1. Introduction
Owing to diverse bioactivities, small molecule secondary metabolites have been major sources of medicines, promising pesticides, contaminants of food, biological probes, and targets of study for synthetic and analytical chemists [
1,
2]. Polyketides encompass a highly structurally diverse group of secondary products. Chemical skeletons of polyketides are assembled through sequential additions of two carbon building blocks derived from acetyl-coenzyme A (CoA) or malonyl-CoA precursors by polyketide synthases (PKSs) [
3,
4]. Based on the structural organization of their functional domains, PKSs are classified into three basic categories: type I PKSs are large multifunctional proteins comprised of several functional domains and found in both bacteria and fungi; type II PKSs are formed by discrete catalytic domains and are typically found in bacteria; type III PKSs are simpler chalcone synthase-type enzymes that catalyze the formation of the product within a single active site, mainly in plants and bacteria. Type I PKSs can be further classified into single modular iterative type I PKS and multi-modular PKS. In the fungi kingdom, the iterative type I PKS is more often encountered. In some cases, modules from type I PKSs are linked to non-ribosomal peptide synthetase (NRPS) modules, which results in the production of polyketide-peptide hybrid metabolites, e.g., cytochalasans [
3,
5,
6,
7].
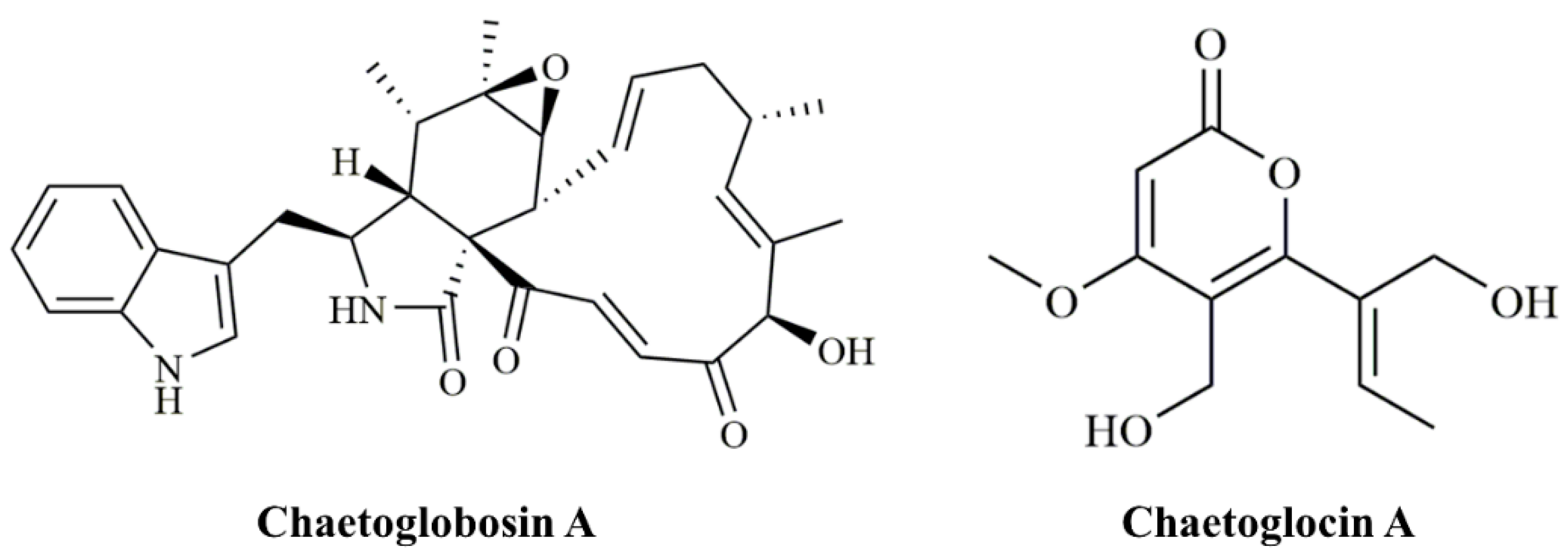
Chaetoglobosin A (ChA,
Figure 1) was initially identified in
Chaetomium globosum [
8]. Structurally, ChA contains a 10-(indol-3-yl) group and a tricyclic core in which a macrocyclic ring is fused to a perhydroisoindolone moiety [
9]. Like most cytochalasins, ChA has been confirmed to target filamentous actin in mammalian cells, and thereby induces cell-cycle arrest and inhibits membrane ruffling and cell migration [
10], endowing ChA strong cytotoxicity against tumor cell lines, immunomodulatory activities, and antifungal activities [
11,
12,
13]. Even more, we previously reported the nematicidal activity of culture filtrates and ChA from
C. globosum NK102 against the nematode
Meloidogyne incognita. Our results showed that both filtrates and purified ChA demonstrated strong adverse effects on the second stage juveniles (J2s) mortality with 99.8% at 300 μg/mL (LC
50= 77.0 μg/mL) [
14]. Later, Ashrafi extracted ChA from the fermentation broth of the destructive parasitic fungus of the cereal cyst nematode
Heterodera filipjevi and demonstrated that ChA caused a temporary inhibition on J2 mobilization [
15]. Thus, it is intriguing to explore the nematicidal activity of
C. globosum, and its use as a biological agent for the control of plant pathogenic microorganisms and pests.
The genetic and molecular basis of ChA biosynthesis in fungi was initially studied in
Penicillium expansum [
16], in which genes involved in ChA biosynthesis are clustered, and the carbon scaffold of ChA is synthesized by a hybrid iterative type I polyketide synthase-non-ribosomal peptide synthetase (PKS-NRPS) CheA and a standalone enoyl reductase CheB. After a spontaneous intramolecular condensation and a Diels–Alder reaction, the key intermediate prochaetoglobosin is generated, which can be furnished into ChA after a set of oxidative modifications. Through gene disruption, homologs of CheA (
CgcheA with a gene locus CHGG_01239) and CheB (
CgcheB with a gene locus CHGG_01238) as well as the enzymes CheD (
CgcheE with a gene locus CHGG_01242-1), CheE (
CgcheF with a gene locus CHGG_01242-2), and CheG (
CgcheG with a gene locus CHGG_01243), involved in the final oxidative transformations, are identified and characterized in
C. globosum [
17,
18]. Nonetheless, we previously observed in
C. globosum NK102 that a putative pigment polyketide synthase gene,
pks-1/alb1, was also required for ChA biosynthesis [
19]. The knock-down of
pks-1 resulted in a dramatic reduction of ChA production and significant inhibition of pigmentation and sporulation. It is proposed that PKS-1 probably provides precursors for ChA biosynthesis.
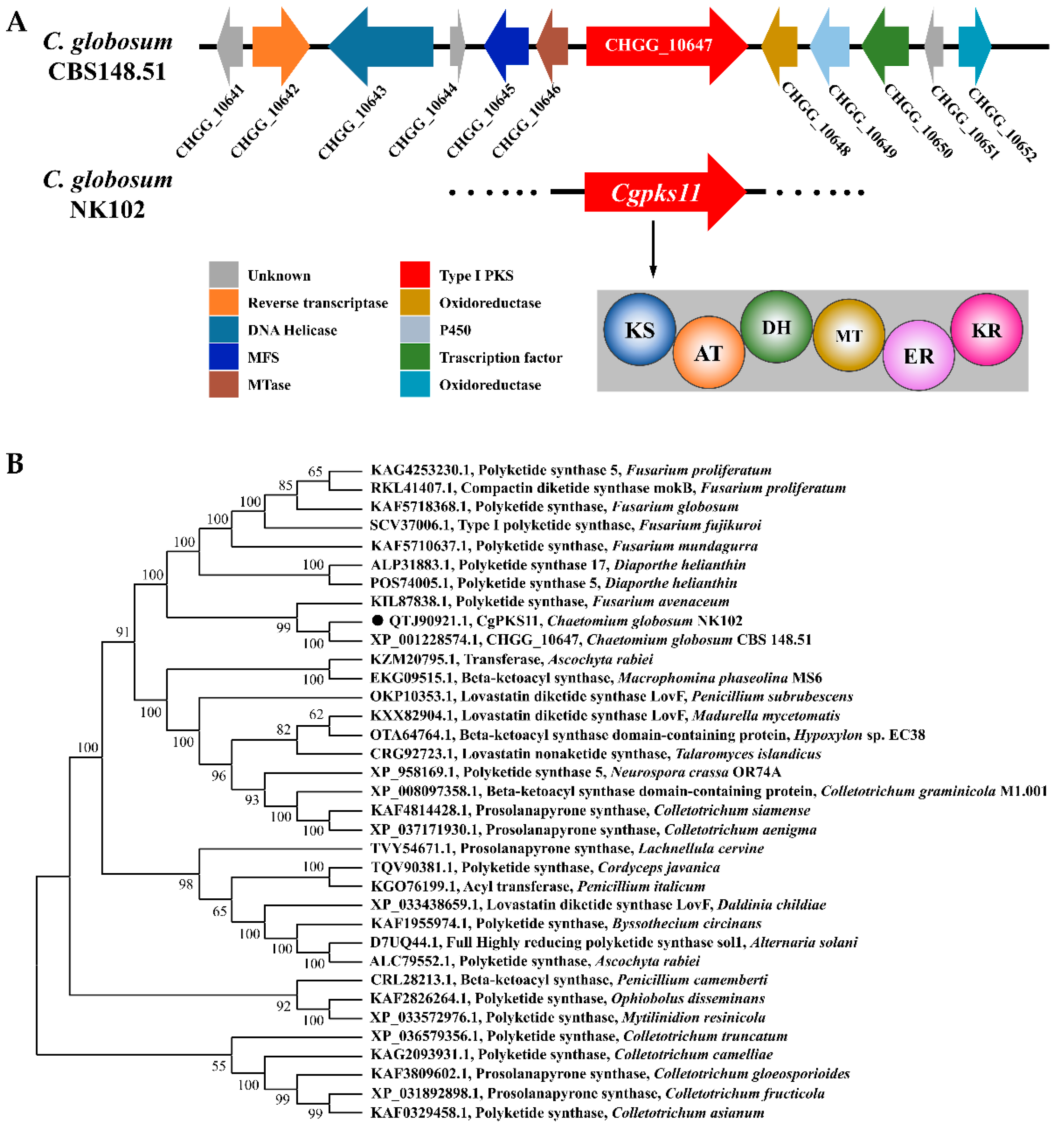
The involvement of
pks-1 in the production of ChA prompts us to the possibility that biosynthesis of polyketides in fungi may have competition for precursors between PKSs. Thus, we conducted bioinformatics analysis using the software antiSMASH (
https://antismash.secondarymetabolites.org, accessed on 17 June 2019) and protein Blast against the NCBI database and identified 28 predicted PKS genes in the genome of
C. globosum (
Table S1). In order to eliminate potential competing polyketide products, we tried to delete 26 putative PKS encoding genes, except for
CgcheA and
pks-1, respectively. A previously reported, PKS encoding ORF in another
C. globosum strain, CHGG_10647, on scaffold_7 was predicted to play a role in the biosynthesis of chaetoglocin A (
Figure 1) [
20]. We confirmed its role in the production of chaetoglocin A. We deleted the homologous gene of CHGG_10647 (named as
Cgpks11) in
C. globosum NK102 via a “suicide” CRISPR-Cas9 system. Interestingly, we found that the deletion of
Cgpks11 leads to a significant increase of ChA production, and yet this dramatically delayed the growth of mycelium, sporulation, and sexual development. We also discuss this new phenomenon as to the role of this gene and its derived polyketide during ChA biosynthesis.
2. Materials and Methods
2.1. Strains and Growth Conditions
The wild-type strain C. globosum NK102, which produces a high level of ChA, was isolated and stocked by our laboratory, and was the host strain for gene knockout in this study. NK102 was grown on potato dextrose agar (PDA) at 28 °C. For preparation of protoplasts, approximately (1–4) ×104 ascospores, collected from a single plate, were used to inoculate 100 mL of potato dextrose broth (PDB) medium, shaken at 28 °C and 200 rpm for 2 days. Ten grown mycelial balls were transferred into 100 mL fresh PDB medium, and then continued to grow for 3 days at 28 °C with a shaking speed of 160 rpm. For DNA isolation, 5 mm agar plaques containing the fungal hyphae were inoculated in 100 mL PDB and incubated for 3 days in a rotary shaker at 28 °C and 200 rpm. For high-performance liquid chromatography (HPLC) analysis or RNA isolation, strains were cultured in 100 mL PDB for 7 days at 28 °C and shaken at 200 rpm. Escherichia coli, used for constructing plasmids and conjugation, was grown in Luria–Bertani (LB) broth (Difco, Detroit, MI, USA) or on LB agar plates at 37 °C.
2.2. Gene Prediction, Alignment, and Phylogenetic Analysis
The PKS gene clusters in
C. globosum CBS148.51 genome (RefSeq: NZ_AAFU00000000.1) were analyzed by using the antiSMASH (
https://antismash.secondarymetabolites.org, accessed on 17 June 2019) [
21]. Peptide sequences of all predicted genes were then submitted to protein BLAST against the NCBI database for further confirmation.
The homologous gene of CHGG_10647 in C. globosum NK102, subsequently named Cgpks11, was obtained by protein BLAST. The putative amino acid sequence of Cgpks11 was then subjected to protein BLAST against the NCBI database for more information. The putative conserved domains of CgPKS11 were defined from the NCBI Conserved Domains database and further confirmed by searching on the UniProtKB database. For the phylogenetic evaluation of CgPKS11, all hits producing E-values below 10−40 generated from protein BLAST were taken into subsequent analysis. Hypothetical protein, uncharacterized protein, and repetitive sequences of the same functional proteins in the same strain were removed. The full-length predicted amino acid sequences of CgPKS11 and the selected well-characterized proteins were aligned using the Muscle program in MEGA Ⅹ (v10.1.8), and the alignment was used to generate a phylogenetic tree by using the Neighbor-Joining algorithm and Jones–Thornton–Taylor (JTT) matrix-based model. The alignment gaps and missing data sites were deleted, and a bootstrap value based on 1000 replications was used to measure statistical confidence of branch nodes of the phylogenetic tree.
2.3. Construction of CRISPR-Cas9 Editing Tool in C. globosum
Previously, we reported a “suicide” CRISPR-Cas9 system to promote gene deletion with a minimized off-target effect by eliminating the system itself upon the occurrence of recombination and a suitable system for gene restoration assay in
Cryptococcus neoformans [
22]. In this study, a similar system was constructed in
C. globosum NK102. Plasmids for gene deletion were constructed based on recombination cloning method using In-Fusion
® HD Cloning kits (Takara Bio, Mountain View, CA, USA). To construct the Cas9 expression cassette, a Cas9 coding region with NLSs followed by a bGHpA terminator was amplified from pBS-URA5-Pact: Cas9 [
22] by primers Cas9in-F/Cas9in-R. An 800 bp ACTIN promoter was amplified from
C. globosum NK102 genomic DNA with primers act1p-F/act1p-R. Vector pUCATPH [
23] was linearized by restriction enzyme
Hind III. Primer act1p-R and Cas9in-F shared 15 homologous bases at each 5′-terminal. Primers act1p-F and Cas9in-R shared 15 homologous bases with the cohesive terminus of linearized pUCATPH, respectively. The amplicons of the expected sizes were purified with the GeneJET Gel Extraction Kit (Thermo Fisher Scientific, Carlsbad, CA, USA) and quantified using NanoDropTM One Spectrophotometer (Thermo Fisher Scientific, Madison, WI, USA). To fuse the ACTIN promoter and CAS9 coding region into the
Hind III site of pUCATPH to generate pUCATPH-Cas9, the In-Fusion reaction was performed in a total volume of 5 μL, containing 1.0 μL of 5× In-Fusion HD Enzyme Premix, 100 ng of each purified PCR fragment, and ddH
2O from the In-Fusion HD PCR Cloning Kit. The reaction mix was incubated at 50 °C for 15 min, and then placed on ice for transformation using
E. coli DH5α competent cells. Transformants were screened with colony PCR using primers act1p-F and CXcas9infu-1. To construct the single guide DNA (gDNA) expression cassette for the production of the single gRNA, a 200 bp U6 promoter was amplified from
C. globosum NK102 genomic DNA with primers u6gdna1-F and u6gdna1-R. A bacterial tracerRNA scaffold was amplified from plasmid pBS-URA5-Pact: Cas9 with primers u6gdna2-F and u6gdna2-R. Vector pUCATPH-Cas9 was linearized by restriction enzyme
Kpn I. In order to reuse the plasmid to target other genes, two
Bbs I restriction sites were designed at the 5′-terminal of the primers u6gdna1-R and u6gdna2-F for insertion of the target sequence GN
19NGG [
22]. primers u6gdna1-F and u6gdna2-R shared 15 homologous bases with the cohesive terminus of linearized pUCATPH-Cas9, respectively. As described above, the amplicons of the expected sizes were purified, quantified, and fused into the
Kpn Ⅰ site of pUCATPH-Cas9 to generate the plasmid pCgCRISPR. The transformants were screened with colony PCR using the primers u6gdna1-F and u6gdna2-R.
In the CRISPR-Cas9 editing system, a GN19NGG guide sequence was designed with the following parameters:
- (1)
The sequence was located close to the 5′ end of the target gene-coding region for cleavage;
- (2)
Similarity to the GN19NGG was searched by BLASTN to the C. globosum genome to avoid cleavage at secondary loci.
2.4. CRISPR-Cas9 Mediated Cgpks11 Deletion
To construct the
Cgpks11 knockout vector, the
HygR cassette flanked by
Xho I and
Xba I sites and amplified from pUCATPH by primers Hyg-
Xho I -F and Hyg-
Xho I -R, was introduced into the
Xho I site of plasmid pCgCRISPR, and the resulting plasmid was named pCRISPR-Hyg. Then, the vector pCRISPR-Hyg was linearized by the restriction of enzyme
Bbs I. The guide GN
19NGG sequence to
Cgpks11 was designed for designing the complementary primers PCgpks11-N19-F and PCgpks11-N19-R. The cohesive terminus of
Bbs I was added at the 5′ terminal of each primer, respectively. These primers were synthesized, denatured (95 °C, 10 min), and then annealed at room temperature to generate the GN
19NGG fragment. The GN
19NGG fragment was ligated into the
Bbs I site of pCRISPR-Hyg to generate plasmid PCgpks11-N19. The upstream (877 bp) and downstream (938 bp) flanking sequences of the
Cgpks11 were amplified from NK102 genomic DNA with primers PCgpks11-UF/PCgpks11-UR and PCgpks11-DF/PCgpks11-DR, respectively. PCgpks11-N19 was cut by
Xba I to generate a large fragment containing Cas9 and a small fragment containing a
HygR cassette bearing the hygromycin B resistance as a selection marker. The 5′ and 3′ flanks of
Cgpks11 and the
HygR cassette were fused with the largest fragment via In-Fusion HD Cloning kits, as mentioned above (
Figure S1). The fused plasmid, designated as pCgpks11, was linearized with
Xba I and introduced into the NK102 via PEG-mediated protoplast transformation. All primers used in this assay are listed in
Table S2.
2.5. Fungal Transformation and Transformants Screening
Protoplasts were prepared and transformed as described [
24]. For transformants selection, hygromycin B (Sigma-Aldrich, St. Louis, MO, USA) was added to the plates at a final concentration of 100 μg/mL.
In screening for Δ
cgpks11 mutants, two PCR reactions per transformant were carried out. Mutants were subjected to PCR analysis using a combination of a marker-specific primer and a primer designed to anneal the outside of the homologous region flanking the
HygR gene. Primers P11-KO-VP-F/iHYG-R and primers P11-KO-VP-R/PHYG-ter-F could amplify fragments of 1550 bp and 1367 bp, respectively. For further confirmation, genomic DNA was extracted and subjected to Southern blot analysis as previously described [
24]. A 2114 bp
HygR cassette, amplified from pUCATPH using primers HYG-F/HYG-R, was labeled as the probe. Experiments involving DNA labeling, hybridization, and detection were carried out according to the instructions of the DIG High Prime DNA Labeling and Detection Starter Kit II (Roche Life Science, Mannheim, Germany). All primers were listed in
Table S2.
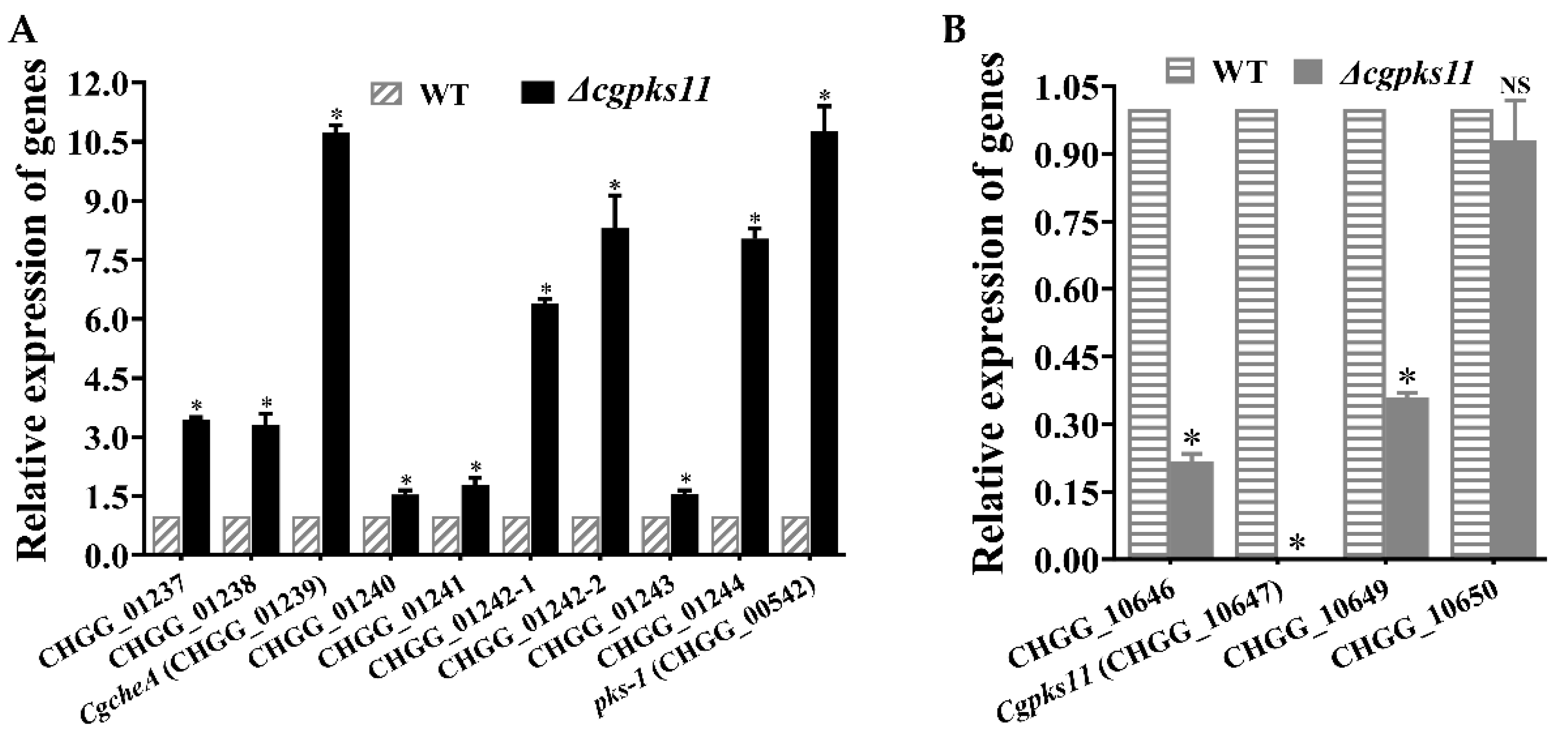
2.6. RNA Preparation and Quantitative Real-Time PCR
Total RNA was extracted from the lyophilized and ground mycelium using an RNAiso Plus kit (Takara Bio, Shiga, Japan). The first-strand cDNA was generated by reverse transcription in a 20 μL reaction using the TransScript First-Strand cDNA Synthesis SuperMix kit (TransGen Biotech, Beijing, China). Quantitative real-time PCR was performed by LightCycler
® 480 instrument III (Roche Diagnostics, Indianapolis, IN, USA). Each reaction of 20 μL PCR was performed with SYBR Green I Master (Roche Life Science, Mannheim, Germany). Reactions were set up in three replicates per sample. Controls without the addition of the templates were included for each primer set. PCR cycling parameters were pre-incubation at 95 °C for 5 min, followed by 45 cycles of denaturation at 95 °C for 10 s, annealing at 55 °C for 15 s, and extension at 72 °C for 20 s. The qRT-PCR data was analyzed using the
relative quantification method [
25] to calculate the relative expression levels of genes. The housekeeping gene encoding glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as reference. The amplification efficiencies of the target and reference genes were compared at different template concentrations. The gene-specific pairs of primers used in the amplifications were as follows: qCgpks11F/qCgpks11-R for
Cgpks11, q10646-F/q10646-R for CHGG_10646, q10649-F/q10649-R for CHGG_10649, q10650-F/q10650-R for CHGG_10650, q01237-F/q01237-R for CHGG_01237, q01238-F/q01238-R for CHGG_01238, q01239-F/q01239-R for
CgcheA, q01240-F/q01240-R for CHGG_01240, q01241-F/q01241-R for CHGG_01241, q01242-1-F/q01242-1-R for CHGG_01242-1, q01242-2-F/q01242-2-R for CHGG_01242-2, q01243-F/q01243-R for CHGG_01243, q01244-F/q01244-R for CHGG_01244, q00542-F/q00542-R for
pks-1, and qGAPDH-F/qGAPDH-R for GAPDH gene (
Table S2).
2.7. Growth Observation
The growth of Δcgpks11 mutants was compared with the WT strain on agar plates and in liquid medium. The linear growth rate of mutants on PDA media at 28 °C was determined in three replicate plates (D = 9 cm) by measuring the diameter of the colony every day over a 7-day period. For the submerged culture, the total mycelium of each strain was isolated from cultures inoculated in 100 mL PDB medium for 7 days at 28 °C, with shaking at 200 rpm, respectively. Fresh mycelium was isolated by vacuum pump and dry mycelium was obtained by vacuum freeze-drying. The weight of the dry mycelium was determined.
2.8. Quantification of Ascospores Production
Ascospores were harvested from 15-day-old cultures on PDA at 28 °C in triplicate for each strain. Firstly, 2 mL of sterile distilled water was added to each plate. Then, ascospores formed on each plate were scrapped using a sterilized spatula and was resuspended in 30 mL of sterile distilled water. After centrifugation at 3000× g for 10 min, the supernatant full of hyphae-debris was discarded, and the ascospores pellet was washed twice with sterile distilled water and finally resuspended in 10 mL sterile distilled water. The concentration of ascospores suspension in sterile distilled water was determined by hemocytometry under the Moticam Ⅹ3 microscope (Motic, Xiamen, China).
2.9. Measurement of the Cell Length of Ascoma Hairs
Ascocarps were scrapped from a 15-day-old culture and observed using a 40×/0.65NA objective lens on a Moticam X3 microscope. MotiConnect software was used to measure the cell length of ascoma hairs and photographs. Ten ascocarps and two mature septate ascoma hairs for each ascocarp were measured.
2.10. Microscopy
Agar plaques containing the fungal hyphae of Δcgpks11, or the WT strain were inoculated on PDA plates and cultured at 28 °C. Perithecia that formed on the plates was scrapped off. To observe the asci and ascospores that developed within a perithecium, we gently tapped the cover slip with the holder of the inoculating loop and crack the perithecium. Images were taken by using 10×/0.25NA and 40×/0.65NA objective lenses on a Moticam Ⅹ3 microscope. For microcopy observation of mature ascospores, a Carl Zeiss Axio Imager Z2 Apotome2 Upright Microscope (Carl Zeiss, Oberkochen, Germany) equipped with EC P1an-NEOFLUAR 40×/0.75NA and Plan-APOCHROMAT 63×/1.40NA objective lenses (Carl Zeiss, Göttingen, Germany) were used. Images were captured with an Axiocam 506 mono microscopy camera (Carl Zeiss, Göttingen, Germany) and processed using ZEN 2.3 lite software.
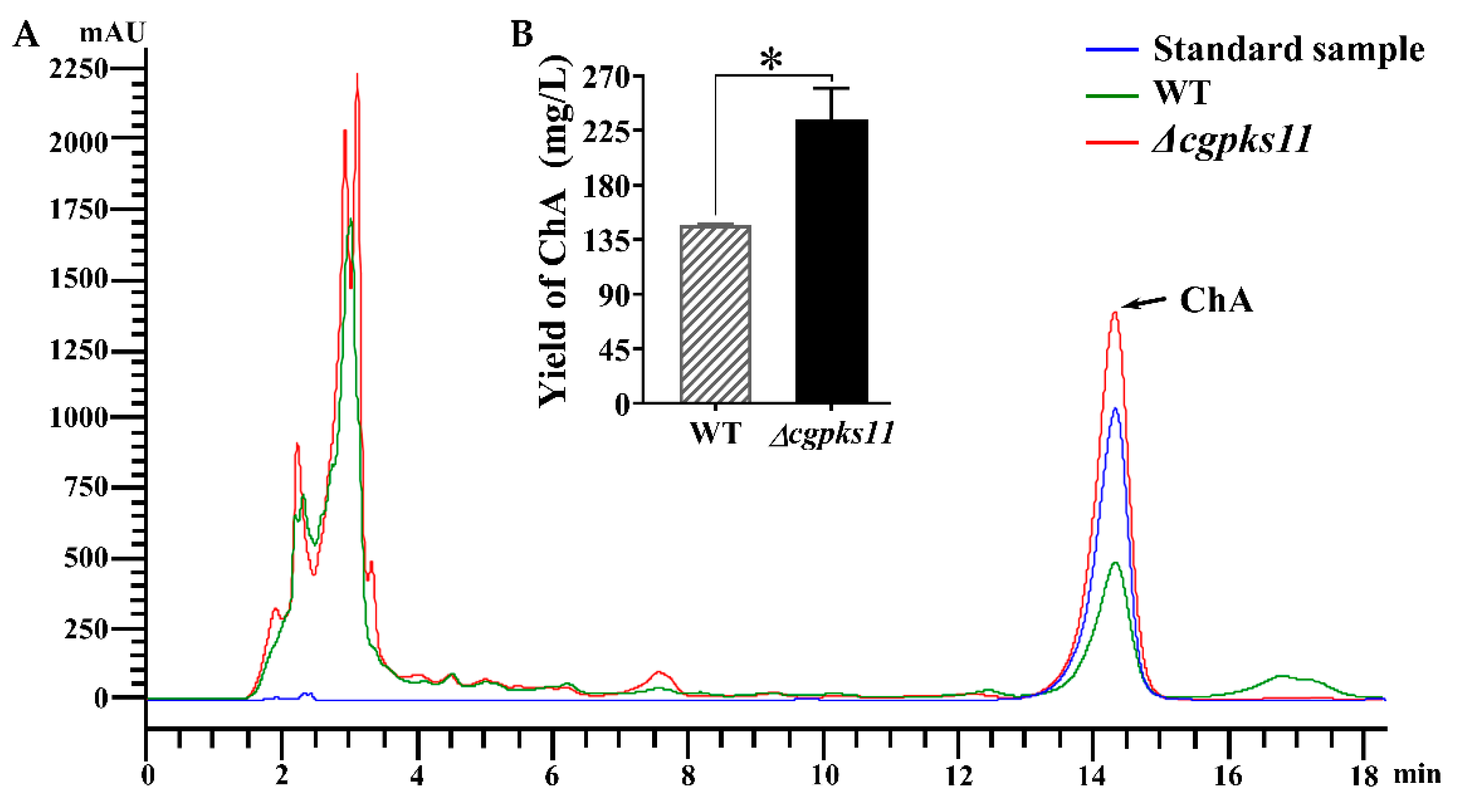
2.11. Detection of Chaetoglobosin A by HPLC
After 7 days of cultivation, the fermentation cultures of the WT and Δ
cgpks11 were passed through a Buchner funnel vacuum filter, and the filter residue, i.e., mycelial pellets were subjected to lyophilized and weighed, respectively. Each sample of the filtrate was added in an equal volume of ethyl acetate and stood for 12 h after agitation to extract ChA through layering. The organic phase was then concentrated, dissolved, and centrifuged as previously reported [
24]. The supernatant was filtered through a 0.22 μm Millipore filter and subjected to HPLC analysis on Agilent 1200 HPLC system (Agilent Technologies, Santa Clara, CA, USA) with Kromasil C18 ODS column (4.6 × 250 mm, AKZO Nobel, Gland, Switzerland). The UV detection wavelength was set at 227 nm, and the sample flow rate was set at 1 mL/min. Standard ChA (Sigma-Aldrich, St. Louis, MO, USA) served as the control. For quantification of ChA, a standard curve was created with known concentrations of the standard sample.
4. Discussion
Polyketides are the common secondary products of fungi. The widely spread fungus
C. globosum is well studied for its capacity of making an abundant polyketide and is used as a biocontrol agent. One of the metabolites, ChA, strongly inhibits the egg hatching of root-knot nematode
M. incognita [
14] and has strong nematicidal activities against the second stage juveniles of
M. incognita or
M. javanica [
14,
27]. Considering the promising application of
C. globosum, we intended to improve the yield of its main active product ChA by eliminating competing polyketide products in NK102.
Polyketide synthases (PKSs) are responsible for the biosynthesis of polyketide backbone. Usually, a single genome of filamentous fungi can harbor a number of PKS genes. For the majority, their biological function is largely uninvestigated. In the search for total PKSs in
C. globosum by using bioinformatics tools, 28 putative PKS encoding genes were identified, which are located in 28 gene clusters with 24 type I PKSs, 3 PKS-NRPSs, and 1 type III PKS genes. A putative PKS with a gene locus CHGG_10647 was previously predicted to be responsible for the biosynthesis of the polyketide chaetoglocin A [
20]. Here, we defined the domain structure of the putative protein product encoded by the homologous gene of CHGG_10647, designated as CgPKS11. As a rare type I PKS, however, CgPKS11 is without the ACP domain. The ACP domain is a small (~80–100 residues) and non-catalytic, either independent, freestanding structures, or covalently bound as part of multi-modular enzymes such as fatty acid synthases (FASs), PKSs, and NRPSs [
28]. In known PKSs, ACP activates the acyl CoA substrates and channels the polyketide intermediates, except for type III PKSs that utilize acyl CoAs as substrates directly [
29]. There is one ACP domain located in the key enzyme of ChA biosynthesis CgCheA [
17]. CgCheA catalyzes the stepwise assembly of a nonaketide from an acetyl-CoA starter unit and eight malonyl-CoA extenders that are loaded onto the ACP domain by means of the acyl transferase (AT) domain. The product is then modified with a tryptophan and delivered to the PCP domain of CgCheA for subsequent steps. In contrast to the canonical loading strategy, a standalone ACP plays an important role in the biosynthesis of
β-amino acid-containing macrolactams. The
β-amino acid unit is first ligated with a standalone ACP VinL and then aminoacylated with L-alanine, prior to loading onto a modular PKS, in the biosynthesis of β-amino acid-containing macrolactams [
30]. In some cases, the ACP domain of PKS is missing. In an analysis of PKS gene clusters in lichenizing fungi, six ACP-less PKS were observed in the genome of
Peltigera membranacea and one ACP-less PKS was located in the genome of
Endocarpon pusillum [
31]. The reason for this unusual observation is still unknown. Some researchers think it is possible that these PKS were inactivated by evolution and have been rendered non-functional through loss of ACP domains to adjust its production to environmental conditions [
31]. In research on macrotetrolide biosynthesis in
Streptomyces griseus, the macrotetrolide type II PKS has been demonstrated to act non-iteratively, lacking of an ACP domain, to utilize acyl-CoA as substrate directly and catalyze both C–C and C–O bond formation [
29]. CgPKS11 is an ACP-less type I PKS as well, although most of PKSs that share a high homolog with CgPKS11 usually contain an ACP domain (
Figure 2B). We have searched for more than a dozen genes on both sides of the CgPKS11 gene cluster, but none where a predictable standalone ACP was located in the nearby loci. We further search for the potential standalone ACP in the genome of
C. globosum CBS148.51 via Blastp. An ORF, CHGG_09364 was obtained. The ORF putatively encodes 1073 amino acids and is annotated as a hypothetical protein. The region covering 1006-1064AA of this protein contains a standalone ACP domain. However, it is silenced according to RNA-seq profiling (
Table S3).
Secondary metabolites are often bioactive, usually of low molecular weight, and are produced as families of related compounds at restricted parts of the life cycle, with production often correlated with a specific stage of morphological differentiation [
32]. We further investigated the function of this structurally unique PKS, CgPKS11, in several aspects, including the sexual development and ChA biosynthesis of
C. globosum. We deleted
Cgpks11 via a CRISPR-Cas9 gene deletion system [
22]. This is likely the first report of CRISPR-Cas9 system applied in
C. globosum and will facilitate genetic engineering in this fungus. Our data revealed that the function of CgPKS11 participated in the sporulation process. Deletion of CgPKS11 resulted in delayed maturation of ascocarps, shortened cell length of ascoma hairs, and significant reduction of ascospores production. This is an unreported phenotypic consequence for a PKS in this fungus, although the mechanism of CgPKS11 on the fungal development is not clear. Substantial data demonstrated that PKS-derived melanin is required for the development of asexual and sexual structures of fungi that might function as protectants against harmful environmental conditions. Deficiency of PKSs responsible for pigment biosynthesis usually had a negative effect on sexual development [
19,
33,
34,
35]. Indeed, we previously demonstrated that downregulation of PKS-1/Alb1, a key enzyme responsible for pigment biosynthesis displayed a pigment-deficient phenotype and lost the ability to produce ascospores in
C. globosum [
19]. In contrast, deletion of CgPKS11 has no effect on morphological characteristics of ascospores, so CgPKS11 is unlikely to regulate sexual development through pigment biosynthesis.
Secondary metabolite production is also responsive to general environmental factors, like carbon and nitrogen sources, temperature, light, and pH. In some cases, polyketides or polyketide non-ribosomal peptide hybrid molecules, may act as signaling molecules to regulate development and physiology. Taking zearalenone as an example, which was an estrogenic mycotoxin synthesized by
Fusarium roseum, it was reported to act as a sex-regulating hormone on perithecial formation [
36]. Differentiation-inducing factor (DIF)-1 is a chlorinated alkyl phenone made from a polyketide and was reported to act as a chlorinated signal molecule regulating
Dictyostelium development [
37]. Nemamides, the hybrid polyketide non-ribosomal peptides produced by
Caenorhabditis elegans, have been shown to act as regulators of starvation-induced larval arrest [
38]. Studies on the function of FluP polyketide synthase in
Aspergillus flavus suggested that FluP is involved in the synthesis of a diffusible metabolite that could serve as a signal molecule to regulate sclerotiogenesis [
39]. Therefore, we believe chaetoglocin A, a small polyketide that is synthesized by CgPKS11, may act in a similar way. As deletion of CgPKS11, which is responsible for the biosynthesis of chaetoglocin A, led to slower growth of the mycelium and retarded sexual development, and in particular showed a dramatic rise of ChA biosynthesis. The yield of ChA from Δ
cgpks11 increased to nearly 1.6-fold of that from the WT strain. According to qRT-PCR analysis, we found that all genes of ChA biosynthesis gene cluster were simultaneously upregulated as expected. In addition, transcription of
pks-1 that previously reported to be involved in ChA biosynthesis was also upregulated. It explained why ChA yield was increased in Δ
cgpks11. It should be noted that CgPKS11 deletion also led to the downregulation of the transcription of two other genes, CHGG_10646 and CHGG_10649, within the chaetoglocin A biosynthesis gene cluster. Taken together, the above studies suggest that either CgPKS11 or its product chaetoglocin A probably act as a regulation factor in sexual development and ChA biosynthesis.
5. Concluding Remarks
The molecular mechanism regulating ChA biosynthesis remains largely uninvestigated. We constructed CRISPR-Cas9 editing system for manipulation of the genome of C. globosum and attempted to improve ChA by a large leap and to study the control of its production at the molecular level. In the attempt to delete all the PKSs in order to eliminate the possible competitive pathways in the biosynthesis of ChA, a rare type I PKS, Cgpks11, which is responsible for the biosynthesis of chaetoglocin A and lacks the ACP domain, was deleted. The deletion of the gene resulted in the loss of chaetoglocin A, but astonishingly increased the production of ChA dramatically. In addition, CgPKS11 deletion led to growth retardation on agar plate and seriously impaired ascospore development, reduced the capacity of sporulation, and shortened ascoma hair cells. More importantly, we found that CgPKS11 modulated the ChA biosynthesis by inhibiting the expression of genes in the CgcheA cluster, which is responsible for ChA biosynthesis, while it downregulated genes for chaetoglocin A modification genes. Deletion of CgPKS11 released the inhibition. The findings revealed a novel mechanism taken by PKSs in coordination of growth, sporulation, and secondary metabolism in C. globosum. We propose that CgPKS11, probably through its product chaetoglocin A, and as a signal molecule, concerts secondary metabolism, growth, and sexual development in C. globosum. Hence, this work provides insights into the regulation of ChA biosynthesis and high-yield engineering construction and also demonstrates that PKSs are not only responsible for polyketide production, but also affect the growth and development of fungi. To construct stable ChA high-yielding engineering strains for ChA application, we perceive a future plan that should be done to uncover the precise crosstalk between ChA and chaetoglocin A biosynthesis, and to scrutinize on a larger scope of ChA biosynthesis in other fungi.