Identification of Bacillus velezensis SBB and Its Antifungal Effects against Verticillium dahliae
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial and Fungal Strains
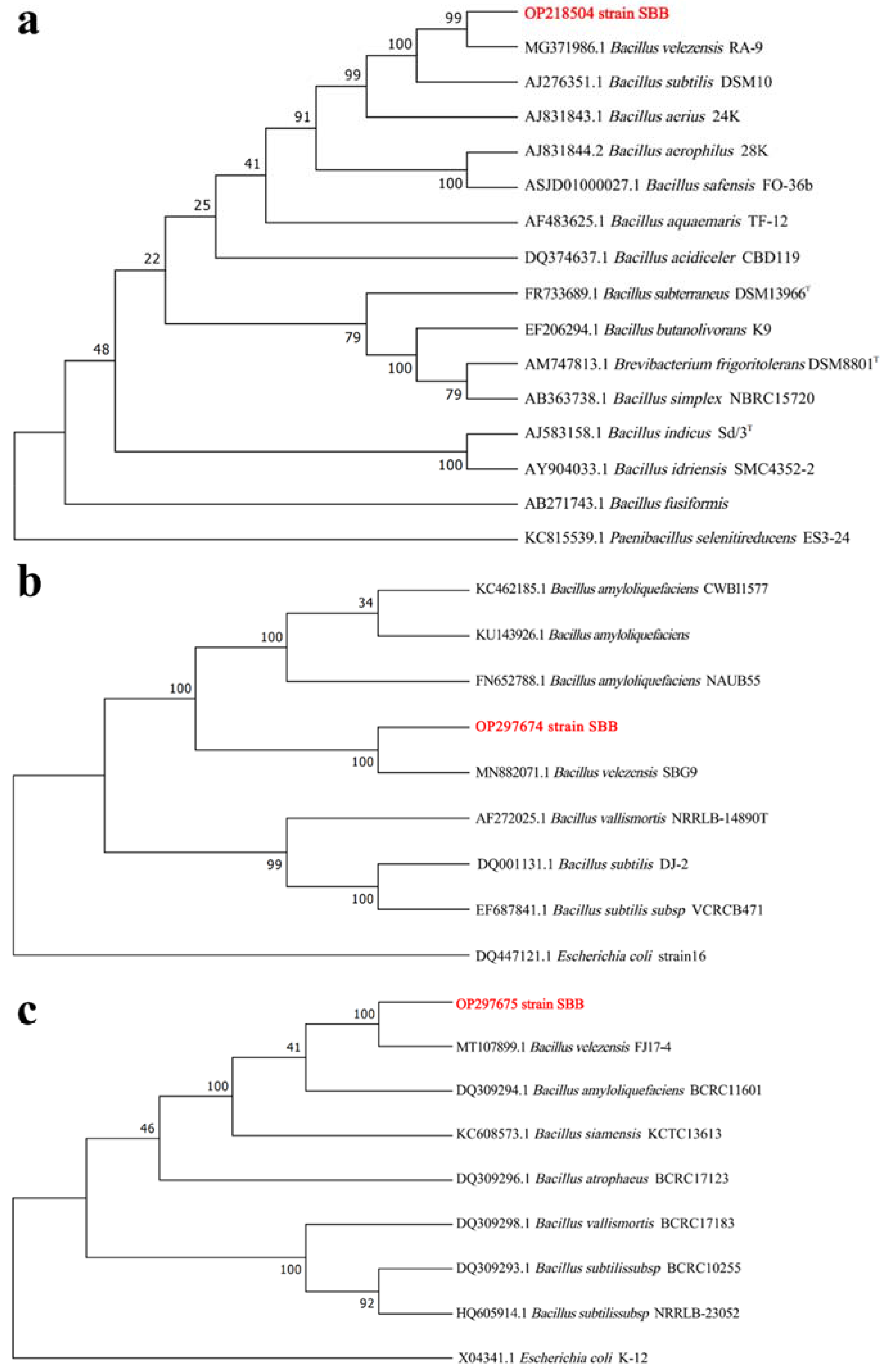
2.2. Identification of Strain SBB
2.3. Determining the Antagonistic Effect of SBB on V. dahliae
2.3.1. Effect of SBB on Mycelial Growth
2.3.2. Effect of SBB VOCs on Mycelial Growth
2.3.3. Effect of SBB Fermentation Filtrate at Different Time Periods on Mycelial Growth
2.4. Effect of SBB Filtrate on Spore Production
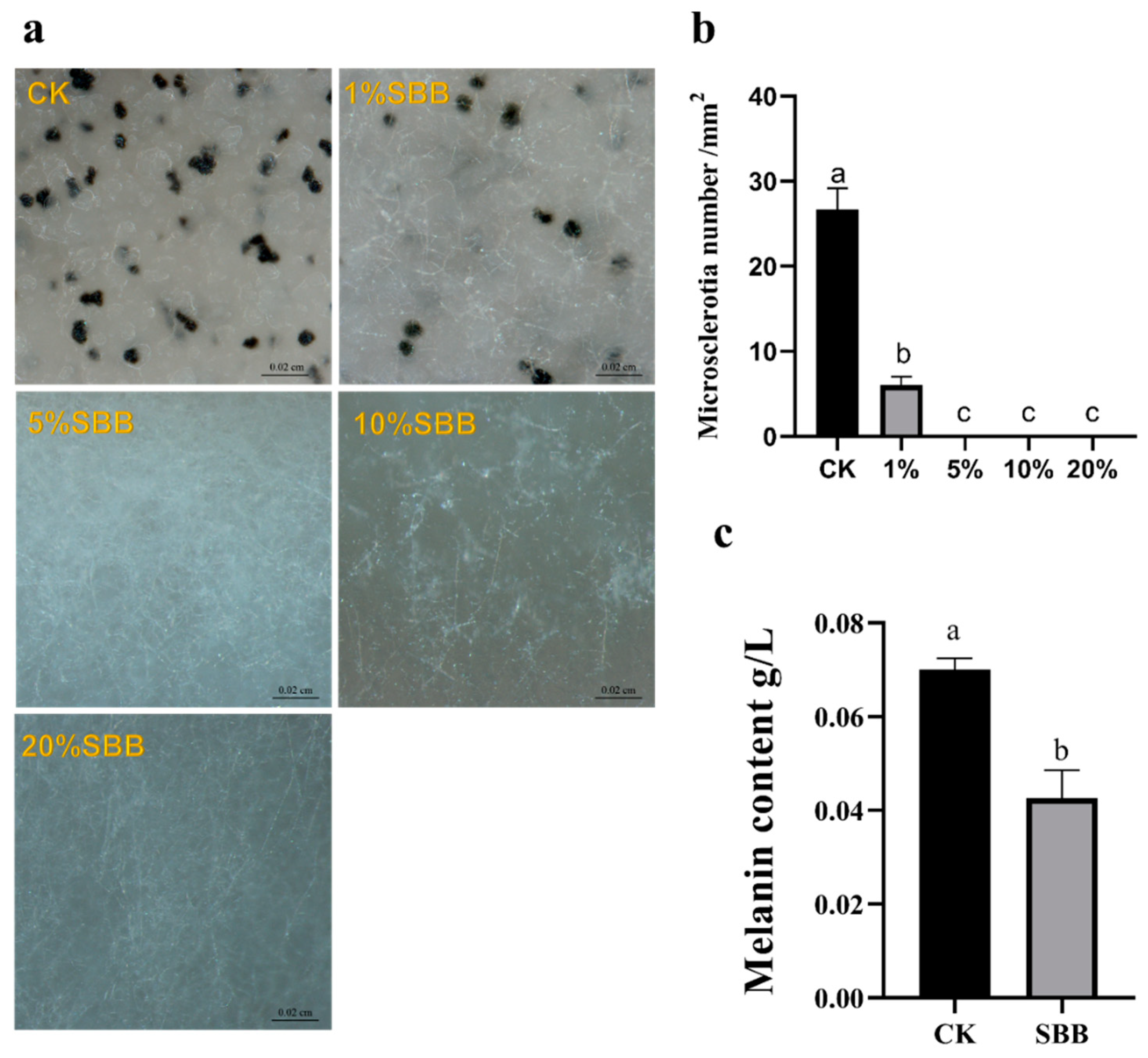
2.5. Effect of SBB Filtrate on Microsclerotia Formation
2.6. Effect of SBB Filtrate on Melanin Formation
2.7. Detection of Antifungal Active Substance Genes in Strain SBB
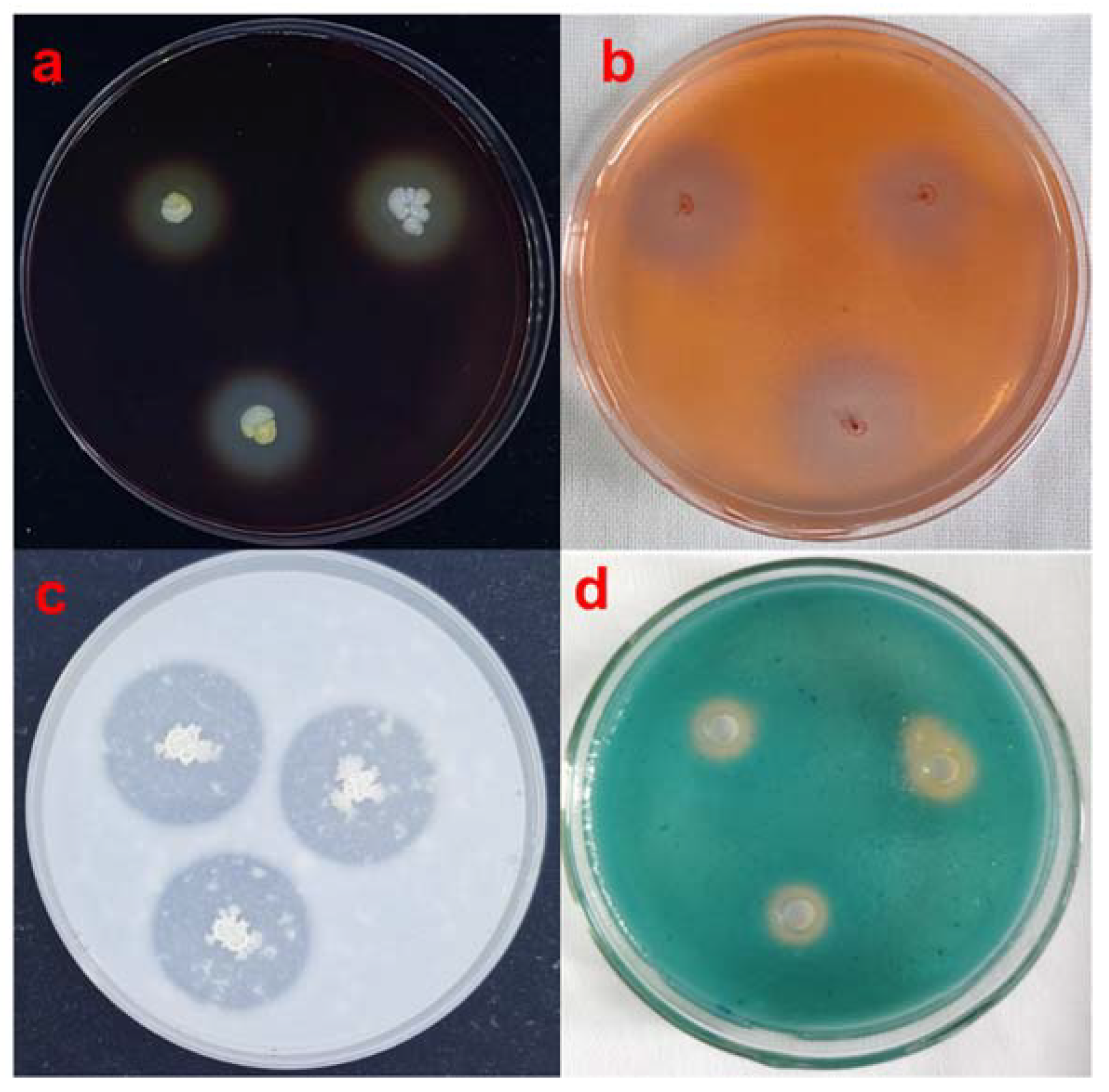
2.8. Extracellular Enzyme and Siderophore Activities
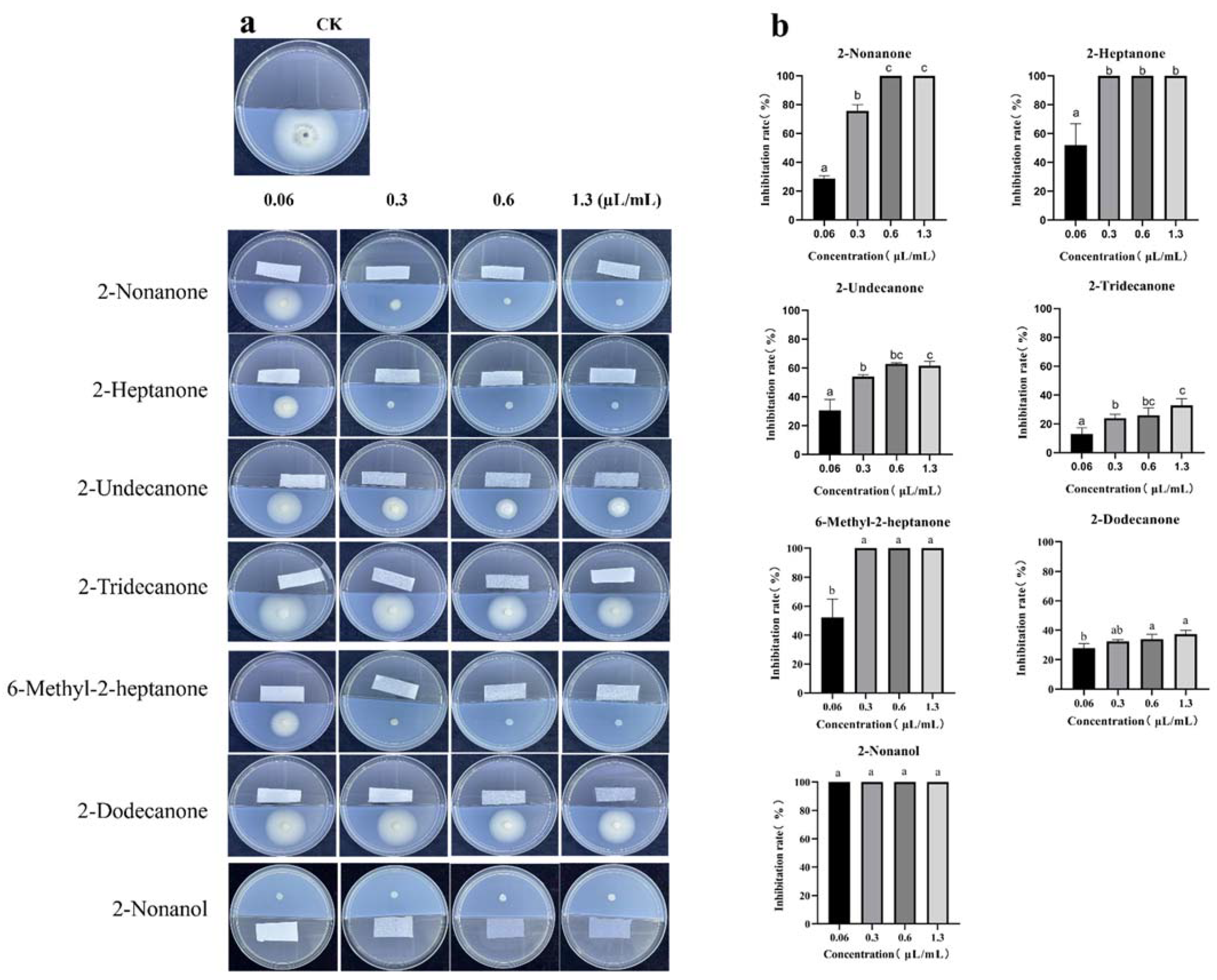
2.9. Gas Chromatograph-Mass Spectrometer (GC-MS) Analysis of VOCs in SBB and Effect of Commercial VOCs on Fungal Growth
2.10. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR) Analysis of SBB
2.11. Statistical Analysis
3. Results
3.1. Identification of Strain SBB
3.2. Inhibition of V. dahliae Growth by SBB, Its VOCs, and Filtrate
3.3. Inhibition of V. dahliae Spore Production by SBB Filtrate
3.4. Effect of SBB Filtrate on V. dahliae Microsclerotia and Melanin Formation
3.5. Identification of Genes Encoding Antimicrobial Substances of SBB
3.6. Detection of Strain SBB Extracellular Enzyme Siderophore Production
3.7. GC–MS Analysis of VOCs Produced by SBB and Antifungal Activity of Commercial VOCs against V. dahliae
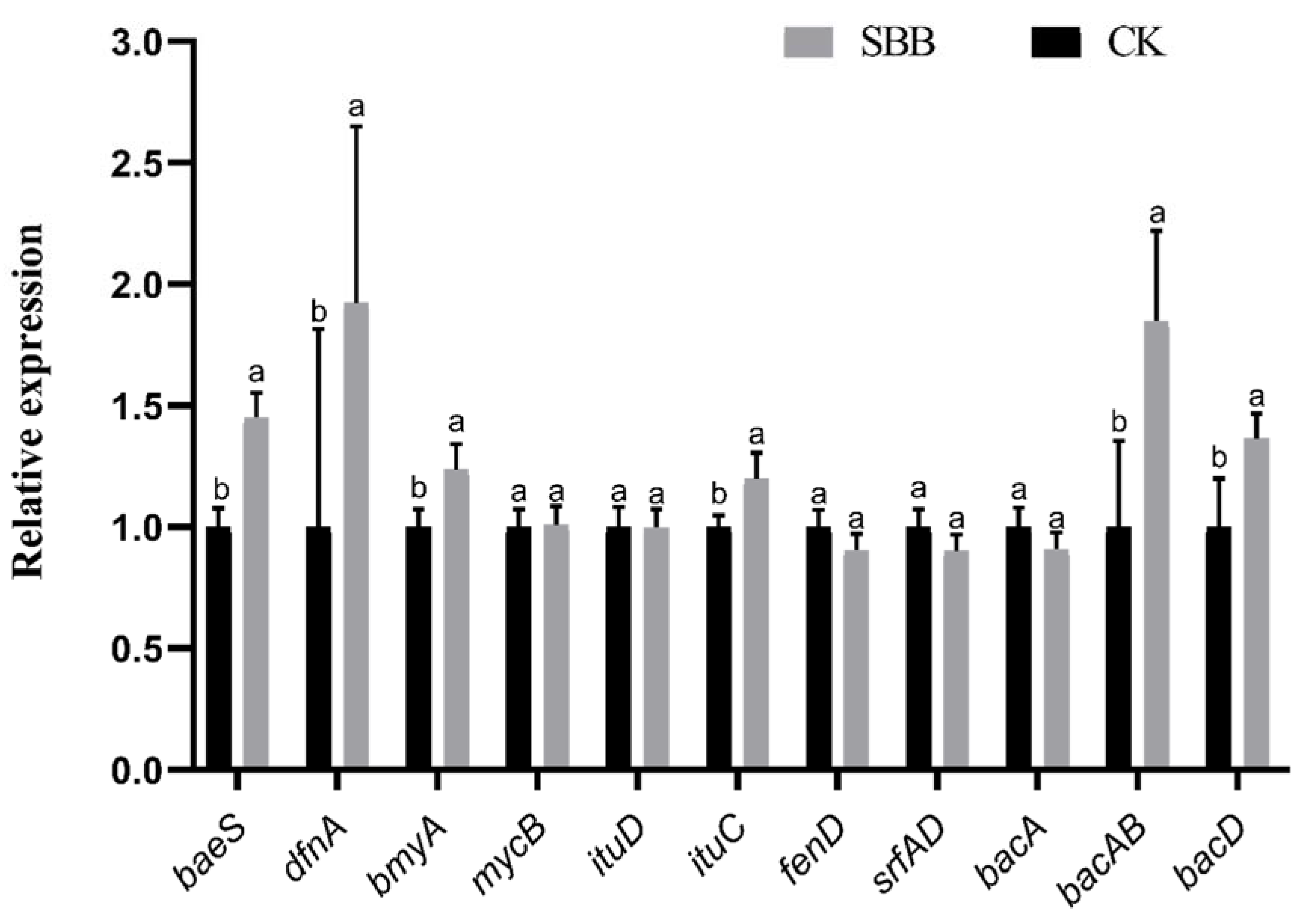
3.8. Gene Expression Analysis of Antifungal Substances in the Antagonistic Process of SBB against V. dahliae
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fradin, E.F.; Thomma, B.P.H.J. Physiology and Molecular Aspects of Verticillium Wilt Diseases Caused by V. Dahliae and V. Albo-Atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J. Infection and Colonization of Potato Roots by Verticillium Dahliae as Affected by Pratylenchus Penetrans and P. Crenatus. Phytopathology 1996, 86, 614. [Google Scholar] [CrossRef]
- Keykhasaber, M.; Thomma, B.P.H.J.; Hiemstra, J.A. Verticillium Wilt Caused by Verticillium Dahliae in Woody Plants with Emphasis on Olive and Shade Trees. Eur. J. Plant Pathol. 2018, 150, 21–37. [Google Scholar] [CrossRef]
- Wang, D.; Su, Z.; Ning, D.; Zhao, Y.; Meng, H.; Dong, B.; Zhao, J.; Zhou, H. Different Appearance Period of Verticillium Wilt Symptoms Affects Sunflower Growth and Production. J. Plant Pathol. 2021, 103, 513–517. [Google Scholar] [CrossRef]
- Zhang, D.; Dai, X.; Klosterman, S.J.; Subbarao, K.V.; Chen, J. The Secretome of Verticillium Dahliae in Collusion with Plant Defence Responses Modulates Verticillium Wilt Symptoms. Biol. Rev. 2022, 97, 1810–1822. [Google Scholar] [CrossRef] [PubMed]
- Dung, J.K.S. Verticillium Wilt of Mint in the United States of America. Plants 2020, 9, 1602. [Google Scholar] [CrossRef]
- Guo, X.; Cai, C.; Yuan, D.; Zhang, R.; Xi, J.; Guo, W. Development and Identification of Verticillium Wilt-Resistant Upland Cotton Accessions by Pyramiding QTL Related to Resistance. J. Integr. Agric. 2016, 15, 512–520. [Google Scholar] [CrossRef]
- Eynck, C.; Koopmann, B.; Grunewaldt-Stoecker, G.; Karlovsky, P.; von Tiedemann, A. Differential Interactions of Verticillium Longisporum and V. Dahliae with Brassica Napus Detected with Molecular and Histological Techniques. Eur. J. Plant Pathol. 2007, 118, 259–274. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Z.; Zhou, X.; Feng, C.; Zhuang, Y. Improving the Resistance of Eggplant (Solanum Melongena) to Verticillium Wilt Using Wild Species Solanum Linnaeanum. Euphytica 2015, 201, 463–469. [Google Scholar] [CrossRef]
- Carroll, C.L.; Carter, C.A.; Goodhue, R.E.; Lawell, C.-Y.C.L.; Subbarao, K.V. The Economics of Managing Verticillium Wilt, an Imported Disease in California Lettuce. Calif. Agric. 2017, 71, 178–183. [Google Scholar] [CrossRef][Green Version]
- Devi, P.; Tymon, L.; Keinath, A.; Miles, C. Progress in Grafting Watermelon to Manage Verticillium Wilt. Plant Pathol. 2021, 70, 767–777. [Google Scholar] [CrossRef]
- Narváez, I.; Martín, C.; Jiménez-Díaz, R.M.; Mercado, J.A.; Pliego-Alfaro, F. Plant Regeneration via Somatic Embryogenesis in Mature Wild Olive Genotypes Resistant to the Defoliating Pathotype of Verticillium Dahliae. Front. Plant Sci. 2019, 10, 1471. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Johnson, D.A. Verticillium Dahliae Infects, Alters Plant Biomass, and Produces Inoculum on Rotation Crops. Phytopathology 2016, 106, 602–613. [Google Scholar] [CrossRef]
- Bjelić, D.; Ignjatov, M.; Marinković, J.; Milošević, D.; Nikolić, Z.; Gvozdanović-Varga, J.; Karaman, M. Bacillus Isolates as Potential Biocontrol Agents of Fusarium Clove Rot of Garlic. Zemdirb.-Agric. 2018, 105, 369–376. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The Significance of Bacillus Spp. in Disease Suppression and Growth Promotion of Field and Vegetable Crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Abbas, A.; Khan, S.U.; Khan, W.U.; Saleh, T.A.; Khan, M.H.U.; Ullah, S.; Ali, A.; Ikram, M. Antagonist Effects of Strains of Bacillus spp. against Rhizoctonia Solani for Their Protection against Several Plant Diseases: Alternatives to Chemical Pesticides. C. R. Biol. 2019, 342, 124–135. [Google Scholar] [CrossRef]
- Timmusk, S.; Grantcharova, N.; Wagner, E.G.H. Paenibacillus Polymyxa Invades Plant Roots and Forms Biofilms. Appl. Environ. Microbiol. 2005, 71, 7292–7300. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Johri, B.N. Interactions of Bacillus Spp. and Plants—With Special Reference to Induced Systemic Resistance (ISR). Microbiol. Res. 2009, 164, 493–513. [Google Scholar] [CrossRef]
- Vellore Sunder, A.; Kumar, A.; Naik, N.; Pundle, A.V. Characterization of a New Bacillus Cereus ATUAVP1846 Strain Producing Penicillin V Acylase, and Optimization of Fermentation Parameters. Ann. Microbiol. 2012, 62, 1287–1293. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Sha, Y.; Zeng, Q.; Sui, S. Screening and Application of Bacillus Strains Isolated from Nonrhizospheric Rice Soil for the Biocontrol of Rice Blast. Plant Pathol. J. 2020, 36, 231–243. [Google Scholar] [CrossRef]
- Hu, X.; Roberts, D.P.; Xie, L.; Qin, L.; Li, Y.; Liao, X.; Han, P.; Yu, C.; Liao, X. Seed Treatment Containing Bacillus Subtilis BY-2 in Combination with Other Bacillus Isolates for Control of Sclerotinia Sclerotiorum on Oilseed Rape. Biol. Control 2019, 133, 50–57. [Google Scholar] [CrossRef]
- Han, X.; Shen, D.; Xiong, Q.; Bao, B.; Zhang, W.; Dai, T.; Zhao, Y.; Borriss, R.; Fan, B. The Plant-Beneficial Rhizobacterium Bacillus Velezensis FZB42 Controls the Soybean Pathogen Phytophthora Sojae Due to Bacilysin Production. Appl. Environ. Microbiol. 2021, 87, e01601-21. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, D.F.; Tjamos, S.E.; Antoniou, P.P.; Rafeletos, P.; Tjamos, E.C. Effect of Paenibacillus Alvei, Strain K165, on the Germination of Verticillium Dahliae Microsclerotia in Planta. Biol. Control 2008, 46, 166–170. [Google Scholar] [CrossRef]
- Li, H.; Zhou, L.; Wang, L.; Zhao, X.; Liang, L.; Chen, F. Wilt of Shantung Maple Caused by Verticillium Dahliae in China. Plant Dis. 2018, 102, 249. [Google Scholar] [CrossRef]
- Chen, F.; Ye, J.; Chio, C.; Liu, W.; Shi, J.; Qin, W. A Simplified Quick Microbial Genomic DNA Extraction via Freeze–Thawing Cycles. Mol. Biol. Rep. 2020, 47, 703–709. [Google Scholar] [CrossRef]
- Feng, J.; Qiu, L.; Liang, X.; Chen, B.; Xia, H.; Peng, C.; Zhong, Y. Identification of antagonistic bacteria Bacillus velezensis JK3 against anthracnose of strawberry and its antipathogenic activity. Acta Agric. Zhejiangensis 2020, 32, 831–839. [Google Scholar] [CrossRef]
- Yamamoto, S.; Harayama, S. PCR Amplification and Direct Sequencing of GyrB Genes with Universal Primers and Their Application to the Detection and Taxonomic Analysis of Pseudomonas Putida Strains. Appl. Environ. Microbiol. 1995, 61, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Nakamura, L.K.; Cohan, F.M. Bacillus Vallismortis Sp. Nov., a Close Relative of Bacillus Subtilis, Isolated from Soil in Death Valley, California. Int. J. Syst. Bacteriol. 1996, 46, 470–475. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, B.; Liu, H.; Han, J.; Zhang, Y. Identification of Endophytic Bacillus Velezensis ZSY-1 Strain and Antifungal Activity of Its Volatile Compounds against Alternaria Solani and Botrytis Cinerea. Biol. Control 2017, 105, 27–39. [Google Scholar] [CrossRef]
- Bashyal, B.M.; Chand, R.; Kushwaha, C.; Sen, D.; Prasad, L.C.; Joshi, A.K. Association of Melanin Content with Conidiogenesis in Bipolaris Sorokiniana of Barley (Hordeum Vulgare L.). World J. Microbiol. Biotechnol. 2010, 26, 309–316. [Google Scholar] [CrossRef]
- Ma, S.; Cao, K.; Liu, N.; Meng, C.; Cao, Z.; Dai, D.; Jia, H.; Zang, J.; Li, Z.; Hao, Z.; et al. The StLAC2 Gene Is Required for Cell Wall Integrity, DHN-Melanin Synthesis and the Pathogenicity of Setosphaeria Turcica. Fungal Biol. 2017, 121, 589–601. [Google Scholar] [CrossRef]
- Arguelles-Arias, A.; Ongena, M.; Halimi, B.; Lara, Y.; Brans, A.; Joris, B.; Fickers, P. Bacillus Amyloliquefaciens GA1 as a Source of Potent Antibiotics and Other Secondary Metabolites for Biocontrol of Plant Pathogens. Microb. Cell Factories 2009, 8, 63. [Google Scholar] [CrossRef]
- Mechanism of Antibacterial Activity of Bacillus Amyloliquefaciens C-1 Lipopeptide toward Anaerobic Clostridium Difficile—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7073505/ (accessed on 5 August 2022).
- Zhou, L.; Wang, J.; Wu, F.; Yin, C.; Kim, K.H.; Zhang, Y. Termite Nest Associated Bacillus Siamensis YC-9 Mediated Biocontrol of Fusarium Oxysporum f. Sp. Cucumerinum. Front. Microbiol. 2022, 13, 893393. [Google Scholar] [CrossRef]
- Xu, B.-H.; Ye, Z.-W.; Zheng, Q.-W.; Wei, T.; Lin, J.-F.; Guo, L.-Q. Isolation and Characterization of Cyclic Lipopeptides with Broad-Spectrum Antimicrobial Activity from Bacillus Siamensis JFL15. 3 Biotech 2018, 8, 444. [Google Scholar] [CrossRef]
- Zhu, M.; He, Y.; Li, Y.; Ren, T.; Liu, H.; Huang, J.; Jiang, D.; Hsiang, T.; Zheng, L. Two New Biocontrol Agents Against Clubroot Caused by Plasmodiophora Brassicae. Front. Microbiol. 2020, 10, 3099. [Google Scholar] [CrossRef]
- Kumar, R.S.; Ayyadurai, N.; Pandiaraja, P.; Reddy, A.V.; Venkateswarlu, Y.; Prakash, O.; Sakthivel, N. Characterization of Antifungal Metabolite Produced by a New Strain Pseudomonas Aeruginosa PUPa3 That Exhibits Broad-Spectrum Antifungal Activity and Biofertilizing Traits. J. Appl. Microbiol. 2005, 98, 145–154. [Google Scholar] [CrossRef]
- Xu, S.J.; Kim, B.S. Biocontrol of Fusarium Crown and Root Rot and Promotion of Growth of Tomato by Paenibacillus Strains Isolated from Soil. Mycobiology 2014, 42, 158–166. [Google Scholar] [CrossRef]
- Shin, S.H.; Lim, Y.; Lee, S.E.; Yang, N.W.; Rhee, J.H. CAS Agar Diffusion Assay for the Measurement of Siderophores in Biological Fluids. J. Microbiol. Methods 2001, 44, 89–95. [Google Scholar] [CrossRef]
- Kong, W.-L.; Li, P.-S.; Wu, X.-Q.; Wu, T.-Y.; Sun, X.-R. Forest Tree Associated Bacterial Diffusible and Volatile Organic Compounds against Various Phytopathogenic Fungi. Microorganisms 2020, 8, 590. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.-L.; Rui, L.; Ni, H.; Wu, X.-Q. Antifungal Effects of Volatile Organic Compounds Produced by Rahnella Aquatilis JZ-GX1 Against Colletotrichum Gloeosporioides in Liriodendron Chinense × Tulipifera. Front. Microbiol. 2020, 11, 1114. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.-L.; Wang, Y.-H.; Wu, X.-Q. Enhanced Iron Uptake in Plants by Volatile Emissions of Rahnella Aquatilis JZ-GX1. Front. Plant Sci. 2021, 12, 704000. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Liu, J.; Bilal, M.; Liu, X.; Wang, X.; Dong, F.; Liu, Y.; Zang, S.; Yin, X.; Yang, X.; et al. Extracellular Lipopeptide Bacillomycin L Regulates Serial Expression of Genes for Modulating Multicellular Behavior in Bacillus Velezensis Bs916. Appl. Microbiol. Biotechnol. 2021, 105, 6853–6870. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Wang, Y.; Ma, J.; Klosterman, S.J.; Xiao, S.; Tian, C. Deep MRNA Sequencing Reveals Stage-Specific Transcriptome Alterations during Microsclerotia Development in the Smoke Tree Vascular Wilt Pathogen, Verticillium Dahliae. BMC Genom. 2014, 15, 324. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, C.A.; Kim, S.-J.; Kwon, S.-W.; Rooney, A.P. Bacillus Velezensis Is Not a Later Heterotypic Synonym of Bacillus Amyloliquefaciens; Bacillus Methylotrophicus, Bacillus Amyloliquefaciens Subsp. Plantarum and ‘Bacillus Oryzicola’ Are Later Heterotypic Synonyms of Bacillus Velezensis Based on Phylogenomics. Int. J. Syst. Evol. Microbiol. 2016, 66, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Tian, A. Antagonistic strain of TJB-8 and its antifungal substances. J. Zhejiang Agric. For. Univ. 2017, 34, 1071–1078. [Google Scholar] [CrossRef]
- Yu, J.; Li, T.; Tian, L.; Tang, C.; Klosterman, S.J.; Tian, C.; Wang, Y. Two Verticillium Dahliae MAPKKKs, VdSsk2 and VdSte11, Have Distinct Roles in Pathogenicity, Microsclerotial Formation, and Stress Adaptation. mSphere 2019, 4, e00426-19. [Google Scholar] [CrossRef]
- Li, J.; Zhou, L.; Yin, C.; Zhang, D.; Klosterman, S.J.; Wang, B.; Song, J.; Wang, D.; Hu, X.; Subbarao, K.V.; et al. The Verticillium Dahliae Sho1-MAPK Pathway Regulates Melanin Biosynthesis and Is Required for Cotton Infection. Environ. Microbiol. 2019, 21, 4852–4874. [Google Scholar] [CrossRef]
- Zheng, J.; Tang, C.; Deng, C.; Wang, Y. Involvement of a Response Regulator VdSsk1 in Stress Response, Melanin Biosynthesis and Full Virulence in Verticillium Dahliae. Front. Microbiol. 2019, 10, 606. [Google Scholar] [CrossRef]
- Cheng, F.; Li, G.; Peng, Y.; Wang, A.; Zhu, J. Mixed Bacterial Fermentation Can Control the Growth and Development of Verticillium Dahliae. Biotechnol. Biotechnol. Equip. 2020, 34, 58–69. [Google Scholar] [CrossRef]
- Ling, L.; Luo, H.; Yang, C.; Wang, Y.; Cheng, W.; Pang, M.; Jiang, K. Volatile Organic Compounds Produced by Bacillus Velezensis L1 as a Potential Biocontrol Agent against Postharvest Diseases of Wolfberry. Front. Microbiol. 2022, 13, 987844. [Google Scholar] [CrossRef]
- Wang, H.; Cai, X.-Y.; Xu, M.; Tian, F. Enhanced Biocontrol of Cucumber Fusarium Wilt by Combined Application of New Antagonistic Bacteria Bacillus Amyloliquefaciens B2 and Phenolic Acid-Degrading Fungus Pleurotus Ostreatus P5. Front. Microbiol. 2021, 12, 700142. [Google Scholar] [CrossRef]
- Dhouib, H.; Zouari, I.; Ben Abdallah, D.; Belbahri, L.; Taktak, W.; Triki, M.A.; Tounsi, S. Potential of a Novel Endophytic Bacillus Velezensis in Tomato Growth Promotion and Protection against Verticillium Wilt Disease. Biol. Control 2019, 139, 104092. [Google Scholar] [CrossRef]
- Liu, C.; Yin, X.; Wang, Q.; Peng, Y.; Ma, Y.; Liu, P.; Shi, J. Antagonistic Activities of Volatiles Produced by Two Bacillus Strains against Monilinia Fructicola in Peach Fruit: Antagonistic Activities of Volatiles Produced by Two Bacillus Strains. J. Sci. Food Agric. 2018, 98, 5756–5763. [Google Scholar] [CrossRef]
- Sokol-Anderson, M.L.; Brajtburg, J.; Medoff, G. Amphotericin B-Induced Oxidative Damage and Killing of Candida Albicans. J. Infect. Dis. 1986, 154, 76–83. [Google Scholar] [CrossRef]
- Zhang, X.; Kong, F.; Wang, J.; Zhang, C.; Li, B. Isolation, Purification and Antagonism of Extracellular Protein from Bacillus subtilis Strain SH7. Chin. Tob. Sci. 2010, 31, 13–15. [Google Scholar] [CrossRef]
- Chaiharn, M.; Chunhaleuchanon, S.; Lumyong, S. Screening Siderophore Producing Bacteria as Potential Biological Control Agent for Fungal Rice Pathogens in Thailand. World J. Microbiol. Biotechnol. 2009, 25, 1919–1928. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Qiao, X.; Li, Z.; Li, F.; Chen, M.; Wang, Y.; Huang, Y.; Cui, H. Antifungal Activity of Volatile Organic Compounds from Streptomyces Alboflavus TD-1. FEMS Microbiol. Lett. 2013, 341, 45–51. [Google Scholar] [CrossRef]
- Yuan, J.; Raza, W.; Shen, Q.; Huang, Q. Antifungal Activity of Bacillus Amyloliquefaciens NJN-6 Volatile Compounds against Fusarium Oxysporum f. Sp. Cubense. Appl. Environ. Microbiol. 2012, 78, 5942–5944. [Google Scholar] [CrossRef]
- Lee, T.; Park, D.; Kim, K.; Lim, S.M.; Yu, N.H.; Kim, S.; Kim, H.-Y.; Jung, K.S.; Jang, J.Y.; Park, J.-C.; et al. Characterization of Bacillus Amyloliquefaciens DA12 Showing Potent Antifungal Activity against Mycotoxigenic Fusarium Species. Plant Pathol. J. 2017, 33, 499–507. [Google Scholar] [CrossRef]
- Ren, L.; Zhou, J.; Yin, H.; Qin, N.; Yao, F.; Ma, D.; Zhao, X. Antifungal Activity and Control Efficiency of Endophytic Bacillus Velezensis ZJ1 Strain and Its Volatile Compounds against Alternaria Solani and Botrytis Cinerea. J. Plant Pathol. 2022, 104, 575–589. [Google Scholar] [CrossRef]




| Lipopeptides | Gene | Primers | Sequences (5′–3′) | PCR Products Size (bp) | Reference |
|---|---|---|---|---|---|
| Surfactin | srfAA | srfAA-F | GCCCGTGAGCCGAATGGATAAG | 1600 | [34] |
| srfAA-R | CCGTTTCAGGGACACAAGCTCCG | ||||
| srfAD | srfAD-F | CCGTTCGCAGGAGGCTATTCC | 1300 | [34] | |
| srfAD-R | CCGTTCGCAGGAGGCTATTCC | ||||
| Fengycin | fenB | fenB-F | CTATAGTTTGTTGACGGCTC | 1600 | [35] |
| fenB-R | CAGCACTGGTTCTTGTCGCA | ||||
| fenD | fenD-F | TTTGGCAGCAGGAGAAGTTT | 1600 | [36] | |
| fenD-R | GACAGTGCTGCCTGATGAAA | ||||
| ituA | ituA-F | ATGTATACCAGTCAATTCC | 1047 | [37] | |
| ituA-R | GATCCGAAGCTGACAATAG | ||||
| Iturin | ituC | ituC-F | CCCCCTCGGTCAA GTGAATA | 594 | [37] |
| ituC-R | TTGGTTAAGCCCTGATGCTC | ||||
| ituD | ituD-F | ATGAACAATCTTGCCTTTTTA | 1203 | [35] | |
| ituD-R | TTATTTTAAAATCCGCAATT | ||||
| Bacillomycin | bmyA | bmyA-F | AAAGCGGCTCAAGAAGCGAAACCC | 1200 | [37] |
| bmyA-R | CGATTCAGCTCATCGACCAGGTAGGC | ||||
| Mycosubtilin | mycB | mycB-F | ATGTCGGTGTTTAAAAATCAAGTAACG | 2000 | [38] |
| mycB-R | TTAGGACGCCAGCAGTTCTTCTATTGA | ||||
| bacAB | bacAB-F | CAGCTCATGGGAATGCTTTT | 500 | [38] | |
| bacAB-R | CTCGGTCCTGAAGGGACAAG | ||||
| Bacilysin | bacD | bacD-F | CTTCTCCAAGGGGTGAACAG | 815 | [38] |
| bacD-R | TGTAGGTTTCACCGGCTTTC | ||||
| bacA | bacA-F | GTGAAGGCCGTACTTTTGTCTGGC | 1200 | [34] | |
| bacA-R | GGGGGGAAATACAGCTTCAGGGC | ||||
| Bacillaene | baeS | baeS-F | CGCAAAAGCTCTTCGACCGCCGTC | 1550 | [34] |
| baeS-R | CTCTCGTGCCGTCGGAATATCCGC | ||||
| Difficidin | dfnA | dfnA-F | GGTGCGGCATGAAGATTTGAGATCACCG | 1950 | [34] |
| dfnA-R | GGAGAGCACTTCAATTCCGACGTTGACC | ||||
| Bacillibactin | dhbA | dhbA-F | CGCCTAAAGTAGCGCCGCCATCAACGC | 1350 | [34] |
| dhbA-R | CCGCGATGGAGCGGGATTATCCG |
| Gene | Primers | Sequences (5′–3′) | Function |
|---|---|---|---|
| YvzC | qYvzC-F | GTGGTCGAAAAGATACA | Internal reference gene |
| qYvzC-R | TCATTGACTTTTGTCAAC | ||
| srfAD | qsrfAD-F | AATGCTGTCTGTCATATCCTATA | Regulating the synthesis of surfactin |
| qsrfAD-R | GTGGCGGAACGAATCTAT | ||
| fenD | qfenD-F | TCATTACCGACTCCATCA | Regulating the synthesis of fengycin |
| qfenD-R | TTCTGTTTCCTGTGTTCAA | ||
| ituC | qituC-F | CGGAGACACATACACTTC | Regulating the synthesis of iturin |
| qituC-R | GTTCGTTTCATCTGTTCTTC | ||
| ituD | qituD-F | GAATCACATTGTACCTTATC | Regulating the synthesis of iturin |
| qituD-R | CGTCGTCATATTGGAATA | ||
| bmyA | qbmyA-F | CGATTTCCTTCCAATACG | Regulating the synthesis of bacillomycin |
| qbmyA-R | AACAATATACGGACAACAC | ||
| mycB | qmycB-F | AATTGAACGCTGGTCTAA | Regulating the synthesis of mycosubtilin |
| qmycB-R | AATGCTGAAGGTGAAGTC | ||
| bacAB | qbacAB-F | ATGTCATCTGTATATTCAA | Regulating the synthesis of bacilysin |
| qbacAB-R | CATTAAGCACTTCTACAT | ||
| bacD | qbacD-F | GATAACGGAGTAAGACAATA | Regulating the synthesis of bacilysin |
| qbacD-R | GACTTCCTTATGCTGATG | ||
| bacA | qbacA-F | CAAGGCTTATTCAATATCTA | Regulating the synthesis of bacilysin |
| qbacA-R | CACGATTCAAATGTATCA | ||
| baeS | qbaeS-F | AACGCATTCATTCACATC | Regulating the synthesis of bacillaene |
| qbaeS-R | ACAACGGCTCATAAGTAT | ||
| dfnA | qdfnA-F | ATAACGCATTATATTCTC | Regulating the synthesis of difficidin |
| qdfnA-R | CATATTAGGCTACTCTAT |
| Retention Time (min) | Relative Peak Area (%) | CAS# | Compound |
|---|---|---|---|
| 5.25 | 14.92 | 110-43-0 | 2-Heptanone |
| 6.19 | 9.45 | 928-68-7 | 6-Methyl-2-heptanone |
| 8.71 | 8.51 | 821-55-6 | 2-Nonanone |
| 8.93 | 2.76 | 628-99-9 | 2-Nonanol |
| 10.07 | 2.63 | 693-54-9 | 2-Decanone |
| 13.27 | 4.89 | 112-12-9 | 2-Undecanone |
| 14.79 | 7.47 | 6175-49-1 | 2-Dodecanone |
| 18.11 | 3.22 | 593-08-8 | 2-Tridecanone |
| 19.59 | 1.66 | 2345-27-9 | 2-Tetradecanone |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-Y.; Kong, W.-L.; Liao, Y.-C.-Z.; Zhu, L.-H. Identification of Bacillus velezensis SBB and Its Antifungal Effects against Verticillium dahliae. J. Fungi 2022, 8, 1021. https://doi.org/10.3390/jof8101021
Wang W-Y, Kong W-L, Liao Y-C-Z, Zhu L-H. Identification of Bacillus velezensis SBB and Its Antifungal Effects against Verticillium dahliae. Journal of Fungi. 2022; 8(10):1021. https://doi.org/10.3390/jof8101021
Chicago/Turabian StyleWang, Wei-Yu, Wei-Liang Kong, Yang-Chun-Zi Liao, and Li-Hua Zhu. 2022. "Identification of Bacillus velezensis SBB and Its Antifungal Effects against Verticillium dahliae" Journal of Fungi 8, no. 10: 1021. https://doi.org/10.3390/jof8101021
APA StyleWang, W.-Y., Kong, W.-L., Liao, Y.-C.-Z., & Zhu, L.-H. (2022). Identification of Bacillus velezensis SBB and Its Antifungal Effects against Verticillium dahliae. Journal of Fungi, 8(10), 1021. https://doi.org/10.3390/jof8101021






