Native Endophytic Pseudomonas putida as a Biocontrol Agent against Common Bean Rust Caused by Uromyces appendiculatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Uromyces appendiculatus and Spores Collection and Preservation
2.2. Isolation of Endophytic Strain ASU15
2.3. Phenotypic Characterization of Endophytic pseudomonas putida Strain ASU15
2.4. Genotypic Identification of Endophytic pseudomonas putida Strain ASU15
2.5. Determination of Suppressive Impact of Pseudomonas putida ASU15 on Uredospores Germination
2.6. Scanning Electron Microscopy (SEM) Analysis
2.7. Evaluation of Extracellular Enzymatic Activities
2.7.1. Chitinase Activity
2.7.2. Protease Activity
2.7.3. Lipase Activity
2.7.4. Protein Assay
2.8. Efficacy of Bacterium Isolate as Spraying Treatments for Controlling Common Bean Rust Disease under Greenhouse Conditions
2.8.1. Inoculum Preparation, Inoculation Methods, and Evaluation of Disease Severity
2.8.2. Determination of Suppressive Impact of P. putida ASU15 on Incidence of Common Bean Rust
2.9. Statistical Analysis
3. Results
3.1. Source of Uromyces appendiculatus
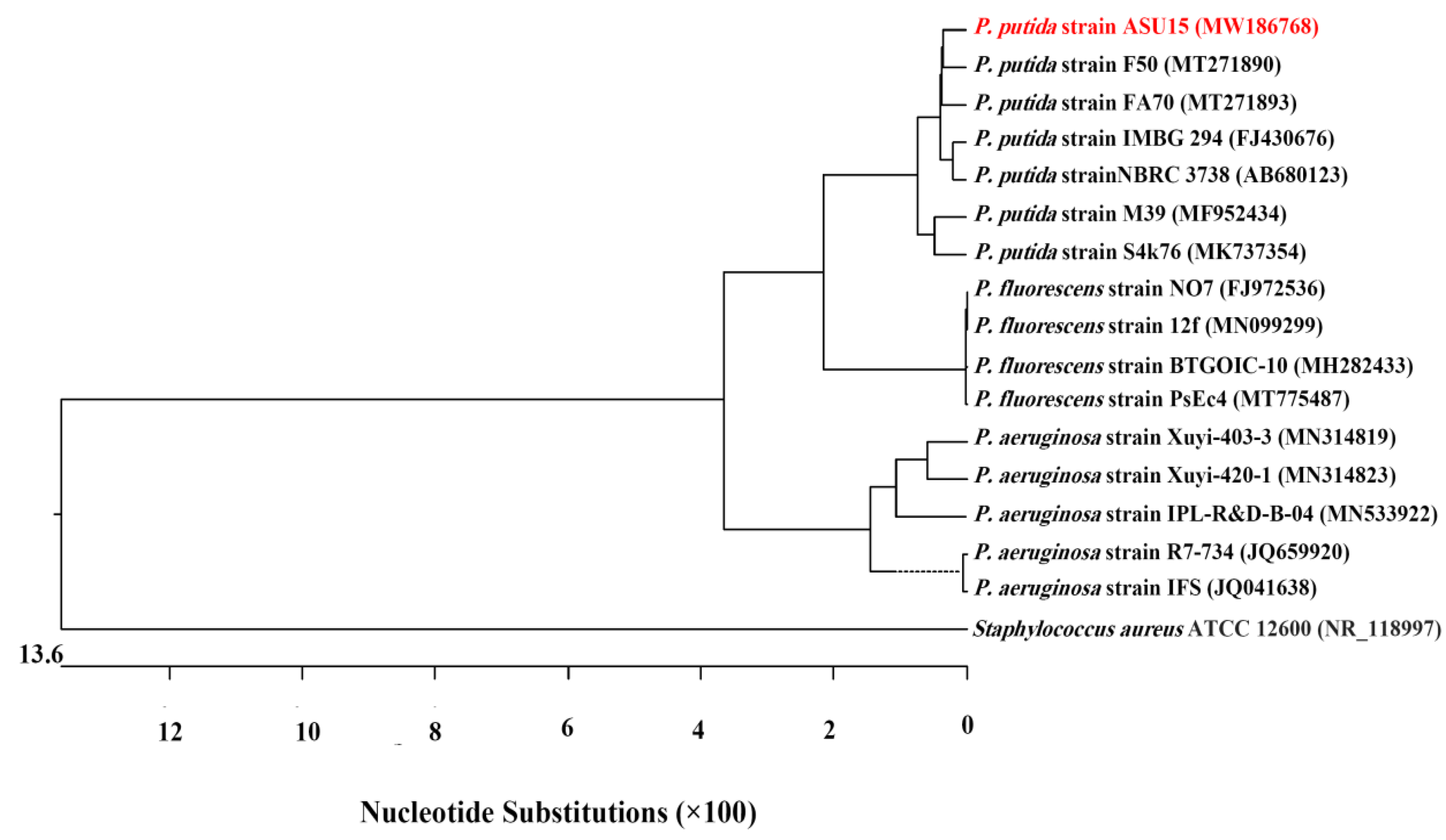
3.2. Identification of Endophytic Bacterium
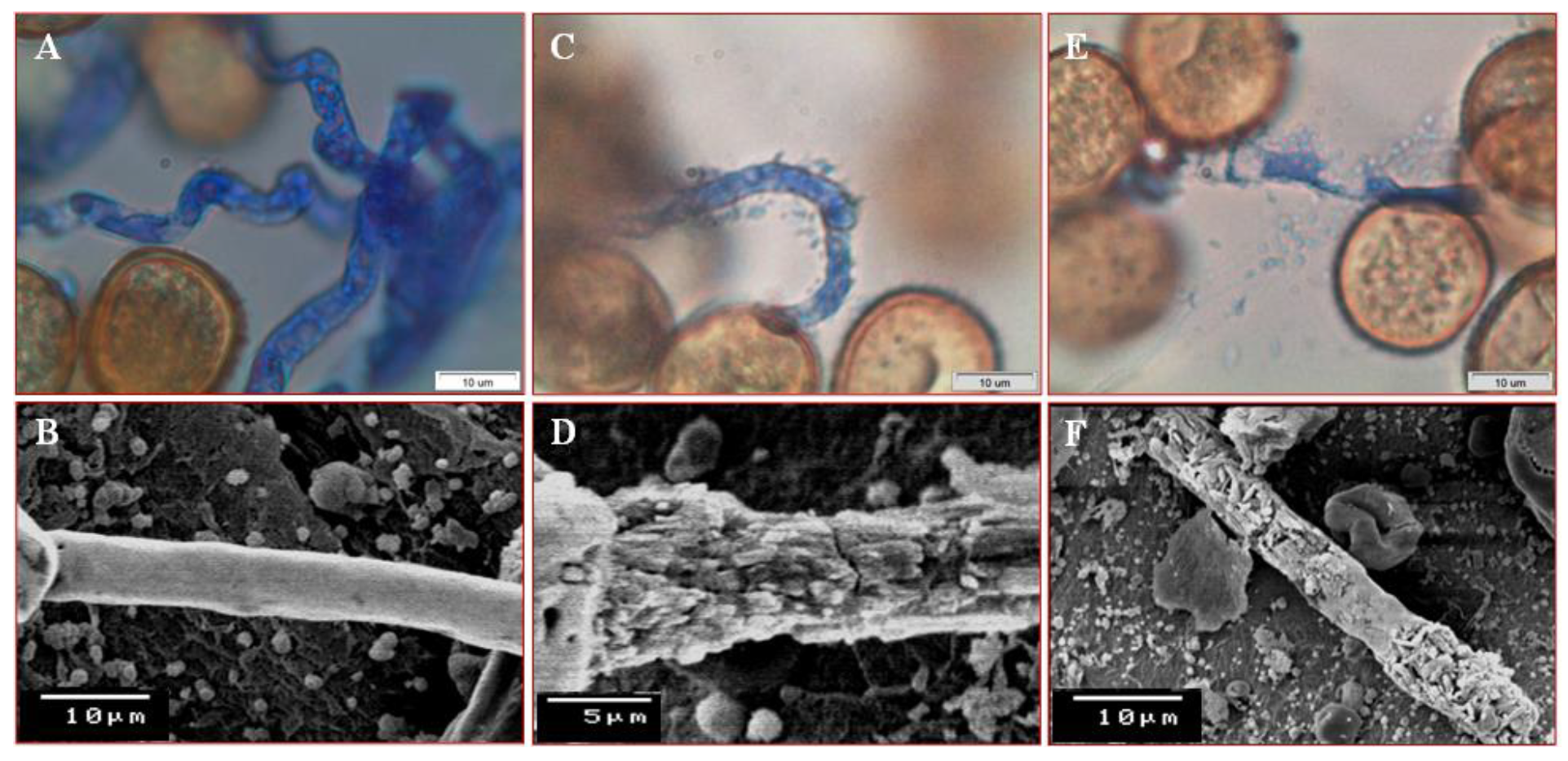
3.3. Inhibitory Impact of P. putida ASU15 on Germination Rate of Uredospores
3.4. Scanning Electron Microscopy (SEM) Analysis
3.5. Extracellular Enzymatic Activity
3.6. Suppressive Impact of P. putida ASU15 on Incidence of Common Bean Rust
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartz, H.F.; Corrales, M.A.P.; Centro Internacional de Agricultura Tropical (CIAT). Bean Production Problems in the Tropics; Centro Internacional de Agricultura Tropical (CIAT): Palmira, Colombia, 1989. [Google Scholar]
- El-Fawy, M.M.; Abo-Elyousr, K.A.M. Efficacy of certain chemical compounds on common bean rust disease. Arch. Phytopathol. Plant Prot. 2016, 49, 522–532. [Google Scholar] [CrossRef]
- Steadman, J.; Pastor-Corrales, M.; Beaver, J. An overview of the 3rd bean rust and 2nd bean common bacterial blight international workshops, 4–8 March 2002, Pietermaritzburg, South Africa. Annu. Rep. Bean Improv. Coop. 2002, 45, 120–124. [Google Scholar]
- Acevedo, M.; Steadman, J.R.; Rosas, J.C. Uromyces appendiculatus in Honduras: Pathogen diversity and host resistance screening. Plant Dis. 2012, 97, 652–661. [Google Scholar] [CrossRef]
- Jochua, C.; Amane, M.I.V.; Steadman, J.R.; Xue, X.; Eskridge, K.M. Virulence diversity of the common bean rust pathogen within and among individual bean fields and development of sampling strategies. Plant Dis. 2008, 92, 401–408. [Google Scholar] [CrossRef]
- Ismail, A.M.; Afifi, M.M. Efficacy of some biotic and abiotic factors in controlling common bean rust disease caused by Uromyces appendiculatus. Egypt. J. Phytopathol. 2019, 47, 313–329. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Sallam, N.M.A.; Mohamed, A.A.; Hassan, M.H.A. Effect of mycorrhiza and biofertilisers on reducing the incidence of Fusarium root and pod rot diseases of peanut. Arch. Phytopathol. Plant Prot. 2013, 46, 868–881. [Google Scholar] [CrossRef]
- Souza, T.L.P.; Faleiro, F.G.; Dessaune, S.N.; Paula-Junior, T.J.d.; Moreira, M.A.; Barros, E.G.d. Breeding for common bean (Phaseolus vulgaris L.) rust resistance in Brazil. Trop. Plant Pathol. 2013, 38, 361–374. [Google Scholar] [CrossRef]
- Yuen, G.Y.; Steadman, J.R.; Lindgren, D.T.; Schaff, D.; Jochum, C. Bean rust biological control using bacterial agents. Crop. Prot. 2001, 20, 395–402. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Vlami, M.; De Souza, J.T. Antibiotic production by bacterial biocontrol agents. Antonie Leeuwenhoek 2002, 81, 537. [Google Scholar] [CrossRef]
- Sallam, N.A.; Riad, S.N.; Mohamed, M.S.; El-eslam, A.S. Formulations of Bacillus spp. and Pseudomonas fluorescens for biocontrol of cantaloupe root rot caused by Fusarium Solani. J. Plant Prot. Res. 2013, 53, 295–300. [Google Scholar]
- Lodewyckx, C.; Vangronsveld, J.; Porteous, F.; Moore, E.R.; Taghavi, S.; Mezgeay, M.; Der Lelie, D.V. Endophytic bacteria and their potential applications. Crit. Rev. Plant Sci. 2002, 21, 583–606. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; Del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Lodewyckx, C.; Mergeay, M.; Vangronsveld, J.; Clijsters, H.; Van Der Lelie, D. Isolation, characterization, and identification of bacteria associated with the zinc hyperaccumulator Thlaspi caerulescens subsp. calaminaria. Int. J. Phytoremediation 2002, 4, 101–115. [Google Scholar] [CrossRef]
- Chanway, C. Inoculation of tree roots with plant growth promoting soil bacteria: An emerging technology for reforestation. For. Sci. 1997, 43, 99–112. [Google Scholar]
- Bent, E.; Chanway, C.P. The growth-promoting effects of a bacterial endophyte on lodgepole pine are partially inhibited by the presence of other rhizobacteria. Can. J. Microbiol. 1998, 44, 980–988. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B.; Castillo, U.; Harper, J. Natural products from endophytic microorganisms. J. Nat. Prod. 2004, 67, 257–268. [Google Scholar] [CrossRef]
- Zaidi, A.; Ahmad, E.; Khan, M.S.; Saif, S.; Rizvi, A. Role of plant growth promoting rhizobacteria in sustainable production of vegetables: Current perspective. Sci. Hortic. 2015, 193, 231–239. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.; Bagy, H.M.K.; Hashem, M.; Alamri, S.A.; Mostafa, Y.S. Biological control of the tomato wilt caused by Clavibacter michiganensis subsp. michiganensis using formulated plant growth-promoting bacteria. Egypt. J. Biol. Pest Control 2019, 29, 54. [Google Scholar] [CrossRef]
- Barahona, E.; Navazo, A.; Martínez-Granero, F.; Zea-Bonilla, T.; Pérez-Jiménez, R.M.; Martín, M.; Rivilla, R. Pseudomonas fluorescens F113 mutant with enhanced competitive colonization ability and improved biocontrol activity against fungal root pathogens. Appl. Environ. Microbiol. 2011, 77, 5412–5419. [Google Scholar] [CrossRef]
- Mohamed, B.F.; Sallam, N.M.; Alamri, S.A.; Abo-Elyousr, K.A.; Mostafa, Y.S.; Hashem, M. Approving the biocontrol method of potato wilt caused by Ralstonia solanacearum (Smith) using Enterobacter cloacae PS14 and Trichoderma asperellum T34. Egypt. J. Biol. Pest Control 2020, 30, 1–13. [Google Scholar] [CrossRef]
- Panpatte, D.G.; Jhala, Y.K.; Shelat, H.N.; Vyas, R.V. Pseudomonas fluorescens: A promising biocontrol agent and PGPR for sustainable agriculture. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: Berlin/Heidelberg, Germany, 2016; pp. 257–270. [Google Scholar]
- Ohno, M.; Kataoka, S.; Numata, S.; Yamamoto-Tamura, K.; Fujii, T.; Nakajima, M.; Akutsu, K.; Hasebe, A. Biological control of Rhizoctonia damping-off of cucumber by a transformed Pseudomonas putida strain expressing a chitinase from a marine bacterium. Jpn. Agric. Res. Q. JARQ 2011, 45, 91–98. [Google Scholar] [CrossRef][Green Version]
- Lee, S.-W.; Ahn, I.-P.; Lim, J.-W.; Lee, Y.-H. Pseudomonas putida strain 17 Isolated from replant soil promotes tomato growth and Inhibits conidial germination of soilborne plant pathogens. Plant Pathol. J. 2005, 21, 244–251. [Google Scholar] [CrossRef][Green Version]
- Oliver, C.; Hernández, I.; Caminal, M.; Lara, J.M.; Fernàndez, C. Pseudomonas putida strain B2017 produced as technical grade active ingredient controls fungal and bacterial crop diseases. Biocontrol Sci. Technol. 2019, 29, 1053–1068. [Google Scholar] [CrossRef]
- Andreote, F.D.; De Araújo, W.L.; De Azevedo, J.L.; Van Elsas, J.D.; Da Rocha, U.N.; Van Overbeek, L.S. Endophytic colonization of potato (Solanum tuberosum L.) by a novel competent bacterial endophyte, Pseudomonas putida strain P9, and its effect on associated bacterial communities. Appl. Environ. Microbiol. 2009, 75, 3396–3406. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhuo, T.; Hu, X.; Fan, X.; Zou, H. Identification of a Pseudomonas putida as biocontrol agent for tomato bacterial wilt disease. Biol. Control. 2017, 114, 45–50. [Google Scholar] [CrossRef]
- Lopes, D.B.; Berger, R.D. The effects of rust and anthracnose on the photosynthetic competence of diseased bean leaves. Phytopathology 2001, 91, 212–220. [Google Scholar] [CrossRef]
- Li, L.; Mohamad, O.A.A.; Ma, J.; Friel, A.D.; Su, Y.; Wang, Y.; Musa, Z.; Liu, Y.; Hedlund, B.P.; Li, W. Synergistic plant–microbe interactions between endophytic bacterial communities and the medicinal plant Glycyrrhiza uralensis F. Antonie Leeuwenhoek 2018, 111, 1735–1748. [Google Scholar] [CrossRef] [PubMed]
- Abdelshafy, M.O.A.; Ma, J.-B.; Liu, Y.-H.; Zhang, D.; Hua, S.; Bhute, S.; Hedlund, B.P.; Li, W.-J.; Li, L. Beneficial endophytic bacterial populations associated with medicinal plant Thymus vulgaris alleviate salt stress and confer resistance to Fusarium oxysporum. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Garrity, G.; Brenner, D.J.; Krieg, N.R.; Staley, J.R. Bergey’s Manual® of Systematic Bacteriology: Volume 2: The Proteobacteria, Part B: The Gammaproteobacteria; Springer: New York, NY, USA, 2007. [Google Scholar]
- Li, H.; Zhao, J.; Feng, H.; Huang, L.; Kang, Z. Biological control of wheat stripe rust by an endophytic Bacillus subtilis strain E1R-j in greenhouse and field trials. Crop Protect. 2013, 43, 201–206. [Google Scholar] [CrossRef]
- Chen, J.-P.; Lee, M.-S. Simultaneous production and partition of chitinase during growth of Serratia marcescens in an aqueous two-phase system. Biotechnol. Tech. 1994, 8, 783–788. [Google Scholar] [CrossRef]
- El-Tarabily, K.A. An endophytic chitinase-producing isolate of Actinoplanes missouriensis, with potential for biological control of root rot of lupin caused by Plectosporium tabacinum. Aust. J. Bot. 2003, 51, 257–266. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Palanivel, P.; Ashokkumar, L.; Balagurunathan, R. Production, purification and fibrinolytic characterization of alkaline protease from extremophilic soil fungi. Int. J. Pharma Bio Sci. 2013, 4, 101–110. [Google Scholar]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Pereira, M.G.; Vici, A.C.; Facchini, F.D.A.; Tristão, A.P.; Cursino-Santos, J.R.; Sanches, P.R.; Jorge, J.A.; Polizeli, M.d.L.T.d.M. Screening of filamentous fungi for lipase production: Hypocrea pseudokoningii a new producer with a high biotechnological potential. Biocatal. Biotransform. 2014, 32, 74–83. [Google Scholar] [CrossRef]
- Prazeres, J.N.d.; Cruz, J.A.B.; Pastore, G.M. Characterization of alkaline lipase from Fusarium oxysporum and the effect of different surfactants and detergents on the enzyme activity. Braz. J. Microbiol. 2006, 37, 505–509. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Sackston, W. Studies on sunflower rust: II. Longevity of urediospores of Puccinia helianthi. Can. J. Bot. 1960, 38, 883–889. [Google Scholar] [CrossRef]
- McInroy, J.A.; Kloepper, J.W. Survey of indigenous bacterial endophytes from cotton and sweet corn. Plant Soil 1995, 173, 337–342. [Google Scholar] [CrossRef]
- Gardner, J.M.; Feldman, A.W.; Zablotowicz, R.M. Identity and behavior of xylem-residing bacteria in rough lemon roots of Florida citrus trees. Appl. Environ. Microbiol. 1982, 43, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.J.; Bugbee, W.M.; Gabrielson, D.A. Enumeration, location, and characterization of endophytic bacteria within sugar beet roots. Can. J. Bot. 1985, 63, 1262–1265. [Google Scholar] [CrossRef]
- Pang, F.; Wang, T.; Zhao, C.; Tao, A.; Yu, Z.; Huang, S.; Yu, G. Novel bacterial endophytes isolated from winter wheat plants as biocontrol agent against stripe rust of wheat. BioControl 2016, 61, 207–219. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.; Yadav, A.; Giri, D.; Singh, P.; Pandey, K.D. Isolation and characterization of bacterial endophytes of Curcuma longa L. 3 Biotech 2016, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Tao, A.; Pang, F.; Huang, S.; Yu, G.; Li, B.; Wang, T. Characterisation of endophytic Bacillus thuringiensis strains isolated from wheat plants as biocontrol agents against wheat flag smut. Biocontrol Sci. Technol. 2014, 24, 901–924. [Google Scholar] [CrossRef]
- Abeysinghe, S. Induced systemic resistance (ISR) in bean (Phaseolus vulgaris L.) mediated by rhizobacteria against bean rust caused by Uromyces appendiculatus under greenhouse and field conditions. Arch. Phytopathol. Plant Prot. 2009, 42, 1079–1087. [Google Scholar] [CrossRef]
- Haddad, F.; Saraiva, R.M.; Mizubuti, E.S.; Romeiro, R.S.; Maffia, L.A. Antifungal compounds as a mechanism to control Hemileia vastatrix by antagonistic bacteria. Trop. Plant Pathol. 2013, 38, 398–405. [Google Scholar] [CrossRef]
- Vieira, A.; Talhinhas, P.; Loureiro, A.; Duplessis, S.; Fernandez, D.; Do Céu Silva, M.; Paulo, O.S.; Azinheira, H.G. Validation of RT-qPCR reference genes for in planta expression studies in Hemileia vastatrix, the causal agent of coffee leaf rust. Fungal Biol. 2011, 115, 891–901. [Google Scholar] [CrossRef]
- Weller, D.M.; Mavrodi, D.V.; Van Pelt, J.A.; Pieterse, C.M.; Van Loon, L.C.; Bakker, P.A. Induced systemic resistance in Arabidopsis thaliana against Pseudomonas syringae pv. tomato by 2, 4-diacetylphloroglucinol-producing Pseudomonas fluorescens. Phytopathology 2012, 102, 403–412. [Google Scholar] [CrossRef]
- Rajendran, L.; Samiyappan, R. Endophytic Bacillus species confer increased resistance in cotton against damping off disease caused by Rhizoctonia solani. Plant Pathol. J. 2008, 7, 1–12. [Google Scholar] [CrossRef]
- Vinayarani, G.; Prakash, H. Growth promoting rhizospheric and endophytic bacteria from Curcuma longa L. as biocontrol agents against rhizome rot and leaf blight diseases. Plant Pathol. J. 2018, 34, 218. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, B.; Bazaid, S.; Gherbawy, Y.; Elhariry, H. Characterization of endophytic bacteria associated with rose plant (Rosa damascena trigintipeta) during flowering stage and their plant growth promoting traits. J. Plant Interact. 2012, 7, 248–253. [Google Scholar] [CrossRef]
- Castro, R.A.; Quecine, M.C.; Lacava, P.T.; Batista, B.D.; Luvizotto, D.M.; Marcon, J.; Ferreira, A.; Melo, I.S.; Azevedo, J.L. Isolation and enzyme bioprospection of endophytic bacteria associated with plants of Brazilian mangrove ecosystem. SpringerPlus 2014, 3, 382. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; Monchy, S.; Cardinale, M.; Taghavi, S.; Crossman, L.; Avison, M.B.; Berg, G.; Van Der Lelie, D.; Dow, J.M. The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat. Rev. Microbiol. 2009, 7, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, J.; Quadt-Hallmann, A.; Mahaffee, W.; Kloepper, J. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 1997, 43, 895–914. [Google Scholar] [CrossRef]
- Prasom, P.; Sikhao, P.; Koohakan, P. In vitro study of endophytic bacteria isolated from tomato plant against Fusarium oxysporum. Int. J. Agric. Technol. 2017, 13, 1217–1230. [Google Scholar]
- Fouda, A.; Eid, A.M.; Elsaied, A.; El-Belely, E.F.; Barghoth, M.G.; Azab, E.; Gobouri, A.A.; Hassan, S.E.-D. Plant growth-promoting endophytic bacterial community inhabiting the leaves of Pulicaria incisa (Lam.) DC inherent to arid regions. Plants 2021, 10, 76. [Google Scholar] [CrossRef] [PubMed]

| Characteristics and Tests | Results | Characteristics and Tests | Results |
|---|---|---|---|
| Morphological characteristics | |||
| Colony morphology | Circular, convex and translucent | Colony color | Yellowish |
| Colony surface | Smooth | Colony margin | Entire |
| Colony diameter | 2–3 mm | Fluorescent | + |
| Cell shape | Short rods | Motility | + |
| Gram Stain | Gram-ve | Spore formation | - |
| The growth and utilization of different carbon sources | |||
| Glucose | + | d-Galacturonic acid | + |
| Mannose | + | d-Gluconic acid | + |
| Fructose | + | d-Glucuronic acid | + |
| Galactose | + | Methyl Pyruvate | + |
| Maltose | - | l-Lactic acid | + |
| Raffinose | - | Citric acid | + |
| Xylose | + | d-Malic acid | - |
| Cellobiose | - | l-Malic acid | + |
| Sucrose | - | Acetoacetic acid | - |
| Lactose | - | Propionic acid | + |
| Melibiose | + | Acetic acid | + |
| l-arabinose | + | Formic acid | + |
| Rhamnose | - | Tween 40 | + |
| Inulin | - | d-Serine | - |
| Dextrose | + | l-Serine | + |
| Inosine | + | l-Alanine | + |
| Gelatin | + | l-Arginine | + |
| Pectin | - | d-Aspartic acid | - |
| Sorbitol | - | l-Aspartic acid | + |
| Mannitol | + | l-Glutamic acid | + |
| d-Arabitol | - | l-Pyroglutamic acid | + |
| Glycerol | - | Inositol | - |
| Growth at pH 5 | + | Sodium butyrate | + |
| Growth at pH 6 | + | Sodium bromate | - |
| Growth at 1% NaCl | + | 1% Sodium lactate | + |
| Growth at 4% NaCl | + | ||
| Growth at 8% NaCl | - | ||
| Physiological and enzymatic activities | |||
| Glucose fermentation | - | Casein hydrolysis | + |
| Cellulase | - | Starch hydrolysis | - |
| Catalase | + | HCN production | - |
| Oxidase | + | H2S production | - |
| Urease | + | Nitrate reduction | + |
| Indole acetic acid | + | ||
| Treatments (Concentration) | Germination Rate | % Inhibition |
|---|---|---|
| P. putida (10 × 103 CFU mL−1) | 16.00 ± 1.30 b | 83.89 |
| P. putida (10 × 105 CFU mL−1) | 11.33 ± 1.60 c | 88.59 |
| P. putida (10 × 106 CFU mL−1) | 4.67 ± 1.20 d | 95.30 |
| Fungicide Ortiva (0.01%) | 0.00 ± 0.00 e | 100.00 |
| Control | 99.33 ± 0.88 a |
| Extracellular Enzymes | Chitinase | Lipase | Protease |
|---|---|---|---|
| Enzymatic activity (U/mL) | 55.26 ± 0.03 | 26.12 ± 0.02 | 3.87 ± 0.03 |
| Protein (mg/mL) | 6.17 ± 0.04 | 2.84 ± 0.02 | 0.46 ± 0.04 |
| Specific activity (U/mg protein) | 8.96 ± 0.20 | 9.20 ± 0.20 | 8.41 ± 0.12 |
| Treatments | Two Days before the Pathogen | At the Same Time of Pathogen Inoculation | Mean | ||
|---|---|---|---|---|---|
| Inoculation | |||||
| Severity | Reduction % | Severity | Reduction % | ||
| P. putida ASU15 | 26.67 b | 69.9 | 21.67 b | 54.8 | 31.67 b |
| Ortiva | 5.00 c | 93 | 5.00 c | 92.9 | 5.00 c |
| Control | 70.83 a | - | 71.67 a | - | 70.00 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abo-Elyousr, K.A.M.; Abdel-Rahim, I.R.; Almasoudi, N.M.; Alghamdi, S.A. Native Endophytic Pseudomonas putida as a Biocontrol Agent against Common Bean Rust Caused by Uromyces appendiculatus. J. Fungi 2021, 7, 745. https://doi.org/10.3390/jof7090745
Abo-Elyousr KAM, Abdel-Rahim IR, Almasoudi NM, Alghamdi SA. Native Endophytic Pseudomonas putida as a Biocontrol Agent against Common Bean Rust Caused by Uromyces appendiculatus. Journal of Fungi. 2021; 7(9):745. https://doi.org/10.3390/jof7090745
Chicago/Turabian StyleAbo-Elyousr, Kamal A. M., Ismail R. Abdel-Rahim, Najeeb M. Almasoudi, and Sameera A. Alghamdi. 2021. "Native Endophytic Pseudomonas putida as a Biocontrol Agent against Common Bean Rust Caused by Uromyces appendiculatus" Journal of Fungi 7, no. 9: 745. https://doi.org/10.3390/jof7090745
APA StyleAbo-Elyousr, K. A. M., Abdel-Rahim, I. R., Almasoudi, N. M., & Alghamdi, S. A. (2021). Native Endophytic Pseudomonas putida as a Biocontrol Agent against Common Bean Rust Caused by Uromyces appendiculatus. Journal of Fungi, 7(9), 745. https://doi.org/10.3390/jof7090745








