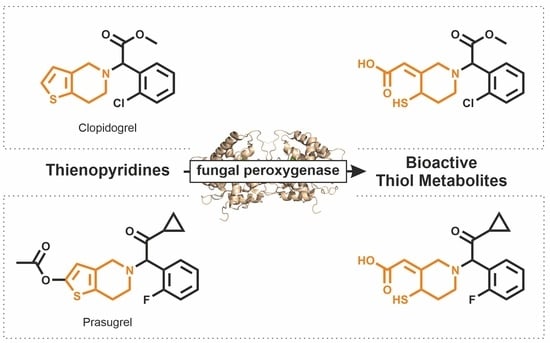
Biocatalytic Syntheses of Antiplatelet Metabolites of the Thienopyridines Clopidogrel and Prasugrel Using Fungal Peroxygenases
Abstract
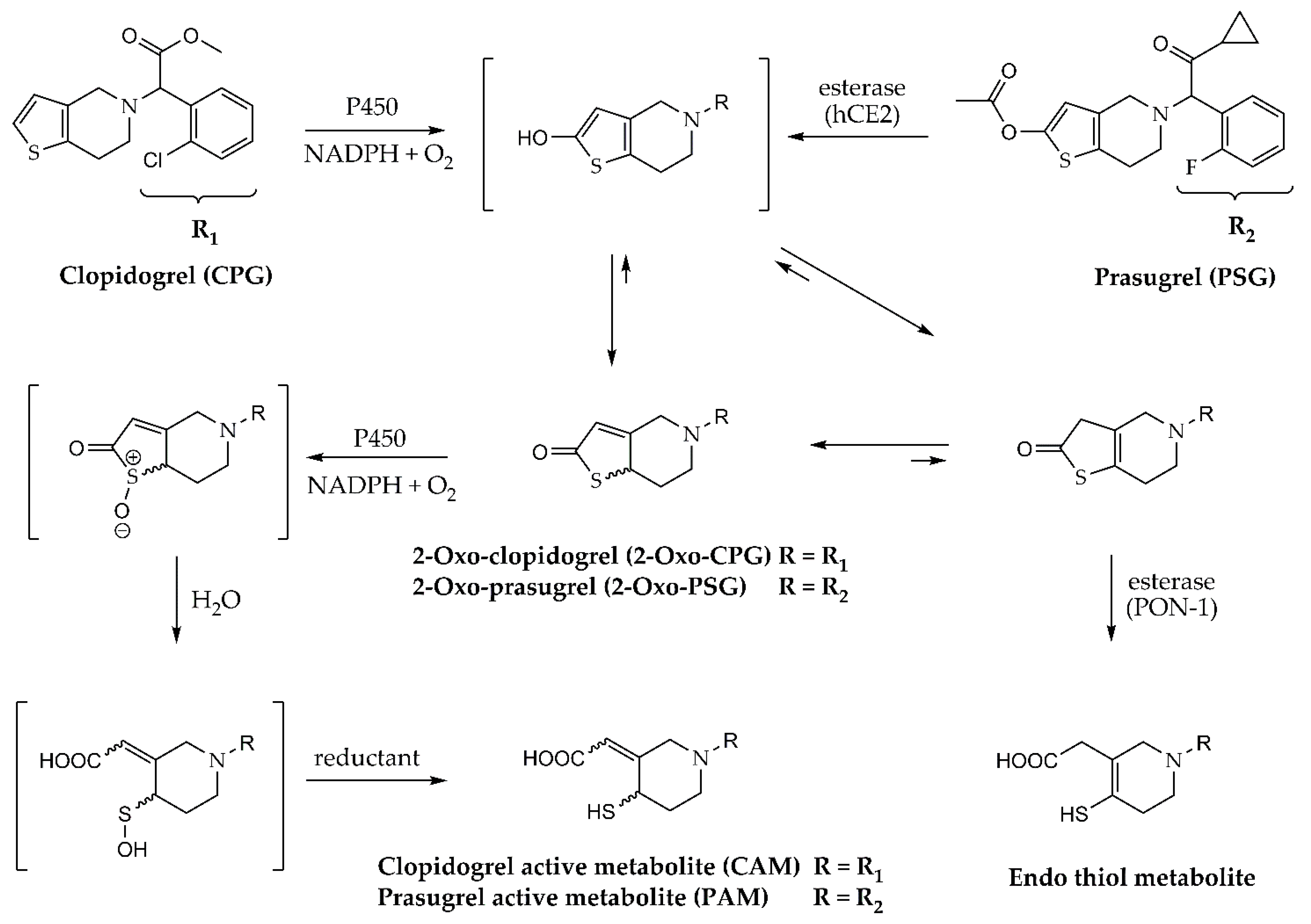
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Enzymes
2.3. Enzymatic Reactions
2.4. Semi-Preparative Synthesis of Thienopyridine Metabolites
2.4.1. Clopidogrel Active Metabolite (CAM)
2.4.2. 2-Oxo-Prasugrel (2-Oxo-PSG, R-95913)
2.4.3. Prasugrel Active Metabolite (PAM, R-138727)
2.4.4. Preparative Liquid Chromatography
2.5. Analytical Methods
2.5.1. High-Performance Liquid Chromatography
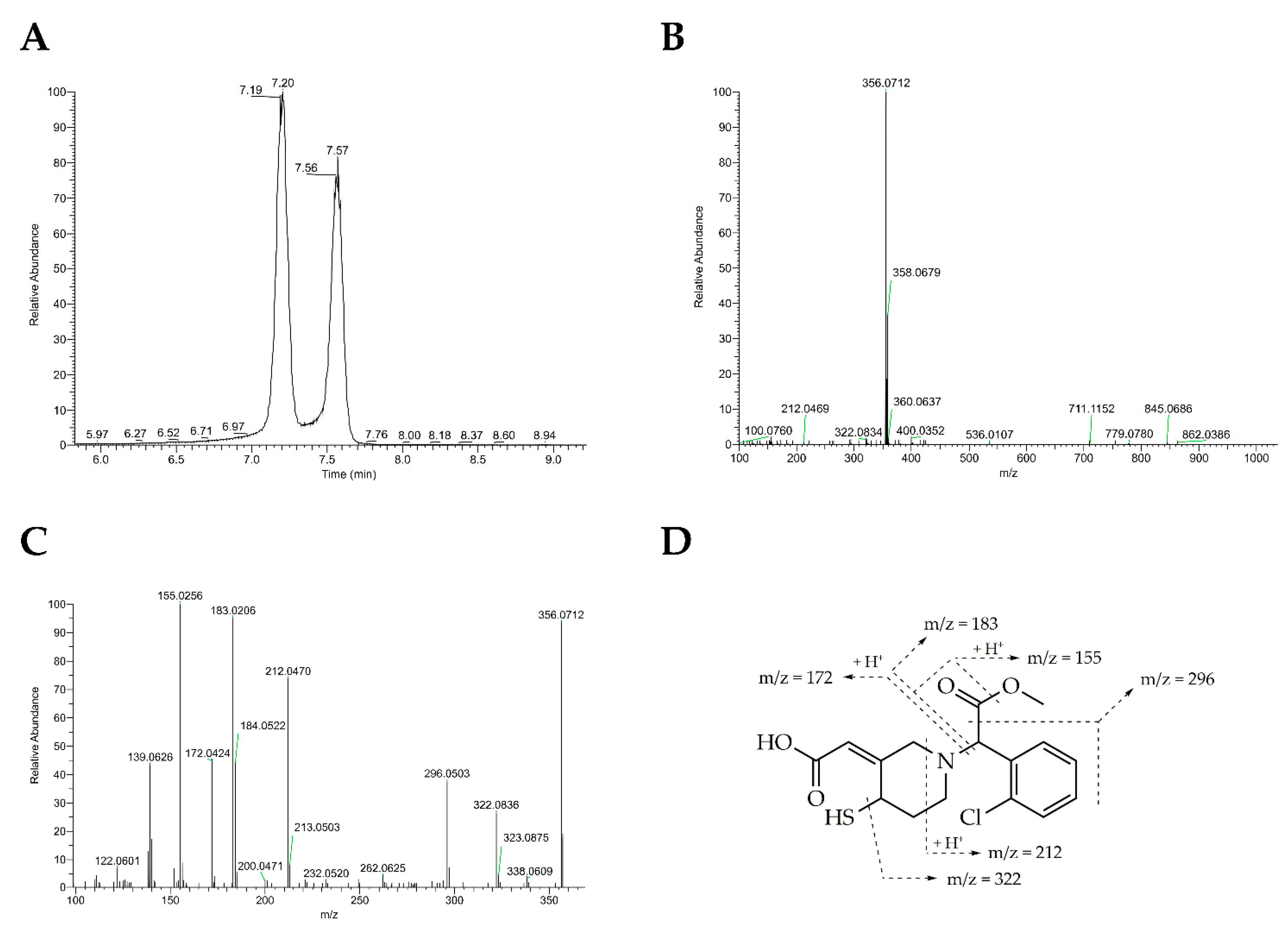
2.5.2. High-Resolution Mass Spectrometry
2.5.3. NMR Studies
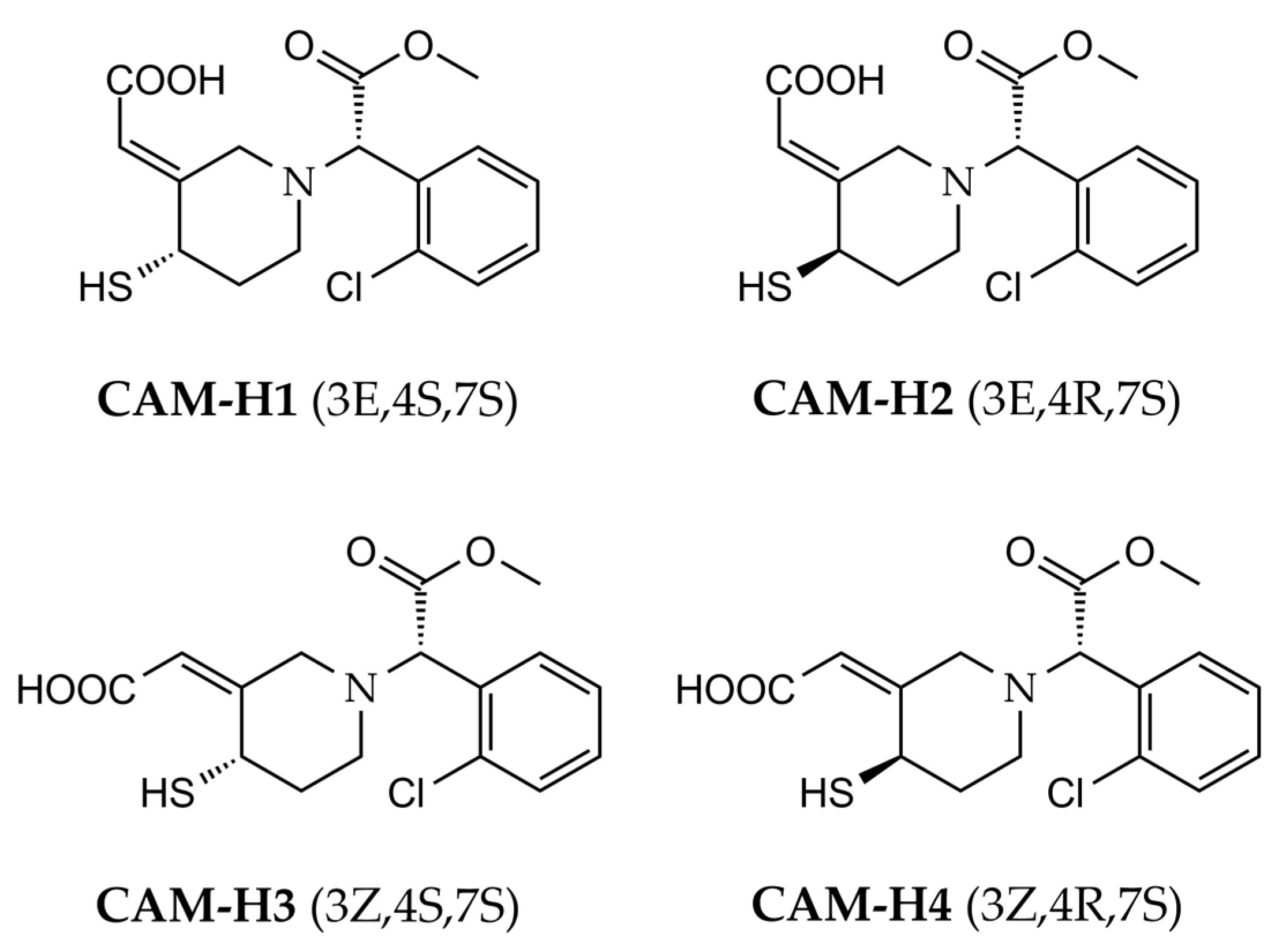
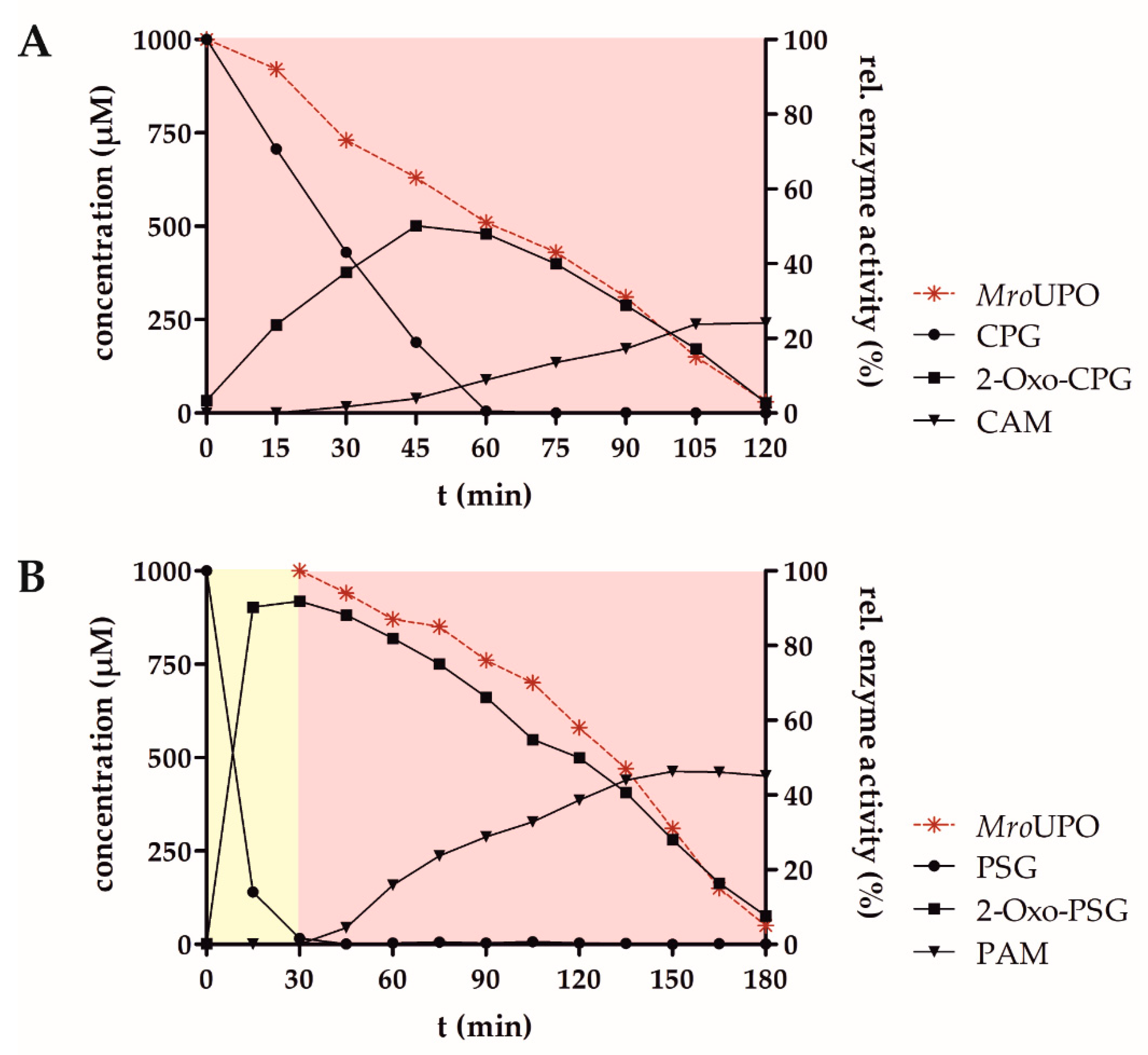
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jarvis, B.; Simpson, K. Clopidogrel. Drugs 2000, 60, 347–377. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.-J.; Ardissino, D.; De Servi, S.; Murphy, S. Prasugrel Versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [Green Version]
- Farid, N.A.; Kurihara, A.; Wrighton, S.A. Metabolism and Disposition of the Thienopyridine Antiplatelet Drugs Ticlopidine, Clopidogrel, and Prasugrel in Humans. J. Clin. Pharmacol. 2010, 50, 126–142. [Google Scholar] [CrossRef]
- Pereillo, J.-M.; Maftouh, M.; Andrieu, A.; Uzabiaga, M.-F.; Fedeli, O.; Savi, P.; Pascal, M.; Herbert, J.-M.; Maffrand, J.-P.; Picard, C. Structure and Stereochemistry of the Active Metabolite of Clopidogrel. Drug Metab. Dispos. 2002, 30, 1288–1295. [Google Scholar] [CrossRef]
- Kazui, M.; Nishiya, Y.; Ishizuka, T.; Hagihara, K.; Farid, N.A.; Okazaki, O.; Ikeda, T.; Kurihara, A. Identification of the Human Cytochrome P450 Enzymes Involved in the Two Oxidative Steps in the Bioactivation of Clopidogrel to Its Pharmacologically Active Metabolite. Drug Metab. Dispos. 2010, 38, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Williams, E.T.; Jones, K.O.; Ponsler, G.D.; Lowery, S.M.; Perkins, E.J.; Wrighton, S.A.; Ruterbories, K.J.; Kazui, M.; Farid, N.A. The Biotransformation of Prasugrel, a New Thienopyridine Prodrug, by the Human Carboxylesterases 1 and 2. Drug Metab. Dispos. 2008, 36, 1227–1232. [Google Scholar] [CrossRef] [Green Version]
- Dansette, P.M.; Rosi, J.; Bertho, G.; Mansuy, D. Paraoxonase-1 and Clopidogrel Efficacy. Nat. Med. 2011, 17, 1040–1041. [Google Scholar] [CrossRef] [PubMed]
- Dansette, P.M.; Rosi, J.; Bertho, G.; Mansuy, D. Cytochromes P450 Catalyze Both Steps of the Major Pathway of Clopidogrel Bioactivation, Whereas Paraoxonase Catalyzes the Formation of a Minor Thiol Metabolite Isomer. Chem. Res. Toxicol. 2012, 25, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Dansette, P.M.; Libraire, J.; Bertho, G.; Mansuy, D. Metabolic Oxidative Cleavage of Thioesters: Evidence for the Formation of Sulfenic Acid Intermediates in the Bioactivation of the Antithrombotic Prodrugs Ticlopidine and Clopidogrel. Chem. Res. Toxicol. 2009, 22, 369–373. [Google Scholar] [CrossRef]
- Dansette, P.M.; Thébault, S.; Bertho, G.; Mansuy, D. Formation and Fate of a Sulfenic Acid Intermediate in the Metabolic Activation of the Antithrombotic Prodrug Prasugrel. Chem. Res. Toxicol. 2010, 23, 1268–1274. [Google Scholar] [CrossRef]
- Hagihara, K.; Kazui, M.; Kurihara, A.; Iwabuchi, H.; Ishikawa, M.; Kobayashi, H.; Tanaka, N.; Okazaki, O.; Farid, N.A.; Ikeda, T. Biotransformation of Prasugrel, a Novel Thienopyridine Antiplatelet Agent, to the Pharmacologically Active Metabolite. Drug Metab. Dispos. 2010, 38, 898–904. [Google Scholar] [CrossRef] [Green Version]
- Dansette, P.M.; Levent, D.; Hessani, A.; Bertho, G.; Mansuy, D. Thiolactone Sulfoxides as New Reactive Metabolites Acting as Bis-Electrophiles: Implication in Clopidogrel and Prasugrel Bioactivation. Chem. Res. Toxicol. 2013, 26, 794–802. [Google Scholar] [CrossRef]
- Mega, J.L.; Close, S.L.; Wiviott, S.D.; Shen, L.; Hockett, R.D.; Brandt, J.T.; Walker, J.R.; Antman, E.M.; Macias, W.; Braunwald, E. Cytochrome P-450 Polymorphisms and Response to Clopidogrel. N. Engl. J. Med. 2009, 360, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Shuldiner, A.R.; O’Connell, J.R.; Bliden, K.P.; Gandhi, A.; Ryan, K.; Horenstein, R.B.; Damcott, C.M.; Pakyz, R.; Tantry, U.; Gibson, Q. Association of Cytochrome P450 2c19 Genotype with the Antiplatelet Effect and Clinical Efficacy of Clopidogrel Therapy. JAMA 2009, 302, 849–857. [Google Scholar] [CrossRef]
- Gong, I.Y.; Crown, N.; Suen, C.M.; Schwarz, U.I.; Dresser, G.K.; Knauer, M.J.; Sugiyama, D.; DeGorter, M.K.; Woolsey, S.; Tirona, R.G. Clarifying the Importance of Cyp2c19 and Pon1 in the Mechanism of Clopidogrel Bioactivation and in Vivo Antiplatelet Response. Eur. Heart J. 2012, 33, 2856–2864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.-J.; Wang, X.; Gawronski, B.E.; Brinda, B.J.; Angiolillo, D.J.; Markowitz, J.S. Carboxylesterase 1 as a Determinant of Clopidogrel Metabolism and Activation. J. Pharmacol. Exp. Ther. 2013, 344, 665–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zetterberg, F.; Svensson, P. State of Affairs: Design and Structure–Activity Relationships of Reversible P2y12 Receptor Antagonists. Bioorg. Med. Chem. Lett. 2016, 26, 2739–2754. [Google Scholar] [CrossRef]
- Tuffal, G.; Roy, S.; Lavisse, M.; Brasseur, D.; Schofield, J.; Touchard, N.D.; Savi, P.; Bremond, N.; Rouchon, M.-C.; Hurbin, F. An Improved Method for Specific and Quantitative Determination of the Clopidogrel Active Metabolite Isomers in Human Plasma. Thromb. Haemost. 2011, 105, 696–705. [Google Scholar] [PubMed]
- Karaźniewicz-Łada, M.; Danielak, D.; Teżyk, A.; Żaba, C.; Tuffal, G.; Główka, F. HPLC–MS/MS Method for the Simultaneous Determination of Clopidogrel, Its Carboxylic Acid Metabolite and Derivatized Isomers of Thiol Metabolite in Clinical Samples. J. Chromatogr. B 2012, 911, 105–112. [Google Scholar] [CrossRef]
- Shaw, S.A.; Balasubramanian, B.; Bonacorsi, S.; Cortes, J.C.; Cao, K.; Chen, B.-C.; Dai, J.; Decicco, C.; Goswami, A.; Guo, Z. Synthesis of Biologically Active Piperidine Metabolites of Clopidogrel: Determination of Structure and Analyte Development. J. Org. Chem. 2015, 80, 7019–7032. [Google Scholar] [CrossRef]
- Bluet, G.; Blankenstein, J.; Brohan, E.; Prevost, C.; Cheve, M.; Schofield, J.; Roy, S. Synthesis of the Stabilized Active Metabolite of Clopidogrel. Tetrahedron 2014, 70, 3893–3900. [Google Scholar] [CrossRef]
- Wickremsinhe, E.R.; Tian, Y.; Ruterbories, K.J.; Verburg, E.M.; Weerakkody, G.J.; Kurihara, A.; Farid, N.A. Stereoselective Metabolism of Prasugrel in Humans Using a Novel Chiral Liquid Chromatography-Tandem Mass Spectrometry Method. Drug Metab. Dispos. 2007, 35, 917–921. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, M.; Sugidachi, A.; Ogawa, T.; Isobe, T.; Jakubowski, J.A.; Asai, F. Stereoselective Inhibition of Human Platelet Aggregation by R-138727, the Active Metabolite of Cs-747 (Prasugrel, Ly640315), a Novel P2y12 Receptor Inhibitor. Thromb. Haemost. 2005, 94, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Farid, N.A.; Smith, R.L.; Gillespie, T.A.; Rash, T.J.; Blair, P.E.; Kurihara, A.; Goldberg, M.J. The Disposition of Prasugrel, a Novel Thienopyridine, in Humans. Drug Metab. Dispos. 2007, 35, 1096–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigmund, M.-C.; Poelarends, G.J. Current State and Future Perspectives of Engineered and Artificial Peroxygenases for the Oxyfunctionalization of Organic Molecules. Nat. Catal. 2020, 3, 690–702. [Google Scholar] [CrossRef]
- Kiebist, J.; Hofrichter, M.; Zuhse, R.; Scheibner, K. Oxyfunctionalization of Pharmaceuticals by Fungal Peroxygenases. In Pharmaceutical Biocatalysis; Jenny Stanford Publishing: Singapore, 2019; Volume 5, pp. 643–680. [Google Scholar]
- Hobisch, M.; Holtmann, D.; Gomez de Santos, P.; Alcalde, M.; Hollmann, F.; Kara, S. Recent Developments in the Use of Peroxygenases–Exploring Their High Potential in Selective Oxyfunctionalisations. Biotechnol. Adv. 2020, 51, 107615. [Google Scholar] [CrossRef]
- Hofrichter, M.; Kellner, H.; Herzog, R.; Karich, A.; Liers, C.; Scheibner, K.; Kimani, V.W.; Ullrich, R. Fungal Peroxygenases: A Phylogenetically Old Superfamily of Heme Enzymes with Promiscuity for Oxygen Transfer Reactions. In Grand Challenges in Fungal Biotechnology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 369–403. [Google Scholar]
- Hofrichter, M.; Kellner, H.; Pecyna, M.J.; Ullrich, R. Fungal Unspecific Peroxygenases: Heme-Thiolate Proteins That Combine Peroxidase and Cytochrome P450 Properties. Adv. Exp. Med. Biol. 2015, 851, 341–368. [Google Scholar]
- Kiebist, J.; Holla, W.; Heidrich, J.; Poraj-Kobielska, M.; Sandvoss, M.; Gröbe, G.; Atzrodt, J.; Hofrichter, M.; Scheibner, K. One-Pot Synthesis of Human Metabolites of SAR548304 by Fungal Peroxygenases. Bioorg. Med. Chem. 2015, 23, 4324–4332. [Google Scholar] [CrossRef]
- Steinbrecht, S.; Kiebist, J.; König, R.; Thiessen, M.; Schmidtke, K.-U.; Kammerer, S.; Küpper, J.-H.; Scheibner, K. Synthesis of Cyclophosphamide Metabolites by a Peroxygenase from Marasmius Rotula for Toxicological Studies on Human Cancer Cells. AMB Express 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gomez de Santos, P.; Cañellas, M.; Tieves, F.; Younes, S.; Molina-Espeja, P.; Hofrichter, M.; Hollmann, F.; Guallar, V.; Alcalde, M. Selective Synthesis of the Human Drug Metabolite 5′-Hydroxypropranolol by an Evolved Self-Sufficient Peroxygenase. ACS Catal. 2018, 8, 4789–4799. [Google Scholar] [CrossRef] [Green Version]
- Kiebist, J.; Schmidtke, K.-U.; Zimmermann, J.; Kellner, H.; Jehmlich, N.; Ullrich, R.; Zänder, D.; Hofrichter, M.; Scheibner, K. A Peroxygenase from Chaetomium Globosum Catalyzes the Selective Oxygenation of Testosterone. ChemBioChem 2017, 18, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Ullrich, R.; Poraj-Kobielska, M.; Scholze, S.; Halbout, C.; Sandvoss, M.; Pecyna, M.J.; Scheibner, K.; Hofrichter, M. Side Chain Removal from Corticosteroids by Unspecific Peroxygenase. J. Inorg. Biochem. 2018, 183, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, R.; Nüske, J.; Scheibner, K.; Spantzel, J.; Hofrichter, M. Novel Haloperoxidase from the Agaric Basidiomycete Agrocybe Aegerita Oxidizes Aryl Alcohols and Aldehydes. Appl. Environ. Microbiol. 2004, 70, 4575–4581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anh, D.H.; Ullrich, R.; Benndorf, D.; Svatos, A.; Muck, A.; Hofrichter, M. The Coprophilous Mushroom Coprinus Radians Secreted a Haloperoxidase That Catalyzes Aromatic Peroxgenation. Appl. Environ. Microbiol. 2007, 73, 5477–5485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gröbe, G.; Ullrich, R.; Pecyna, M.J.; Kapturska, D.; Friedrich, S.; Hofrichter, M.; Scheibner, K. High-Yield Production of Aromatic Peroxygenase by the Agaric Fungus Marasmius Rotula. AMB Express 2011, 1, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meunier, B.; De Visser, S.P.; Shaik, S. Mechanism of Oxidation Reactions Catalyzed by Cytochrome P450 Enzymes. Chem. Rev. 2004, 104, 3947–3980. [Google Scholar] [CrossRef]
- De Visser, S.P.; Shaik, S. A Proton-Shuttle Mechanism Mediated by the Porphyrin in Benzene Hydroxylation by Cytochrome P450 Enzymes. J. Am. Chem. Soc. 2003, 125, 7413–7424. [Google Scholar] [CrossRef]
- Karich, A.; Kluge, M.; Ullrich, R.; Hofrichter, M. Benzene Oxygenation and Oxidation by the Peroxygenase of Agrocybe aegerita. AMB Express 2013, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Aranda, E.; Kinne, M.; Kluge, M.; Ullrich, R.; Hofrichter, M. Conversion of Dibenzothiophene by the Mushrooms Agrocybe Aegerita and Coprinellus Radians and Their Extracellular Peroxygenases. Appl. Microbiol. Biotechnol. 2009, 82, 1057–1066. [Google Scholar] [CrossRef]
- Treiber, A.; Dansette, P.M.; El Amri, H.; Girault, J.-P.; Ginderow, D.; Mornon, J.-P.; Mansuy, D. Chemical and Biological Oxidation of Thiophene: Preparation and Complete Characterization of Thiophene S-Oxide Dimers and Evidence for Thiophene S-Oxide as an Intermediate in Thiophene Metabolism in Vivo and in Vitro. J. Am. Chem. Soc. 1997, 119, 1565–1571. [Google Scholar] [CrossRef]
- Treiber, A.; Dansette, P.M.; Mansuy, D. Mechanism of the Aromatic Hydroxylation of Thiophene by Acid-Catalyzed Peracid Oxidation. J. Org. Chem. 2002, 67, 7261–7266. [Google Scholar] [CrossRef]
- Rademacher, P.M.; Woods, C.M.; Huang, Q.; Szklarz, G.D.; Nelson, S.D. Differential Oxidation of Two Thiophene-Containing Regioisomers to Reactive Metabolites by Cytochrome P450 2c9. Chem. Res. Toxicol. 2012, 25, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Gomez de Santos, P.; Cervantes, F.V.; Tieves, F.; Plou, F.J.; Hollmann, F.; Alcalde, M. Benchmarking of Laboratory Evolved Unspecific Peroxygenases for the Synthesis of Human Drug Metabolites. Tetrahedron 2019, 75, 1827–1831. [Google Scholar] [CrossRef] [Green Version]
- Karich, A.; Scheibner, K.; Ullrich, R.; Hofrichter, M. Exploring the catalase activity of unspecific peroxygenases and the mechanism of peroxide-dependent heme destruction. J. Mol. Catal. B Enzym. 2016, 134, 238–246. [Google Scholar] [CrossRef]
- Dansette, P.M.; Levent, D.; Hessani, A.; Mansuy, D. Bioactivation of Clopidogrel and Prasugrel: Factors Determining the Stereochemistry of the Thiol Metabolite Double Bond. Chem. Res. Toxicol. 2015, 28, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Molina-Espeja, P.; Ma, S.; Mate, D.M.; Ludwig, R.; Alcalde, M. Tandem-Yeast Expression System for Engineering and Producing Unspecific Peroxygenase. Enzyme Microb. Technol. 2015, 73, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Molina-Espeja, P.; Cañellas, M.; Plou, F.J.; Hofrichter, M.; Lucas, F.; Guallar, V.; Alcalde, M. Synthesis of 1-Naphthol by a Natural Peroxygenase Engineered by Directed Evolution. ChemBioChem 2016, 17, 341–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Diaz, J.; Molina-Espeja, P.; Hofrichter, M.; Hollman, F.; Alcalde, M. Directed Evolution of Unspecific Peroxygenase in Organic Solvents. Biotechnol. Bioeng. 2021, 118, 3002–3014. [Google Scholar] [CrossRef] [PubMed]
- Babot, E.D.; del Río, J.C.; Kalum, L.; Martínez, A.T.; Gutiérrez, A. Oxyfunctionalization of Aliphatic Compounds by a Recombinant Peroxygenase from Coprinopsis cinerea. Biotechnol. Bioeng. 2013, 110, 2323–2332. [Google Scholar] [CrossRef] [Green Version]
- Babot, E.D.; del Río, J.C.; Kalum, L.; Martínez, A.T.; Gutiérrez, A. Regioselective Hydroxylation in the Production of 25-Hydroxyvitamin D by Coprinopsis Cinerea Peroxygenase. ChemCatChem 2015, 7, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Aranda, C.; Municoy, M.; Guallar, V.; Kiebist, J.; Scheibner, K.; Ullrich, R.; José, C.; Hofrichter, M.; Martínez, A.T.; Gutiérrez, A. Selective Synthesis of 4-Hydroxyisophorone and 4-Ketoisophorone by Fungal Peroxygenases. Catal. Sci. Technol. 2019, 9, 1398–1405. [Google Scholar] [CrossRef] [Green Version]
- Carro, J.; Gonzñlez-Benjumea, A.; Fernñndez-Fueyo, E.; Aranda, C.; Guallar, V.; Gutiérrez, A.; Martínez, A.T. Modulating Fatty Acid Epoxidation Vs Hydroxylation in a Fungal Peroxygenase. ACS Catal. 2019, 9, 6234–6242. [Google Scholar] [CrossRef] [Green Version]
- González-Benjumea, A.; Carro, J.; Renau-Mínguez, C.; Linde, D.; Fernández-Fueyo, E.; Gutiérrez, A.; Martínez, A.T. Fatty Acid Epoxidation by Collariella Virescens Peroxygenase and Heme-Channel Variants. Catal. Sci. Technol. 2020, 10, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Linde, D.; Olmedo, A.; González-Benjumea, A.; Estévez, M.; Renau-Mínguez, C.; Carro, J.; Fernández-Fueyo, E.; Gutiérrez, A.; Martínez, A.T. Two New Unspecific Peroxygenases from Heterologous Expression of Fungal Genes in Escherichia coli. Appl. Environ. Microb. 2020, 86, e02899-e19. [Google Scholar] [CrossRef] [Green Version]
- Tonin, F.; Tieves, F.; Willot, S.; van Troost, A.; van Oosten, R.; Breestraat, S.; van Pelt, S.; Alcalde, M.; Hollmann, F. Pilot-Scale Production of Peroxygenase from Agrocybe aegerita. Org. Process Res. Dev. 2021, 25, 1414–1418. [Google Scholar] [CrossRef]
- Aranda, C.; Carro, J.; González-Benjumea, A.; Babot, E.D.; Olmedo, A.; Linde, D.; Martínez, A.T.; Gutiérrez, A. Advances in Enzymatic Oxyfunctionalization of Aliphatic Compounds. Biotechnol. Adv. 2021, 51, 107703. [Google Scholar] [CrossRef]
- Karich, A.; Ullrich, R.; Scheibner, K.; Hofrichter, M. Fungal Unspecific Peroxygenases Oxidize the Majority of Organic EPA Priority Pollutants. Front. Microbiol. 2017, 8, 1463. [Google Scholar] [CrossRef] [PubMed]


| Entry | Substrate | Enzyme(s) | pH | 2-Oxo Metabolite (%) 2 | Active Metabolite (%) 2 |
|---|---|---|---|---|---|
| 1 | CPG | AaeUPO | 7.0 | 29.0 ± 0.5 | <2 |
| 2 | CPG | CraUPO | 7.0 | 12.7 ± 0.3 | <2 |
| 3 | CPG | MroUPO | 7.0 | 10.6 ± 0.2 | 8.6 ± 0.2 |
| 4 | CPG | MroUPO | 5.5 | 46.6 ± 0.5 | 3.6 ± 0.2 |
| 5 | CPG | CglUPO | 7.0 | 3.7 ± 0.1 | <2 |
| 6 | CPG | MweUPO | 7.0 | 8.1 ± 0.9 | 6.4 ± 0.1 |
| 7 | CPG | MweUPO | 5.5 | 31.6 ± 1.2 | <2 |
| 8 | PSG | PLE/AaeUPO | 7.0 | 87.4 ± 3.0 | 5.8 ± 0.6 |
| 9 | PSG | PLE/CraUPO | 7.0 | 87.2 ± 0.4 | <2 |
| 10 | PSG | PLE/MroUPO | 7.0 | 39.1 ± 2.0 | 34.2 ± 0.4 |
| 11 | PSG | PLE/CglUPO | 7.0 | 19.0 ± 0.5 | 28.2 ± 1.2 |
| 12 | PSG | PLE/MweUPO | 7.0 | 46.5 ± 2.7 | 31.0 ± 0.3 |
| Entry | Substrate | Solvent | Reductant | 2-Oxo Metabolite (%) 2 | Active Metabolite (%) 2 |
|---|---|---|---|---|---|
| 13 (3) | CPG | Buffer/acetone 90:10 | Ascorbate | 10.6 ± 0.2 | 8.6 ± 0.2 |
| 14 | CPG | Buffer/acetone 90:10 | Glutathione | 26.2 ± 1.9 | <2 |
| 15 | CPG | Buffer/acetone 90:10 | None | <2 | <2 |
| 16 | CPG | Buffer/MeCN 3 90:10 | Ascorbate | 3.2 ± 0.1 | 4.0 ± 0.1 |
| 17 | CPG | Buffer/MeOH 3 90:10 | Ascorbate | 31.1 ± 0.3 | <2 |
| 18 | CPG | Buffer/DMSO 3 90:10 | Ascorbate | 16.9 ± 1.8 | <2 |
| 19 | CPG | Buffer/DMF 3 90:10 | Ascorbate | 25.5 ± 0.5 | 4.5 ± 0.1 |
| 20 | CPG | Buffer | Ascorbate | 11.5 ± 0.2 | <2 |
| 21 | CPG | Buffer/acetone 80:20 | Ascorbate | 8.7 ± 0.6 | 9.8 ± 0.5 |
| 22 | CPG | Buffer/acetone 60:40 | Ascorbate | 6.3 ± 0.4 | <2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiebist, J.; Schmidtke, K.-U.; Schramm, M.; König, R.; Quint, S.; Kohlmann, J.; Zuhse, R.; Ullrich, R.; Hofrichter, M.; Scheibner, K. Biocatalytic Syntheses of Antiplatelet Metabolites of the Thienopyridines Clopidogrel and Prasugrel Using Fungal Peroxygenases. J. Fungi 2021, 7, 752. https://doi.org/10.3390/jof7090752
Kiebist J, Schmidtke K-U, Schramm M, König R, Quint S, Kohlmann J, Zuhse R, Ullrich R, Hofrichter M, Scheibner K. Biocatalytic Syntheses of Antiplatelet Metabolites of the Thienopyridines Clopidogrel and Prasugrel Using Fungal Peroxygenases. Journal of Fungi. 2021; 7(9):752. https://doi.org/10.3390/jof7090752
Chicago/Turabian StyleKiebist, Jan, Kai-Uwe Schmidtke, Marina Schramm, Rosalie König, Stephan Quint, Johannes Kohlmann, Ralf Zuhse, René Ullrich, Martin Hofrichter, and Katrin Scheibner. 2021. "Biocatalytic Syntheses of Antiplatelet Metabolites of the Thienopyridines Clopidogrel and Prasugrel Using Fungal Peroxygenases" Journal of Fungi 7, no. 9: 752. https://doi.org/10.3390/jof7090752
APA StyleKiebist, J., Schmidtke, K.-U., Schramm, M., König, R., Quint, S., Kohlmann, J., Zuhse, R., Ullrich, R., Hofrichter, M., & Scheibner, K. (2021). Biocatalytic Syntheses of Antiplatelet Metabolites of the Thienopyridines Clopidogrel and Prasugrel Using Fungal Peroxygenases. Journal of Fungi, 7(9), 752. https://doi.org/10.3390/jof7090752