A Molecular Systematics and Taxonomy Research on Trechispora (Hydnodontaceae, Trechisporales): Concentrating on Three New Trechispora Species from East Asia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Herbarium Specimen Preparation
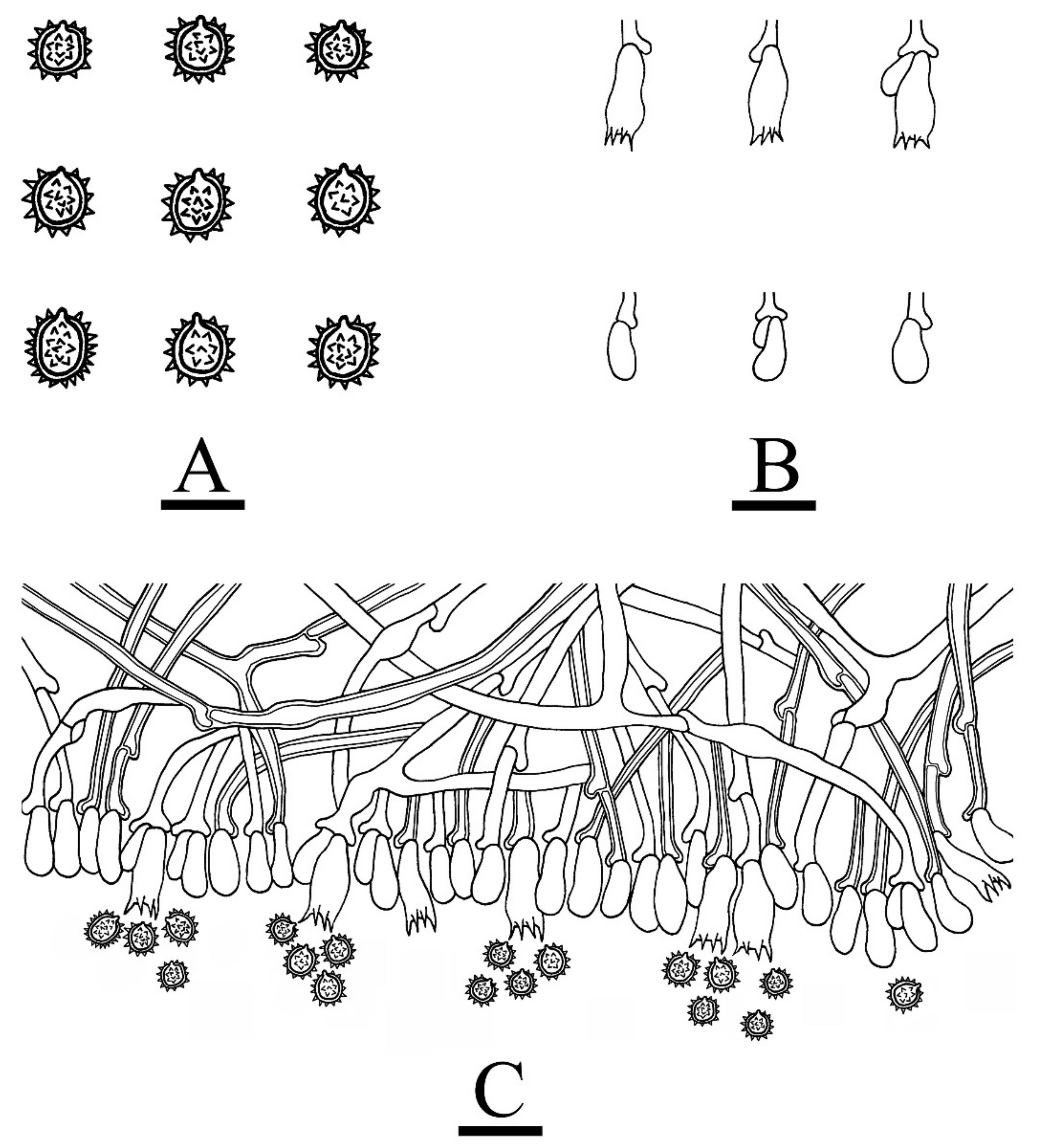
2.2. Morphology
2.3. Molecular Phylogeny
3. Results
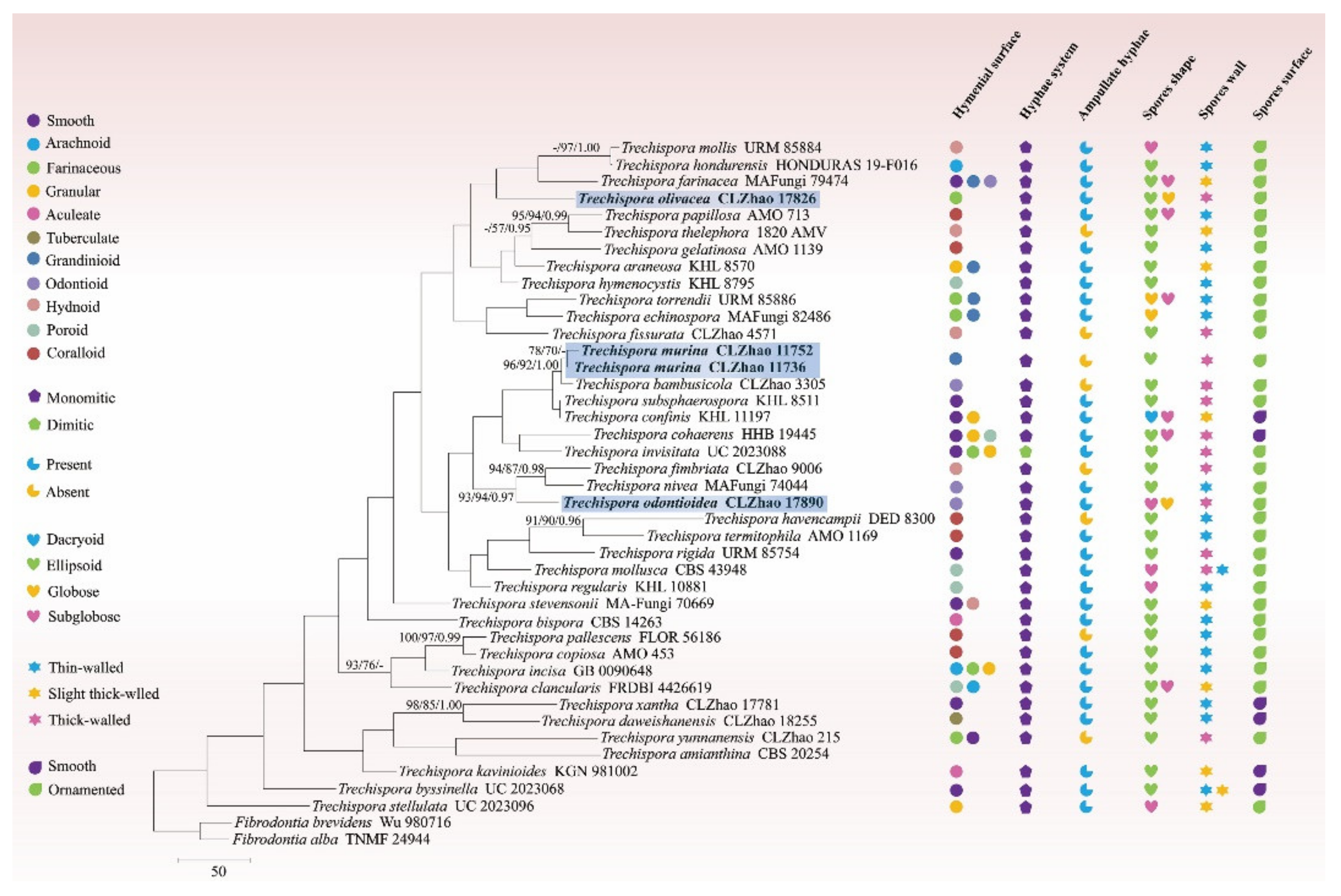
3.1. Molecular Phylogeny
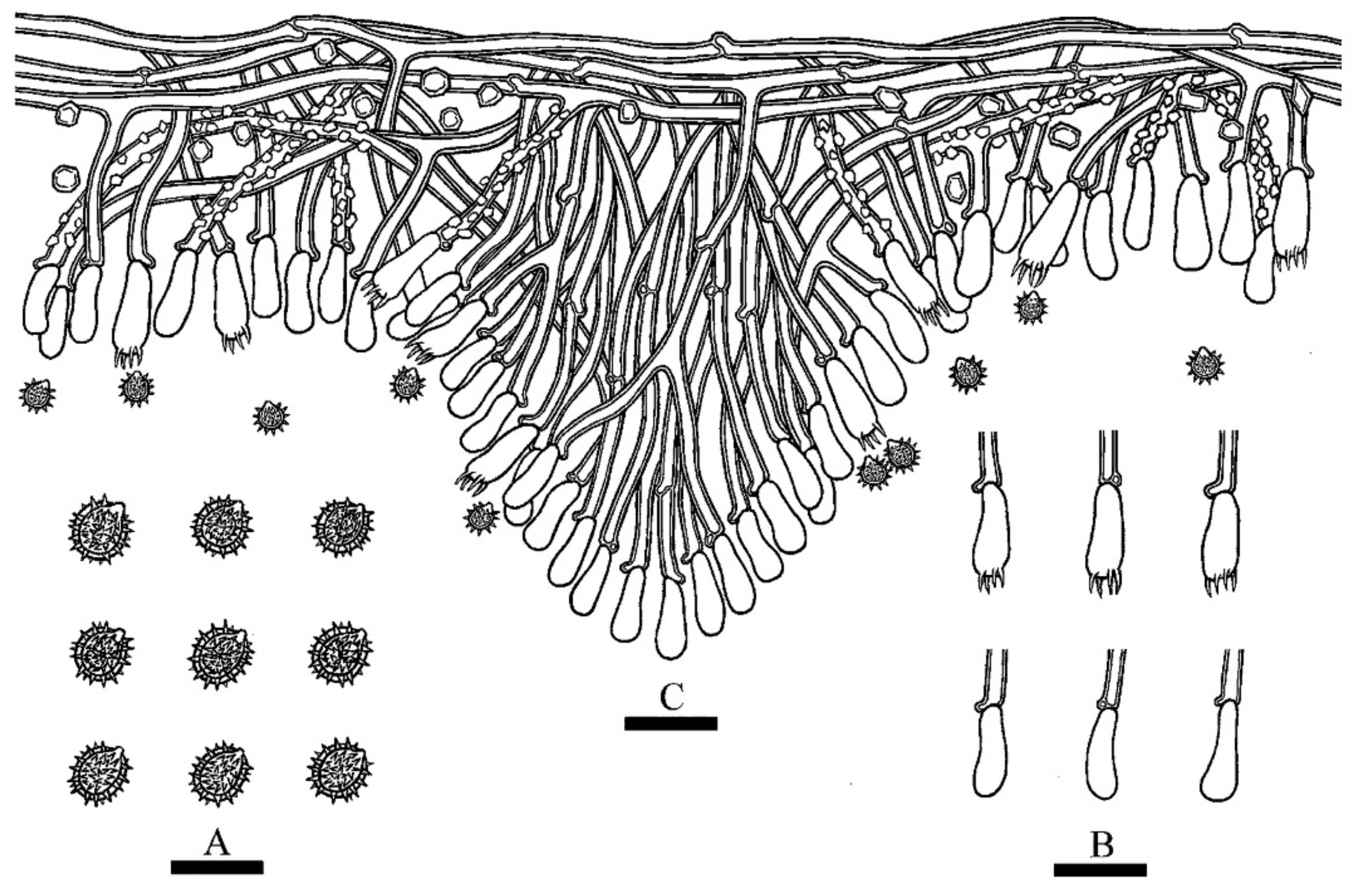
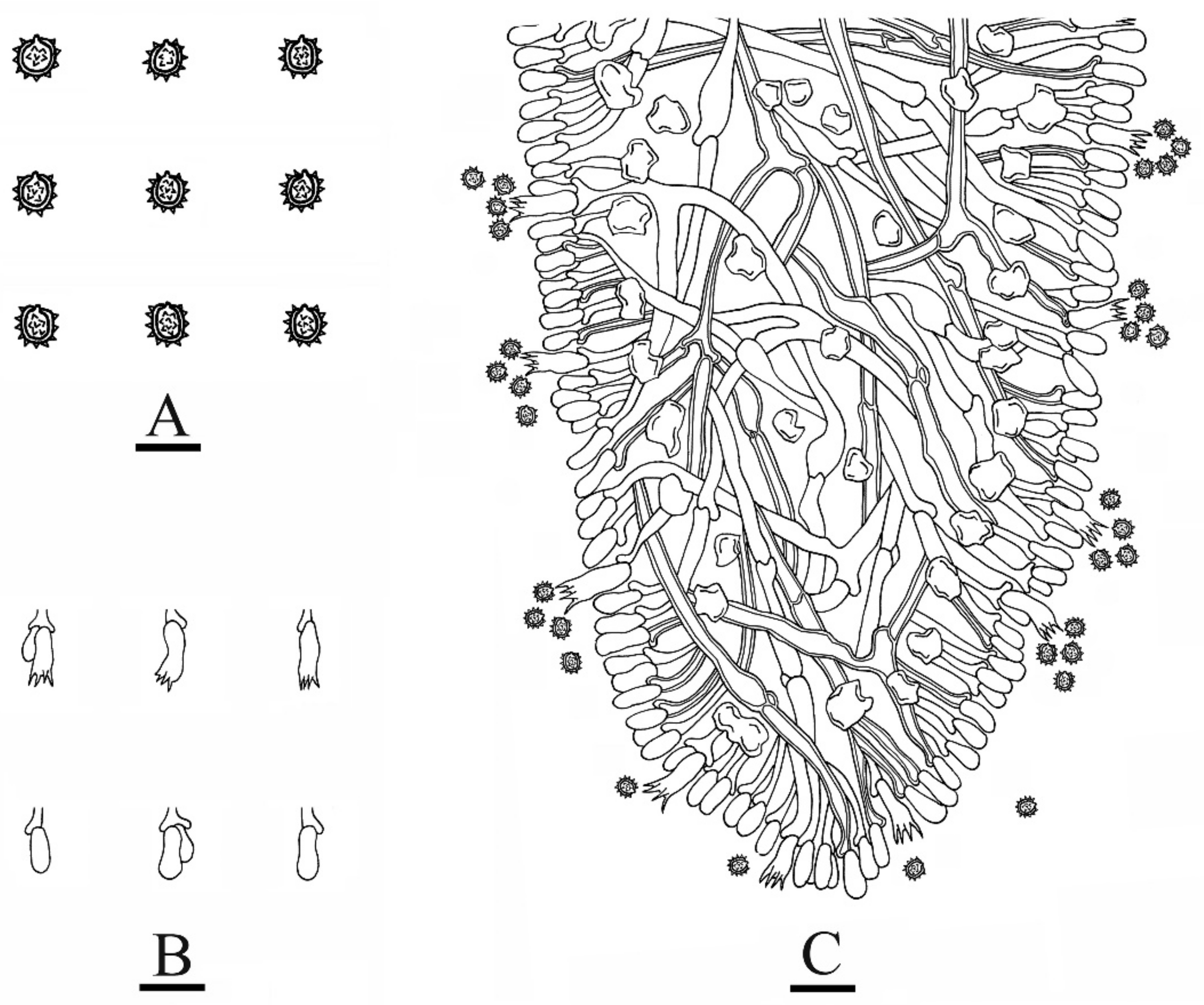
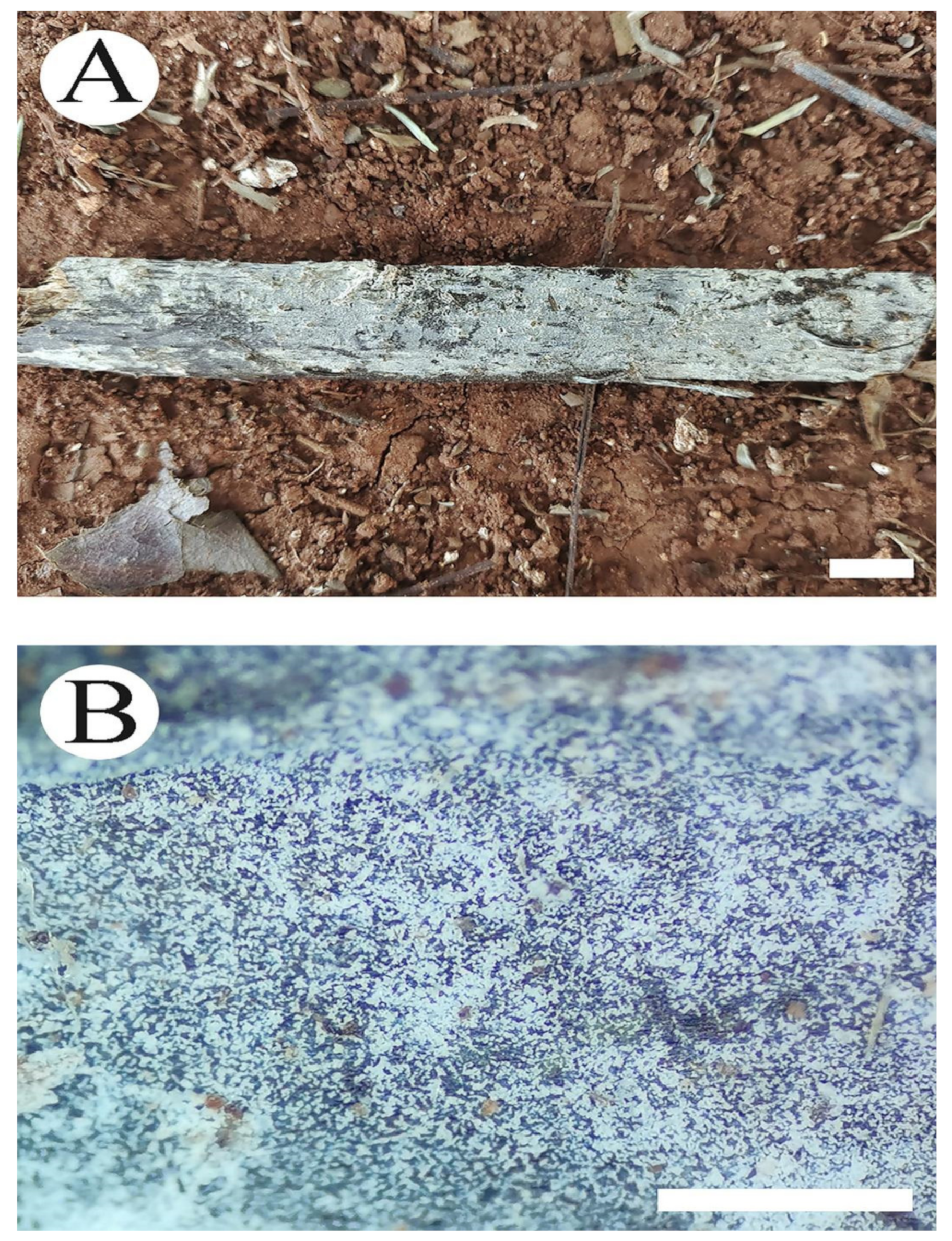
3.2. Taxonomy
4. Discussion
- 1. Basidiospores smooth-------------------------------------------------------------------------------------2
- 1′ Basidiospores aculeate, verrucose or ornamented--------------------------------------------------5
- 2. Ampullate hyphae > 5 μm in width, basidiospores angular----------------T. subsphaerospora
- 2′ Ampullate hyphae < 5 μm in width, basidiospores ellipsoid------------------------------------3
- 3. Basidiospores thick-walled-----------------------------------------------------------------T. cohaerens
- 3′ Basidiospores thin-walled--------------------------------------------------------------------------------4
- 4. Hymenial surface tuberculate-------------------------------------------------------T. daweishanensis
- 4′ Hymenial surface smooth----------------------------------------------------------------------T. xantha
- 5. Hyphal system dimitic------------------------------------------------------------------------T. dimitica
- 5′ Hyphal system monomitic-------------------------------------------------------------------------------6
- 6. Hyphae without ampullate septa----------------------------------------------------------------------7
- 6′ Hyphae with ampullate septa-------------------------------------------------------------------------12
- 7. Basidiospores thin-walled, ovoid to subglobose---------------------------------------T. suberosa
- 7′ Basidiospores thick-walled, ellipsoid-----------------------------------------------------------------8
- 8. Basidiospores > 7 μm in length--------------------------------------------------------T. yunnanensis
- 8′ Basidiospores < 7 μm in length-------------------------------------------------------------------------9
- 9. Basidiomata margin greyish----------------------------------------------------------------T. murina
- 9′ Basidiomata margin white to cream----------------------------------------------------------------10
- 10. Hymenial surface odontioid---------------------------------------------------------T. bambusicola
- 10′ Hymenial surface hydnoid--------------------------------------------------------------------------11
- 11. Hymenophore with blunt aculei--------------------------------------------------------T. fimbriata
- 11′ Hymenophore with sharp aculei--------------------------------------------------------T. fissurata
- 12. Sphaerocysts present, hyphae inflated-------------------------------------------T. hymenocystis
- 12′ Sphaerocysts absent, hyphae uninflated---------------------------------------------------------13
- 13. Ampullate septa > 6 μm in width------------------------------------------------------------------14
- 13′ Ampullate septa < 6 μm in width------------------------------------------------------------------15
- 14. Basidiospores sparsely verrucose-----------------------------------------------T. polygonospora
- 14′ Basidiospores densely aculeate---------------------------------------------------------T. mollusca
- 15. Subhymenium with short-celled hyphae--------------------------------------------------------16
- 15′ Subhymenium with long-celled hyphae---------------------------------------------------------17
- 16. Basidiome thin, ochraceous--------------------------------------------------------------T. farinacea
- 16′ Basidiome thick, dirty white to buff-------------------------------------------------------T. rigida
- 17. Basidiospores thin-walled---------------------------------------------------------------------------18
- 17′ Basidiospores thick-walled--------------------------------------------------------------------------19
- 18. Hymenophore with hydnoid-----------------------------------------------------------------T. nivea
- 18′ Hymenophore without hydnoid------------------------------------------------------T. microspora
- 19. Basidiospores > 5 μm in length---------------------------------------------------------T. praefocata
- 19′ Basidiospores < 5 μm in length---------------------------------------------------------------------20
- 20. Hymenial surface farinaceous with olivaceous--------------------------------------T. olivacea
- 20′ Hymenial surface odontioid with buff--------------------------------------------T. odontioidea
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, T.Y.; Stajich, J.E.; Hittinger, C.T.; Rokas, A. Toward a fully resolved fungal tree of life. Annu. Rev. Microbiol. 2020, 74, 291–313. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Koljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, R.L.; Ryvarden, L. North American Polypores 1–2; Fungiflora: Oslo, Norway, 1987; pp. 1–433. [Google Scholar]
- Núñez, M.; Ryvarden, L. East Asian polypores 2. Synop. Fungorum 2001, 14, 165–522. [Google Scholar]
- Bernicchia, A.; Gorjón, S.P. Fungi Europaei 12: Corticiaceae s.l.; Edizioni Candusso: Alassio, Italy, 2010. [Google Scholar]
- Dai, Y.C. Polypore diversity in China with an annotated checklist of Chinese polypores. Mycoscience 2012, 53, 49–80. [Google Scholar] [CrossRef]
- Ryvarden, L.; Melo, I. Poroid fungi of Europe. Synop. Fungorum 2014, 31, 1–455. [Google Scholar]
- Dai, Y.C.; Cui, B.K.; Si, J.; He, S.H.; Hyde, K.D.; Yuan, H.S.; Liu, X.Y.; Zhou, L.W. Dynamics of the worldwide number of fungi with emphasis on fungal diversity in China. Mycol. Prog. 2015, 14, 62. [Google Scholar] [CrossRef]
- Dai, Y.C.; Yang, Z.L.; Cui, B.K.; Wu, G.; Yuan, H.S.; Zhou, L.W.; He, S.H.; Ge, Z.W.; Wu, F.; Wei, Y.L.; et al. Diversity and systematics of the important macrofungi in Chinese forests. Mycosystema 2021, 40, 770–805. [Google Scholar]
- Cui, B.K.; Yuan, H.S.; Zhou, L.W.; He, S.H.; Wei, Y.L. Diversity of wood-decaying fungi in conifer trees of the Greater and Lesser Khinggan Mountains. Biodivers. Sci. 2019, 27, 887–895. [Google Scholar] [CrossRef]
- Wu, F.; Yuan, H.S.; Zhou, L.W.; Yuan, Y.; Cui, B.K.; Dai, Y.C. Polypore diversity in South China. Mycosystema 2020, 39, 653–682. [Google Scholar]
- Luo, K.Y.; Chen, Z.Y.; Zhao, C.L. Phylogenetic and taxonomic analyses of three new wood-inhabiting fungi of Xylodon (Basidiomycota) in a forest ecological system. J. Fungi 2022, 8, 405. [Google Scholar] [CrossRef]
- Qu, M.H.; Wang, D.Q.; Zhao, C.L. A phylogenetic and taxonomic study on Xylodon (Hymenochaetales): Focusing on three new Xylodon species from southern China. J. Fungi 2022, 8, 35. [Google Scholar] [CrossRef]
- Hibbett, D.S.; Binder, M.; Bischoff, J.F.; Blackwell, M.; Cannon, P.F.; Eriksson, O.E.; Huhndorf, S.; James, T. A higher-level phylogenetic classification of the Fungi. Mycol. Res. 2007, 111, 509–547. [Google Scholar] [CrossRef]
- Larsson, K.H. Re-thinking the classification of corticioid fungi. Mycol. Res. 2007, 111, 1040–1063. [Google Scholar] [CrossRef]
- Karsten, P.A. Fragmenta mycologica XXIX. Nova Hedwig. 1890, 29, 147–149. [Google Scholar]
- Liberta, A.E. On Trechispora. Taxon 1966, 15, 317–319. [Google Scholar] [CrossRef]
- Liberta, A.E. The genus Trechispora (Basidiomycetes, Corticiaceae). Can. J. Bot. 1973, 51, 1871–1892. [Google Scholar] [CrossRef]
- Larsson, K.H. Poroid species in Trechispora and the use of calcium oxalate crystals for species identification. Mycol. Res. 1994, 98, 1153–1172. [Google Scholar] [CrossRef]
- Ryvarden, L. A note on the genus Hydnodon Banker. Synop. Fungorum 2002, 15, 31–33. [Google Scholar]
- Trichiès, G.; Schultheis, B. Trechispora antipus sp. nov., une seconde espèce bisporique du genre Trechispora (Basidiomycota, Stereales). Mycotaxon 2002, 82, 453–458. [Google Scholar]
- Miettinen, O.; Larsson, K.H. Trechispora elongata species nova from North Europe. Mycotaxon 2006, 96, 193–198. [Google Scholar]
- Kirk, P.M.; Cannon, P.F.; David, J.C.; Minter, D.W.; Stalpers, J.A. Ainsworth and Bisby’s Dictionary of the Fungi, 10th ed.; CAB International Press: Wallingford, UK, 2008; p. 783. [Google Scholar] [CrossRef]
- Ordynets, A.; Larsson, K.H.; Langer, E. Two new Trechispora species from La Réunion Island. Mycol. Prog. 2015, 14, 113. [Google Scholar] [CrossRef]
- Phookamsak, R.; Hyde, K.D.; Jeewon, R.; Bhat, D.J.; Jones, E.B.G.; Maharachchikumbura, S.S.N.; Raspe´, O.; Karunarathna, S.C.; Wanasinghe, D.N.; Hongsanan, S.; et al. Fungal diversity notes 929–1035: Taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Divers. 2019, 95, 1–273. [Google Scholar] [CrossRef]
- Chikowski, R.S.; Larsson, K.H.; Gibertoni, T.B. Taxonomic novelties in Trechispora from Brazil. Mycol. Prog. 2020, 19, 1403–1414. [Google Scholar] [CrossRef]
- Meiras-Ottoni, A.; Larsson, K.H.; Gibertoni, T.B. Additions to Trechispora and the status of Scytinopogon (Trechisporales, Basidiomycota). Mycol. Prog. 2021, 20, 203–222. [Google Scholar] [CrossRef]
- Dai, Y.C. A checklist of polypores in China. Mycosystema 2009, 28, 315–327. [Google Scholar]
- Dai, Y.C. A revised checklist of corticioid and hydnoid fungi in China for 2010. Mycoscience 2011, 52, 69–79. [Google Scholar] [CrossRef]
- Yuan, H.S.; Dai, Y.C. Wood-inhabiting fungi in southern China. 6. Polypores from Guangxi Autonomous region. Ann. Bot. Fenn. 2012, 49, 341–351. [Google Scholar] [CrossRef]
- Xu, T.M.; Chen, Y.H.; Zhao, C.L. Trechispora yunnanensis sp. nov. from China. Phytotaxa 2019, 424, 253–261. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, M.J.; Su, J.H.; Yang, L.; Deng, H.; Wang, Y.H.; Wu, H.J.; Li, Y.; Wu, H.M.; Wei, X.D.; et al. The use of checklist of fungi in China database in the red list assessment of macrofungi in China. Biodivers. Sci. 2020, 28, 74–98. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, C.L. The phylogenetic relationship revealed three new wood-inhabiting fungal species from genus Trechispora. Front. Microbiol. 2021, 12, 650195. [Google Scholar] [CrossRef] [PubMed]
- Zong, T.K.; Liu, C.M.; Wu, J.R.; Zhao, C.L. Trechispora daweishanensis and T. xantha spp. nov. (Hydnodontaceae, Trechisporales) found in Yunnan Province of China. Phytotaxa 2021, 479, 147–159. [Google Scholar] [CrossRef]
- Larsson, K.H.; Larsson, E.; Kõljalg, U. High phylogenetic diversity among corticioid homobasidiomycetes. Mycol. Res. 2004, 108, 983–1002. [Google Scholar] [CrossRef]
- Telleria, M.T.; Melo, I.; Dueñas, M.; Larsson, K.H.; Paz, M.M.P. Molecular analyses confifirm Brevicellicium in Trechisporales. IMA Fungus 2013, 4, 21–28. [Google Scholar] [CrossRef]
- Jülich, W. Notes on some Basidiomycetes (Aphyllophorales and Heterobasidiomycetes). Persoonia 1982, 11, 421–428. [Google Scholar]
- Liu, S.L.; Ma, H.X.; He, S.H.; Dai, Y.C. Four new corticioid species in Trechisporales (Basidiomycota) from East Asia and notes on phylogeny of the order. MycoKeys 2019, 48, 97–113. [Google Scholar] [CrossRef]
- Kornerup, A.; Wanscher, J. Metheun Hand Book of Colour, 3rd ed.; Metheun London Ltd.: London, UK, 1978; pp. 144–148. [Google Scholar]
- Wu, F.; Zhou, L.W.; Vlasák, J.; Dai, Y.C. Global diversity and systematics of Hymenochaetaceae with poroid hymenophore. Fungal Divers. 2022, 113, 1–192. [Google Scholar] [CrossRef]
- Zhao, C.L.; Wu, Z.Q. Ceriporiopsis kunmingensis sp. nov. (Polyporales, Basidiomycota) evidenced by morphological characters and phylogenetic analysis. Mycol. Prog. 2017, 16, 93–100. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar] [CrossRef]
- Spirin, V.; Volobuev, S.; Viner, I.; Miettinen, O.; Vlasak, J.; Schoutteten, N.; Motato-Vásquez, V.; Kotiranta, H.; Hernawati; Larsson, K.H. On Sistotremastrum and similar-looking taxa (Trechisporales, Basidiomycota). Mycol. Prog. 2021, 20, 453–476. [Google Scholar] [CrossRef]
- Yurchenko, E.; Wu, S.H. Hyphoderma formosanum sp. nov. (Meruliaceae, Basidiomycota) from Taiwan. Sydowia 2014, 66, 19–23. [Google Scholar]
- Wu, F.; Yuan, Y.; Zhao, C.L. Porpomyces submucidus (Hydnodontaceae, Basidiomycota) from tropical China. Phytotaxa 2015, 230, 61–68. [Google Scholar] [CrossRef]
- Desjardin, D.E.; Perry, B.A. A new species of Scytinopogon from the island of Príncipe, Republic of São Tomé and Príncipe, West Africa. Mycosphere 2015, 6, 434–441. [Google Scholar] [CrossRef]
- Vu, D.; Groenewald, M.; de Vries, M.; Gehrmann, T.; Stielow, B.; Eberhardt, U.; Al-Hatmi, A.; Groenewald, J.Z.; Cardinali, G.; Houbraken, J.; et al. Large-scale generation and analysis of fifilamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2019, 92, 135–154. [Google Scholar] [CrossRef]
- Larsson, K.H.; Parmasto, E.; Fischer, M.; Langer, E.; Nakasone, K.K.; Redhead, S.A. Hymenochaetales: A molecular phylogeny for the hymenochaetoid clade. Mycologia 2006, 98, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Ordynets, A.; Scherf, D.; Pansegrau, F.; Denecke, J.; Langer, E. Short-spored Subulicystidium (Trechisporales, Basidiomycota): High morphological diversity and only partly clear species boundaries. MycoKeys 2018, 35, 41–99. [Google Scholar] [CrossRef]
- Ordynets, A.; Liebisch, R.; Lysenko, L.; Scherf, D.; Volobuev, S.; Saitta, A.; Larsson, K.H.; Yurchenko, E.; Buyck, B.; Bolshakov, S. Morphologically similar but not closely related: The long-spored species of Subulicystidium (Trechisporales, Basidiomycota). Mycol. Prog. 2020, 19, 691–703. [Google Scholar] [CrossRef]
- Vasco-Palacios, A.M.; Lopez-Quintero, C.; Franco-Molano, A.E.; Boekhout, T. Austroboletus amazonicus sp. nov. and Fistulinella campinaranae var. scrobiculata, two commonly occurring boletes from a forest dominated by Pseudomonotes tropenbosii (Dipterocarpaceae) in Colombian Amazonia. Mycologia 2014, 106, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large data sets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Swofford, D.L. PAUP *: Phylogenetic Analysis Using Parsimony (* and Other Methods); Version 4.0b10; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Felsenstein, J. Confidence intervals on phylogenetics: An approach using bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. The CIPRES science gateway: Enabling high-impact science for phylogenetics researchers with limited resources. Assoc. Comput. Mach. 2012, 39, 1–8. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest V2. Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Haelewaters, D.; Dima, B.; Abdel-Hafiz, A.I.; Abdel-Wahab, M.A.; Abul-Ezz, S.R.; Acar, I.; Aguirre-Acosta, E.; Aime, M.C.; Aldemir, S.; Ali, M.; et al. Fungal systematics and evolution: Fuse 6. Sydowia 2020, 72, 231–356. [Google Scholar] [CrossRef]
- Larsson, K.H. New species and combinations in Trechispora (Corticiaceae, Basidiomycotina). Nord. J. Bot. 1996, 16, 83–98. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.Q.; Gan, M.S.; Li, Y.; Shao, S.C.; Qin, W.Q.; Deng, W.Q.; Li, T.H. Diversity of Cantharellus (Cantharellales, Basidiomycota) in China with Description of Some New Species and New Records. J. Fungi 2022, 8, 483. [Google Scholar] [CrossRef]
- Song, C.G.; Chen, Y.Y.; Liu, S.; Xu, T.M.; He, X.L.; Wang, D.; Cui, B.K. A Phylogenetic and Taxonomic Study on Phellodon (Bankeraceae, Thelephorales) from China. J. Fungi 2022, 8, 429. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, G.; Tuo, Y.; Rao, G.; Zhang, Z.; Qi, Z.; Yue, L.; Liu, Y.; Zhang, T.; Li, Y.; et al. Morphological and Molecular Evidence Reveal Eight New Species of Gymnopus from Northeast China. J. Fungi 2022, 8, 349. [Google Scholar] [CrossRef]
- Mao, N.; Xu, Y.Y.; Zhao, T.Y.; Lv, J.C.; Fan, L. New Species of Mallocybe and Pseudosperma from North China. J. Fungi 2022, 8, 256. [Google Scholar] [CrossRef]
- Wu, F.; Tohtirjap, A.; Fan, L.F.; Zhou, L.W.; Alvarenga, R.L.M.; Gibertoni, T.B.; Dai, Y.C. Global diversity and updated phylogeny of Auricularia (Auriculariales, Basidiomycota). J. Fungi 2021, 7, 933. [Google Scholar] [CrossRef]
- Guan, Q.X.; Zhao, C.L. Taxonomy and phylogeny of the wood-inhabiting fungal genus Hyphoderma with descriptions of three new species from East Asia. J. Fungi 2021, 7, 308. [Google Scholar] [CrossRef]
- Wang, D.Q.; Zhao, C.L. Morphological and Phylogenetic Evidence for Recognition of Two New Species of Phanerochaete from East Asia. J. Fungi 2021, 7, 1063. [Google Scholar] [CrossRef]
- He, M.Q.; Zhao, R.L.; Hyde, K.D.; Begerow, D.; Kemler, M.; Yurkov, A.; McKenzie, E.H.C.; Raspe, O.; Kakishima, M.; Sanchez-Ramırez, S.; et al. Notes, outline and divergence times of Basidiomycota. Fungal Divers. 2019, 99, 105–367. [Google Scholar] [CrossRef]



| Species Name | Specimen No. | GenBank Accession No. | References | |
|---|---|---|---|---|
| ITS | nLSU | |||
| Brevicellicium exile | H (Spirin 8370) | MT002322 | MT002338 | [43] |
| B. olivascens | KHL 8571 | HE963792 | HE963793 | [36] |
| Dextrinocystis calamicola | He 5700 | MK204534 | MK204547 | [38] |
| Fibrodontia alba | TNMF 24944 | NR153983 | NG060401 | [24] |
| F. brevidens | Wu 9807-16 | KC928276 | KC928277 | [44] |
| Litschauerella gladiola | He 3171 | MK204555 | MK204556 | [38] |
| Luellia cystidiata | JHP 09455 | MW371211 | Unpublished | |
| Porpomyces mucidus | Dai 12692 | KT157833 | KT157838 | [45] |
| P. submucidus | Cui 5183 | KT152143 | KT152145 | [45] |
| Scytinopogon pallescens | He 5192 | MK204553 | [38] | |
| S. havencampii | DED 8300 | KT253946 | KT253947 | [46] |
| Sistotremastrum guttuliferum | He 3338 | MK204540 | MK204552 | [38] |
| S. niveocremeum | CBS 42854 | MH857381 | MH868921 | [47] |
| S. suecicum | H (Miettinen14550) | MT075860 | MT002336 | [43] |
| Sphaerobasidium minutum | KHL 11714 | DQ873652 | DQ873653 | [48] |
| Subulicystidium brachysporum | KASL 1584b | MH041544 | MH041610 | [49] |
| S. cochleum | KHL 11200 | MN207036 | MN207024 | [50] |
| S. longisporum | Ordynets 00146 | MN207039 | MN207032 | [50] |
| S. meridense | Hjm 16400 | MH041538 | MH041604 | [49] |
| Trechispora amianthina | CBS 202.54 | MH857292 | [47] | |
| T. araneosa | KHL 8570 | AF347084 | [35] | |
| T. bambusicola | CLZhao 3305 | MW544022 | MW520172 | [33] |
| T. bispora | CBS 142.63 | MH858241 | [47] | |
| T. byssinella | UC 2023068 | KP814481 | Unpublished | |
| T. clancularis | FRDBI 4426619 | MW487976 | Unpublished | |
| T. cohaerens | HHB 19445 | MW740327 | Unpublished | |
| T. copiosa | AMO 453 | MN701018 | [27] | |
| T. confinis | KHL 11197 | AY463473 | AY586719 | [35] |
| T. daweishanensis | CLZhao 18255 | MW302338 | [34] | |
| T. echinospora | MA Fungi 82486 | JX392853 | [36] | |
| T. farinacea | MA Fungi 79474 | JX392855 | JX392856 | [36] |
| T. fimbriata | CLZhao 9006 | MW544025 | MW520175 | [33] |
| T. fissurata | CLZhao 4571 | MW544027 | [33] | |
| T. gelatinosa | AMO 1139 | MN701021 | [27] | |
| T. havencampii | DED 8300 | NR154418 | [46] | |
| T. hondurensis | HONDURAS 19-F016 | MT571523 | MT636540 | Unpublished |
| T. hymenocystis | KHL 8795 | AF347090 | [35] | |
| T. incisa | GB 0090648 | KU747095 | Unpublished | |
| T. invisitata | UC 2023088 | KP814425 | Unpublished | |
| T. kavinioides | KGN 981002 | AF347086 | [35] | |
| T. mollis | URM 85884 | MK514945 | [26] | |
| T. mollusca | CBS 43948 | MH856428 | [47] | |
| T. murina | CLZhao 11736 | OL615003 | Present study | |
| T. murina | CLZhao 11752 | OL615004 | OL615009 | Present study |
| T. nivea | MA Fungi 74044 | JX392832 | [36] | |
| T.odontioidea | CLZhao 17890 | ON417458 | Present study | |
| T.olivacea | CLZhao 17826 | ON417457 | Present study | |
| T. pallescens | FLOR 56186 | MK458766 | Unpublished | |
| T. papillosa | AMO 713 | MN701022 | [27] | |
| T. regularis | KHL 10881 | AF347087 | [35] | |
| T. rigida | URM 85754 | MT406381 | [26] | |
| T. stellulata | UC 2023096 | KP814450 | Unpublished | |
| T. stevensonii | MA Fungi 70669 | JX392841 | [36] | |
| T. subsphaerospora | KHL 8511 | AF347080 | [35] | |
| T. termitophila | AMO 1169 | MN701028 | [27] | |
| T. thelephora | 1820 AMV | KF937369 | [51] | |
| T. torrendii | URM 85886 | MK515148 | [26] | |
| T. xantha | CLZhao 17781 | MW302340 | [34] | |
| T. yunnanensis | CLZhao 215 | MN654923 | [31] | |
| Tubulicium raphidisporum | He 3191 | MK204537 | MK204545 | [38] |
| T. vermiferum | KHL 8714 | — | AY463477 | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, K.; Zhao, C. A Molecular Systematics and Taxonomy Research on Trechispora (Hydnodontaceae, Trechisporales): Concentrating on Three New Trechispora Species from East Asia. J. Fungi 2022, 8, 1020. https://doi.org/10.3390/jof8101020
Luo K, Zhao C. A Molecular Systematics and Taxonomy Research on Trechispora (Hydnodontaceae, Trechisporales): Concentrating on Three New Trechispora Species from East Asia. Journal of Fungi. 2022; 8(10):1020. https://doi.org/10.3390/jof8101020
Chicago/Turabian StyleLuo, Kaiyue, and Changlin Zhao. 2022. "A Molecular Systematics and Taxonomy Research on Trechispora (Hydnodontaceae, Trechisporales): Concentrating on Three New Trechispora Species from East Asia" Journal of Fungi 8, no. 10: 1020. https://doi.org/10.3390/jof8101020
APA StyleLuo, K., & Zhao, C. (2022). A Molecular Systematics and Taxonomy Research on Trechispora (Hydnodontaceae, Trechisporales): Concentrating on Three New Trechispora Species from East Asia. Journal of Fungi, 8(10), 1020. https://doi.org/10.3390/jof8101020








