaque Switching Master Regulator Wor1 Alters Lipid Metabolism and Mitochondrial Function in Candida albicans
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Molecular Biology Procedures and Plasmid Constructions
2.3. RNA Isolation and RT-qPCR Analysis
2.4. Mitochondrial Function Assays
2.5. Protein and Immunoblot Methods
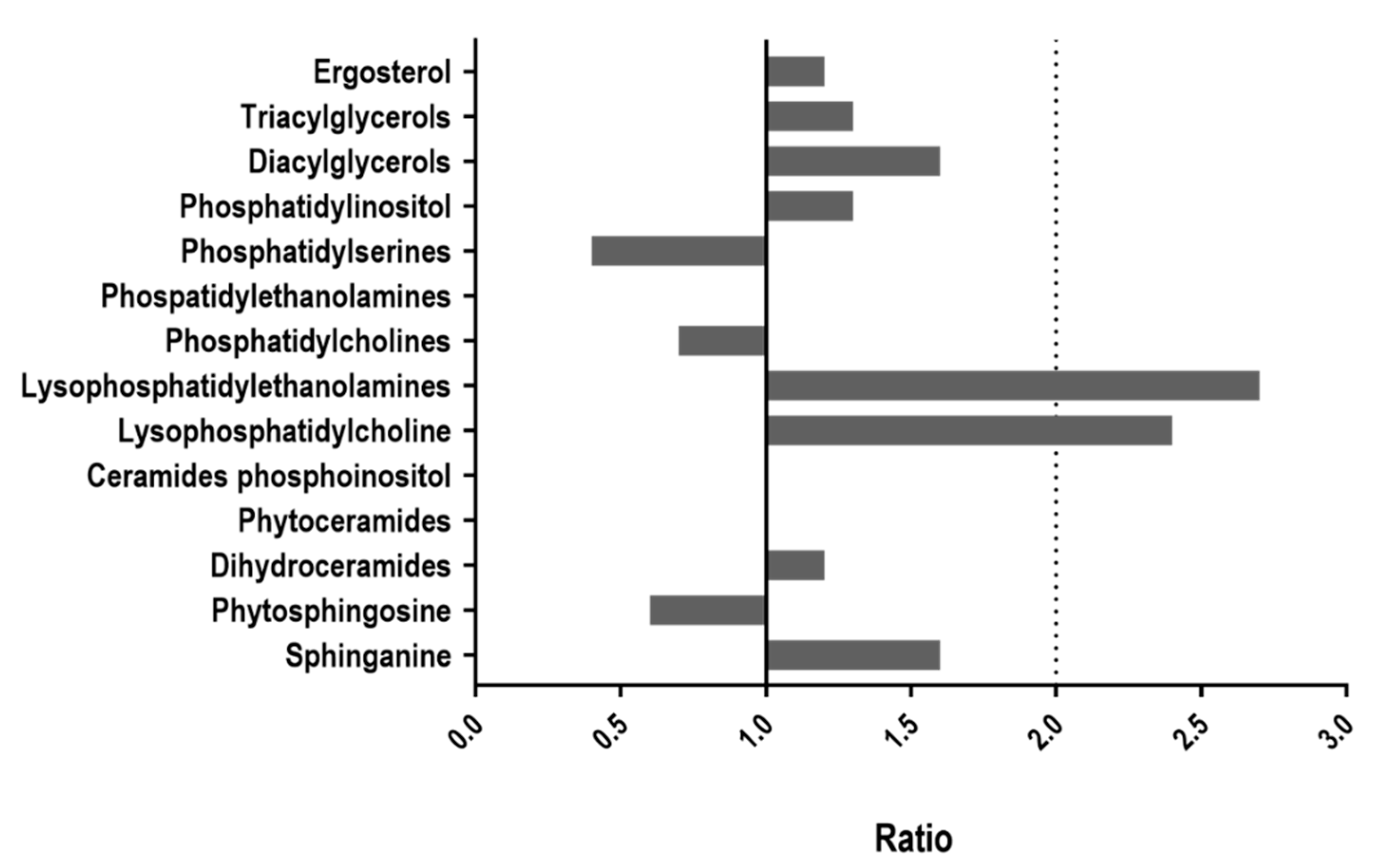
2.6. Analysis of Lipid Content
3. Results
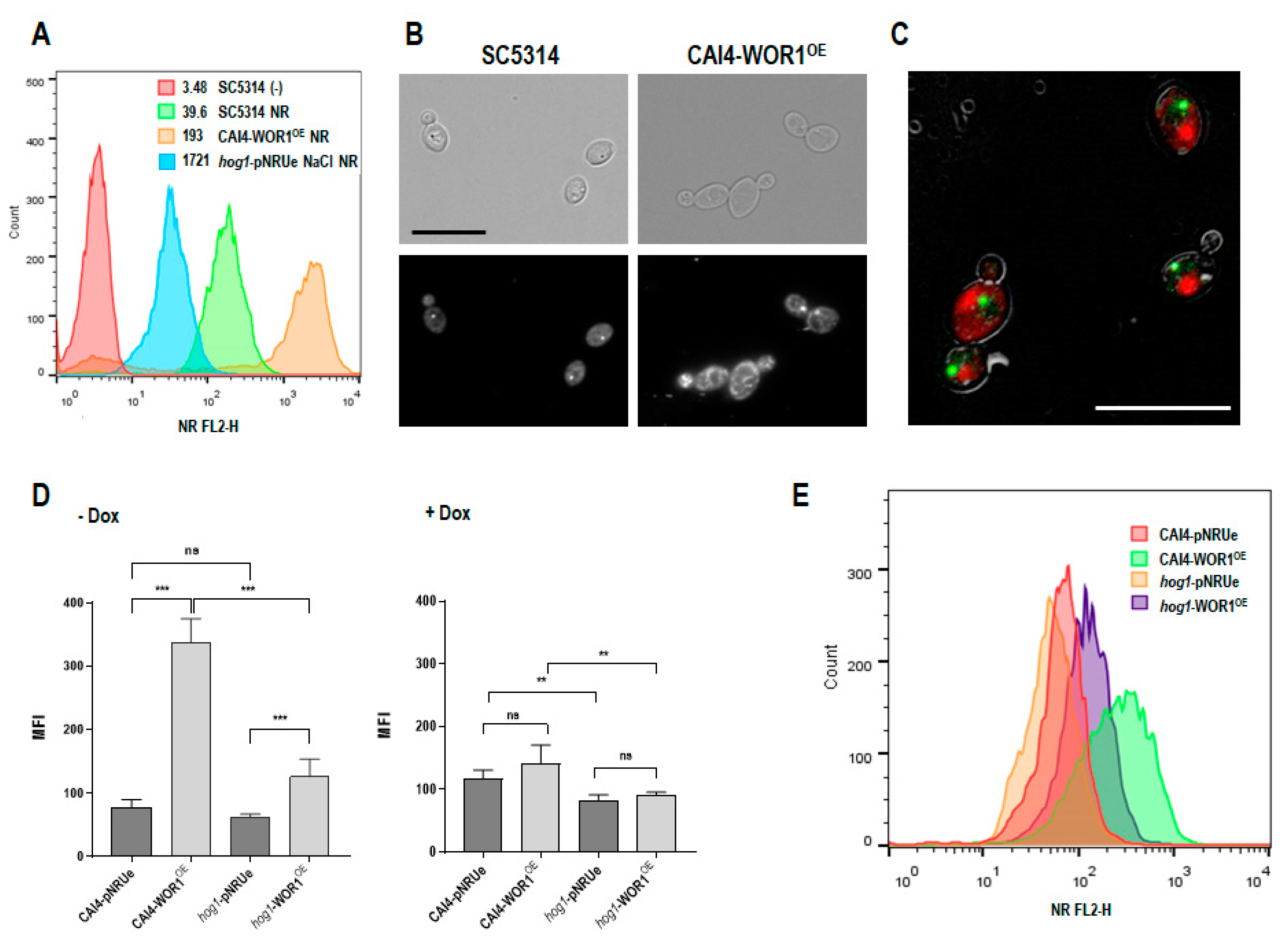
3.1. Lipid Content Is Altered in WOR1OE Strain
3.2. WOR1 Overexpression Enhances the Utilization of Phospholipids and Increases the Number and Size of Peroxisomes
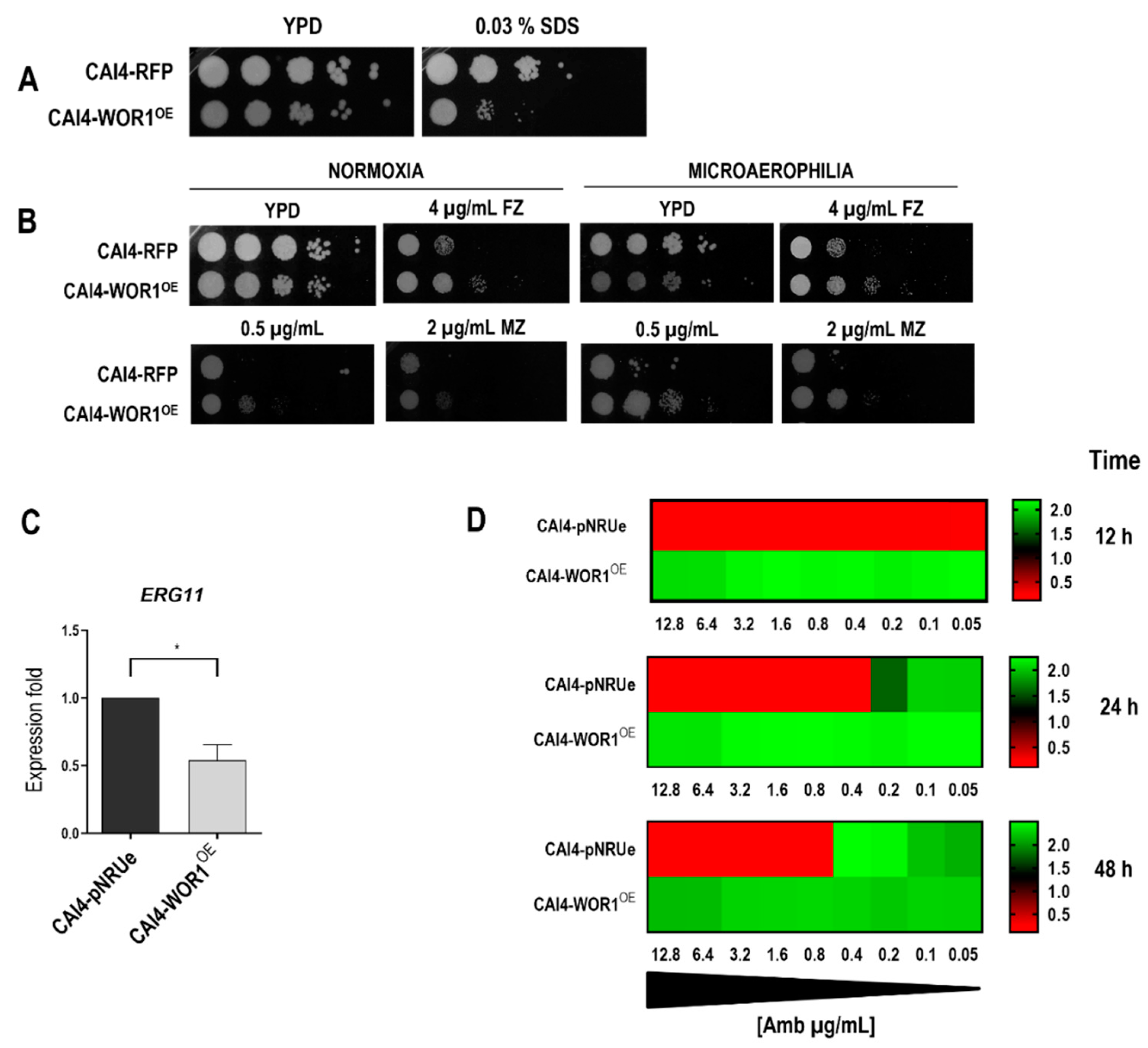
3.3. WOR1 Overexpression Alters the Susceptibility to Certain Membrane Disturbing Compounds
3.4. Mitochondrial Function Is Compromised by WOR1 Overexpression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alonso-Monge, R.; Gresnigt, M.S.; Roman, E.; Hube, B.; Pla, J. Candida albicans colonization of the gastrointestinal tract: A double-edged sword. PLoS Pathog. 2021, 17, e1009710. [Google Scholar] [CrossRef] [PubMed]
- Romo, J.A.; Kumamoto, C.A. On Commensalism of Candida. J. Fungi 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Markey, L.; Shaban, L.; Green, E.R.; Lemon, K.P.; Mecsas, J.; Kumamoto, C.A. Pre-colonization with the commensal fungus Candida albicans reduces murine susceptibility to Clostridium difficile infection. Gut Microbes 2018, 9, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Youssef, E.G.; Zhang, L.; Alkhazraji, S.; Gebremariam, T.; Singh, S.; Yount, N.Y.; Yeaman, M.R.; Uppuluri, P.; Ibrahim, A.S. Monoclonal IgM Antibodies Targeting Candida albicans Hyr1 Provide Cross-Kingdom Protection against Gram-Negative Bacteria. Front. Immunol. 2020, 11, 76. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Prim. 2018, 4, 18026. [Google Scholar] [CrossRef]
- Erwig, L.P.; Gow, N.A. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176. [Google Scholar] [CrossRef]
- Iliev, I.D.; Leonardi, I. Fungal dysbiosis: Immunity and interactions at mucosal barriers. Nat. Rev. Immunol. 2017, 17, 635–646. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef]
- Miranda, L.N.; van der Heijden, I.M.; Costa, S.F.; Sousa, A.P.; Sienra, R.A.; Gobara, S.; Santos, C.R.; Lobo, R.D.; Pessoa, V.P., Jr.; Levin, A.S. Candida colonisation as a source for candidaemia. J. Hosp. Infect. 2009, 72, 9–16. [Google Scholar] [CrossRef]
- Zhai, B.; Ola, M.; Rolling, T.; Tosini, N.L.; Joshowitz, S.; Littmann, E.R.; Amoretti, L.A.; Fontana, E.; Wright, R.J.; Miranda, E.; et al. High-resolution mycobiota analysis reveals dynamic intestinal translocation preceding invasive candidiasis. Nat. Med. 2020, 26, 59–64. [Google Scholar] [CrossRef]
- Prieto, D.; Correia, I.; Pla, J.; Román, E. Adaptation of Candida albicans to commensalism in the gut. Future Microbiol. 2016, 11, 567–583. [Google Scholar] [CrossRef]
- Neville, B.A.; d’Enfert, C.; Bougnoux, M.E. Candida albicans commensalism in the gastrointestinal tract. FEMS Yeast Res. 2015, 15, fov081. [Google Scholar] [CrossRef]
- Perez, J.C.; Kumamoto, C.A.; Johnson, A.D. Candida albicans commensalism and pathogenicity are intertwined traits directed by a tightly knit transcriptional regulatory circuit. PLoS Biol. 2013, 11, e1001510. [Google Scholar] [CrossRef]
- Stoldt, V.R.; Sonneborn, A.; Leuker, C.E.; Ernst, J.F. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997, 16, 1982–1991. [Google Scholar] [CrossRef]
- White, S.J.; Rosenbach, A.; Lephart, P.; Nguyen, D.; Benjamin, A.; Tzipori, S.; Whiteway, M.; Mecsas, J.; Kumamoto, C.A. Self-Regulation of Candida albicans Population Size during GI Colonization. PLoS Pathog. 2007, 3, e184. [Google Scholar] [CrossRef]
- Pierce, J.V.; Dignard, D.; Whiteway, M.; Kumamoto, C.A. Normal adaptation of Candida albicans to the murine gastrointestinal tract requires Efg1p-dependent regulation of metabolic and host defense genes. Eukaryot. Cell 2013, 12, 37–49. [Google Scholar] [CrossRef]
- Sonneborn, A.; Tebarth, B.; Ernst, J.F. Control of white-opaque phenotypic switching in Candida albicans by the Efg1p morphogenetic regulator. Infect. Immun. 1999, 67, 4655–4660. [Google Scholar] [CrossRef]
- Zordan, R.E.; Galgoczy, D.J.; Johnson, A.D. Epigenetic properties of white-opaque switching in Candida albicans are based on a self-sustaining transcriptional feedback loop. Proc. Natl. Acad. Sci. USA 2006, 103, 12807–12812. [Google Scholar] [CrossRef]
- Srikantha, T.; Borneman, A.R.; Daniels, K.J.; Pujol, C.; Wu, W.; Seringhaus, M.R.; Gerstein, M.; Yi, S.; Snyder, M.; Soll, D.R. TOS9 regulates white-opaque switching in Candida albicans. Eukaryot. Cell 2006, 5, 1674–1687. [Google Scholar] [CrossRef]
- Huang, G.; Wang, H.; Chou, S.; Nie, X.; Chen, J.; Liu, H. Bistable expression of WOR1, a master regulator of white-opaque switching in Candida albicans. Proc. Natl. Acad. Sci. USA 2006, 103, 12813–12818. [Google Scholar] [CrossRef]
- Zordan, R.E.; Miller, M.G.; Galgoczy, D.J.; Tuch, B.B.; Johnson, A.D. Interlocking transcriptional feedback loops control white-opaque switching in Candida albicans. PLoS Biol. 2007, 5, e256. [Google Scholar] [CrossRef]
- Hernday, A.D.; Lohse, M.B.; Fordyce, P.M.; Nobile, C.J.; DeRisi, J.L.; Johnson, A.D. Structure of the transcriptional network controlling white-opaque switching in Candida albicans. Mol. Microbiol. 2013, 90, 22–35. [Google Scholar] [CrossRef]
- Wang, H.; Song, W.; Huang, G.; Zhou, Z.; Ding, Y.; Chen, J. Candida albicans Zcf37, a zinc finger protein, is required for stabilization of the white state. FEBS Lett. 2011, 585, 797–802. [Google Scholar] [CrossRef]
- Frazer, C.; Staples, M.I.; Kim, Y.; Hirakawa, M.; Dowell, M.A.; Johnson, N.V.; Hernday, A.D.; Ryan, V.H.; Fawzi, N.L.; Finkelstein, I.J.; et al. Epigenetic cell fate in Candida albicans is controlled by transcription factor condensates acting at super-enhancer-like elements. Nat. Microbiol. 2020, 5, 1374–1389. [Google Scholar] [CrossRef]
- Noble, S.M.; Gianetti, B.A.; Witchley, J.N. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 2017, 15, 96–108. [Google Scholar] [CrossRef]
- Lohse, M.B.; Ene, I.V.; Craik, V.B.; Hernday, A.D.; Mancera, E.; Morschhauser, J.; Bennett, R.J.; Johnson, A.D. Systematic Genetic Screen for Transcriptional Regulators of the Candida albicans White-Opaque Switch. Genetics 2016, 203, 1679–1692. [Google Scholar] [CrossRef][Green Version]
- Pande, K.; Chen, C.; Noble, S.M. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat. Genet. 2013, 45, 1088–1091. [Google Scholar] [CrossRef]
- Kvaal, C.A.; Srikantha, T.; Soll, D.R. Misexpression of the white-phase-specific gene WH11 in the opaque phase of Candida albicans affects switching and virulence. Infect. Immun. 1997, 65, 4468–4475. [Google Scholar] [CrossRef]
- Ene, I.V.; Lohse, M.B.; Vladu, A.V.; Morschhauser, J.; Johnson, A.D.; Bennett, R.J. Phenotypic Profiling Reveals that Candida albicans Opaque Cells Represent a Metabolically Specialized Cell State Compared to Default White Cells. mBio 2016, 7, e01269-16. [Google Scholar] [CrossRef]
- Vico, S.H.; Prieto, D.; Monge, R.A.; Roman, E.; Pla, J. The Glyoxylate Cycle Is Involved in White-Opaque Switching in Candida albicans. J. Fungi 2021, 7, 502. [Google Scholar] [CrossRef]
- Prieto, D.; Román, E.; Alonso-Monge, R.; Pla, J. Overexpression of the Transcriptional Regulator WOR1 Increases Susceptibility to Bile Salts and Adhesion to the Mouse Gut Mucosa in Candida albicans. Front. Cell. Infect. Microbiol. 2017, 7, 389. [Google Scholar] [CrossRef] [PubMed]
- Gillum, A.M.; Tsay, E.Y.H.; Kirsch, D.R. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 1984, 198, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Fonzi, W.A.; Irwin, M.Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics 1993, 134, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Román, E.; Prieto, D.; Martin, R.; Correia, I.; Mesa Arango, A.C.; Alonso-Monge, R.; Zaragoza, O.; Pla, J. Role of catalase overproduction in drug resistance and virulence in Candida albicans. Future Microbiol. 2016, 11, 1279–1297. [Google Scholar] [CrossRef]
- Prieto, A.D.; Román, E.; Correia, I.; Pla, J. The HOG pathway is critical for the colonization of the mouse gastrointestinal tract by Candida albicans. PLoS ONE 2014, 9, e87128. [Google Scholar] [CrossRef]
- Román, E.; Coman, I.; Prieto, D.; Alonso-Monge, R.; Pla, J. Implementation of a CRISPR-based system for gene regulation in Candida albicans. In Proceedings of the FEBS Advanced Lecture Course, La Colle sur Loup, France, 18–24 May 2019. [Google Scholar]
- Arana, D.M.; Alonso-Monge, R.; Du, C.; Calderone, R.; Pla, J. Differential susceptibility of mitogen-activated protein kinase pathway mutants to oxidative-mediated killing by phagocytes in the fungal pathogen Candida albicans. Cell. Microbiol. 2007, 9, 1647–1659. [Google Scholar] [CrossRef]
- Román, E.; Alonso-Monge, R.; Gong, Q.; Li, D.; Calderone, R.; Pla, J. The Cek1 MAPK is a short-lived protein regulated by quorum sensing in the fungal pathogen Candida albicans. FEMS Yeast Res. 2009, 9, 942–955. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Martín, H.; Arroyo, J.; Sánchez, M.; Molina, M.; Nombela, C. Activity of the yeast MAP kinase homologue Slt2 is critically required for cell integrity at 37 degrees C. Mol. Gen. Genet. 1993, 241, 177–184. [Google Scholar] [CrossRef]
- Herrero-de-Dios, C.; Román, E.; Pla, J.; Alonso-Monge, R. Hog1 Controls Lipids Homeostasis Upon Osmotic Stress in Candida albicans. J. Fungi 2020, 6, 355. [Google Scholar] [CrossRef]
- Sharma, M.; Dhamgaye, S.; Singh, A.; Prasad, R. Lipidome analysis reveals antifungal polyphenol curcumin affects membrane lipid homeostasis. Front. Biosci. Elite Ed. 2012, 4, 1195–1209. [Google Scholar] [CrossRef]
- Barbacini, P.; Casas, J.; Torretta, E.; Capitanio, D.; Maccallini, G.; Hirschler, V.; Gelfi, C. Regulation of Serum Sphingolipids in Andean Children Born and Living at High Altitude (3775 m). Int. J. Mol. Sci. 2019, 20, 2835. [Google Scholar] [CrossRef]
- Simbari, F.; McCaskill, J.; Coakley, G.; Millar, M.; Maizels, R.M.; Fabriás, G.; Casas, J.; Buck, A.H. Plasmalogen enrichment in exosomes secreted by a nematode parasite versus those derived from its mouse host: Implications for exosome stability and biology. J. Extracell. Vesicles 2016, 5, 30741. [Google Scholar] [CrossRef]
- Sibirny, A.A. Yeast peroxisomes: Structure, functions and biotechnological opportunities. FEMS Yeast Res. 2016, 16, fow038. [Google Scholar] [CrossRef]
- Urrialde, V.; Alburquerque, B.; Guirao-Abad, J.P.; Pla, J.; Argüelles, J.C.; Alonso-Monge, R. Arsenic inorganic compounds cause oxidative stress mediated by the transcription factor PHO4 in Candida albicans. Microbiol. Res. 2017, 203, 10–18. [Google Scholar] [CrossRef]
- Wong, D.; Plumb, J.; Talab, H.; Kurdi, M.; Pokhrel, K.; Oelkers, P. Genetically Compromising Phospholipid Metabolism Limits Candida albicans’ Virulence. Mycopathologia 2019, 184, 213–226. [Google Scholar] [CrossRef]
- Chen, Y.L.; Montedonico, A.E.; Kauffman, S.; Dunlap, J.R.; Menn, F.M.; Reynolds, T.B. Phosphatidylserine synthase and phosphatidylserine decarboxylase are essential for cell wall integrity and virulence in Candida albicans. Mol. Microbiol. 2010, 75, 1112–1132. [Google Scholar] [CrossRef]
- Cassilly, C.D.; Farmer, A.T.; Montedonico, A.E.; Smith, T.K.; Campagna, S.R.; Reynolds, T.B. Role of phosphatidylserine synthase in shaping the phospholipidome of Candida albicans. FEMS Yeast Res. 2017, 17, fox007. [Google Scholar] [CrossRef]
- Cassilly, C.D.; Reynolds, T.B. PS, It’s Complicated: The Roles of Phosphatidylserine and Phosphatidylethanolamine in the Pathogenesis of Candida albicans and Other Microbial Pathogens. J. Fungi 2018, 4, 28. [Google Scholar] [CrossRef]
- Lv, Q.Z.; Yan, L.; Jiang, Y.Y. The synthesis, regulation, and functions of sterols in Candida albicans: Well-known but still lots to learn. Virulence 2016, 7, 649–659. [Google Scholar] [CrossRef]
- Setiadi, E.R.; Doedt, T.; Cottier, F.; Noffz, C.; Ernst, J.F. Transcriptional response of Candida albicans to hypoxia: Linkage of oxygen sensing and Efg1p-regulatory networks. J. Mol. Biol. 2006, 361, 399–411. [Google Scholar] [CrossRef]
- Prasad, R.; Kapoor, K. Multidrug resistance in yeast Candida. Int. Rev. Cytol. 2005, 242, 215–248. [Google Scholar]
- Banerjee, D.; Martin, N.; Nandi, S.; Shukla, S.; Dominguez, A.; Mukhopadhyay, G.; Prasad, R. A genome-wide steroid response study of the major human fungal pathogen Candida albicans. Mycopathologia 2007, 164, 1–17. [Google Scholar] [CrossRef]
- Prasad, T.; Hameed, S.; Manoharlal, R.; Biswas, S.; Mukhopadhyay, C.K.; Goswami, S.K.; Prasad, R. Morphogenic regulator EFG1 affects the drug susceptibilities of pathogenic Candida albicans. FEMS Yeast Res. 2010, 10, 587–596. [Google Scholar] [CrossRef]
- Ivnitski-Steele, I.; Holmes, A.R.; Lamping, E.; Monk, B.C.; Cannon, R.D.; Sklar, L.A. Identification of Nile red as a fluorescent substrate of the Candida albicans ATP-binding cassette transporters Cdr1p and Cdr2p and the major facilitator superfamily transporter Mdr1p. Anal. Biochem. 2009, 394, 87–91. [Google Scholar] [CrossRef]
- Brun, S.; Aubry, C.; Lima, O.; Filmon, R.; Berges, T.; Chabasse, D.; Bouchara, J.P. Relationships between respiration and susceptibility to azole antifungals in Candida glabrata. Antimicrob. Agents Chemother. 2003, 47, 847–853. [Google Scholar] [CrossRef]
- Chen, X.J.; Clark-Walker, G.D. The petite mutation in yeasts: 50 years on. Int. Rev. Cytol. 2000, 194, 197–238. [Google Scholar] [CrossRef]
- Toffaletti, D.L.; Nielsen, K.; Dietrich, F.; Heitman, J.; Perfect, J.R. Cryptococcus neoformans mitochondrial genomes from serotype A and D strains do not influence virulence. Curr. Genet. 2004, 46, 193–204. [Google Scholar] [CrossRef]
- Wilkie, D.; Saunders, G.; Linnane, A.W. Inhibition of respiratory enzyme synthesis in yeast by chloramphenicol: Relationship between chloramphenicol tolerance and resistance to other antibacterial antibiotics. Genet. Res. 1967, 10, 199–203. [Google Scholar] [CrossRef]
- Bambach, A.; Fernandes, M.P.; Ghosh, A.; Kruppa, M.; Alex, D.; Li, D.; Fonzi, W.A.; Chauhan, N.; Sun, N.; Agrellos, O.A.; et al. Goa1p of Candida albicans localizes to the mitochondria during stress and is required for mitochondrial function and virulence. Eukaryot. Cell 2009, 8, 1706–1720. [Google Scholar] [CrossRef]
- Li, D.; Chen, H.; Florentino, A.; Alex, D.; Sikorski, P.; Fonzi, W.A.; Calderone, R. Enzymatic dysfunction of mitochondrial complex I of the Candida albicans goa1 mutant is associated with increased reactive oxidants and cell death. Eukaryot. Cell 2011, 10, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Monge, R.; Carvaihlo, S.; Nombela, C.; Rial, E.; Pla, J. The Hog1 MAP kinase controls respiratory metabolism in the fungal pathogen Candida albicans. Microbiology 2009, 155, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Mahto, K.K.; Prasad, R. Lipidomics and in vitro azole resistance in Candida albicans. OMICS J. Integr. Biol. 2013, 17, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Singh, A. Lipids of Candida albicans and their role in multidrug resistance. Curr. Genet. 2013, 59, 243–250. [Google Scholar] [CrossRef]
- Park, Y.N.; Pujol, C.; Wessels, D.J.; Soll, D.R. Candida albicans Double Mutants Lacking both EFG1 and WOR1 Can Still Switch to Opaque. mSphere 2020, 5, e.00918-20. [Google Scholar] [CrossRef]
- He, G.; Shankar, R.A.; Chzhan, M.; Samouilov, A.; Kuppusamy, P.; Zweier, J.L. Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc. Natl. Acad. Sci. USA 1999, 96, 4586–4591. [Google Scholar] [CrossRef]
- Park, Y.N.; Conway, K.; Pujol, C.; Daniels, K.J.; Soll, D.R. EFG1 Mutations, Phenotypic Switching, and Colonization by Clinical a/α Strains of Candida albicans. mSphere 2020, 5, e.00795-19. [Google Scholar] [CrossRef]


| Strain Name (Original) | Background Strain and Genotype | Reference |
|---|---|---|
| SC5314 | Clinical isolate | [32] |
| CAI4 | ura3Δ::imm434/ura3Δ::imm434 iro1/iro1::imm434 | [33] |
| CAI4-pNRUe (REP40) | [CAI4] ADH1/adh1::tTATETPR-myc-URA3 | [34] |
| CAI4-RFP | [CAI4] ADH1/adh1::TDH3PRtTATETPR-dTOM2-URA3 | [31] |
| CAI4-WOR1OE | [CAI4] ADH1/adh1::TDH3PRtTATETPR-WOR1-myc-URA3 | [31] |
| SU6 | [CAI4-pNRUe] ARD1/ard1::TDH3PR-GFP-PXP2M SAT1 | This study |
| SU7 | [CAI4-WOR1OE] ARD1/ard1::TDH3PR-GFP-PXP2M SAT1 | This study |
| hog1 ura3 (HI7) | [CAI4] hog1::hisG/hog1::hisG | [35] |
| hog1-pNRUe | [hog1 ura3] ADH1/adh1::tTATETPR-myc-URA3 | Román, submitted |
| hog1-WOR1OE | [hog1 ura3] ADH1/adh1::TDH3PRtTATeTPR-WOR1-myc-URA3 | Román, submitted |
| Primer | Sequence (5′–3′) | Reference |
|---|---|---|
| o-ACTQTup | TGGTGGTTCTATCTTGGCTTCA | [38] |
| o-ACTQTlw | ATCCACATTTGTTGGAAAGTAGA | [38] |
| o-ERG11up-QT | TTACCTCATTATTGGAGACGTGATG | This study |
| o-ERG11lw-QT | CACCACGTTCTCTTCTCAGTTTAATT | This study |
| o1-PXP2 | AATGCGGCCGCATGGCTATGCTTACTAAATCTATACATGATG | This study |
| o2-PXP2 | ACATGGATTTGGTCCCATTGATGGTG | This study |
| o3-PXP2 | CACCATCAATGGGACCAAATCCATGT | This study |
| o4-PXP2 | GAAGCAGCTGCTAAATTATCAAGATAATTAGATCTAAT | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo-Vico, S.; Casas, J.; García, C.; Lillo, M.P.; Alonso-Monge, R.; Román, E.; Pla, J. Overexpression of the White Opaque Switching Master Regulator Wor1 Alters Lipid Metabolism and Mitochondrial Function in Candida albicans. J. Fungi 2022, 8, 1028. https://doi.org/10.3390/jof8101028
Hidalgo-Vico S, Casas J, García C, Lillo MP, Alonso-Monge R, Román E, Pla J. Overexpression of the White Opaque Switching Master Regulator Wor1 Alters Lipid Metabolism and Mitochondrial Function in Candida albicans. Journal of Fungi. 2022; 8(10):1028. https://doi.org/10.3390/jof8101028
Chicago/Turabian StyleHidalgo-Vico, Susana, Josefina Casas, Carolina García, M. Pilar Lillo, Rebeca Alonso-Monge, Elvira Román, and Jesús Pla. 2022. "Overexpression of the White Opaque Switching Master Regulator Wor1 Alters Lipid Metabolism and Mitochondrial Function in Candida albicans" Journal of Fungi 8, no. 10: 1028. https://doi.org/10.3390/jof8101028
APA StyleHidalgo-Vico, S., Casas, J., García, C., Lillo, M. P., Alonso-Monge, R., Román, E., & Pla, J. (2022). Overexpression of the White Opaque Switching Master Regulator Wor1 Alters Lipid Metabolism and Mitochondrial Function in Candida albicans. Journal of Fungi, 8(10), 1028. https://doi.org/10.3390/jof8101028








