Resistance to the SDHI Fungicides Boscalid and Fluopyram in Podosphaera xanthii Populations from Commercial Cucurbit Fields in Spain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolates
2.2. Fungicide Sensitivity Studies for the SDHI Fungicides Boscalid and Fluopyram
2.3. In Vivo Fungicide Sensitivity Tests to Boscalid and Fluopyram in Greenhouse Experiments
2.4. Determination of Mutations in the SdhB, SdhC and SdhD Genes
2.5. Fitness Assays
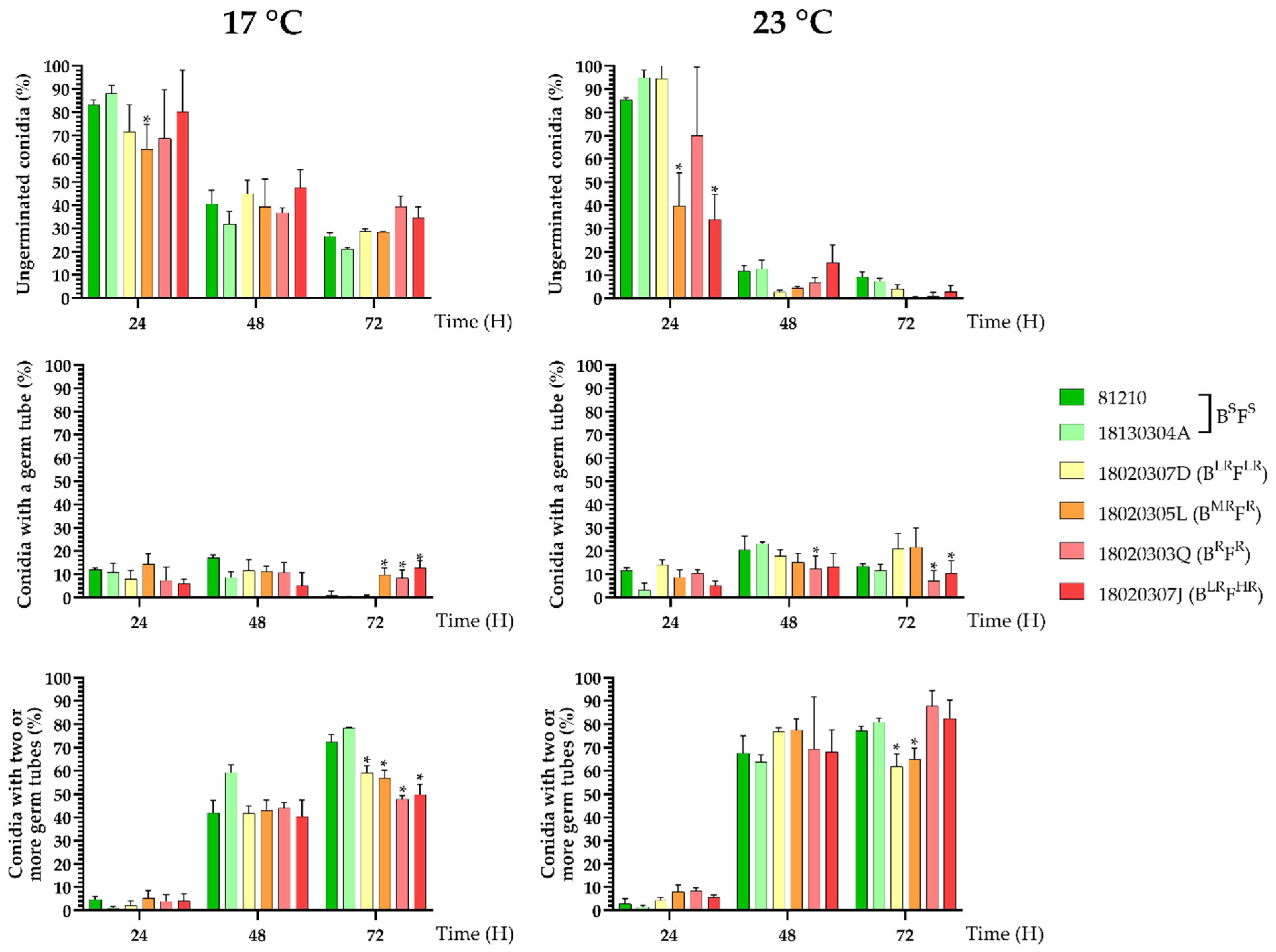
2.5.1. Conidial Germination
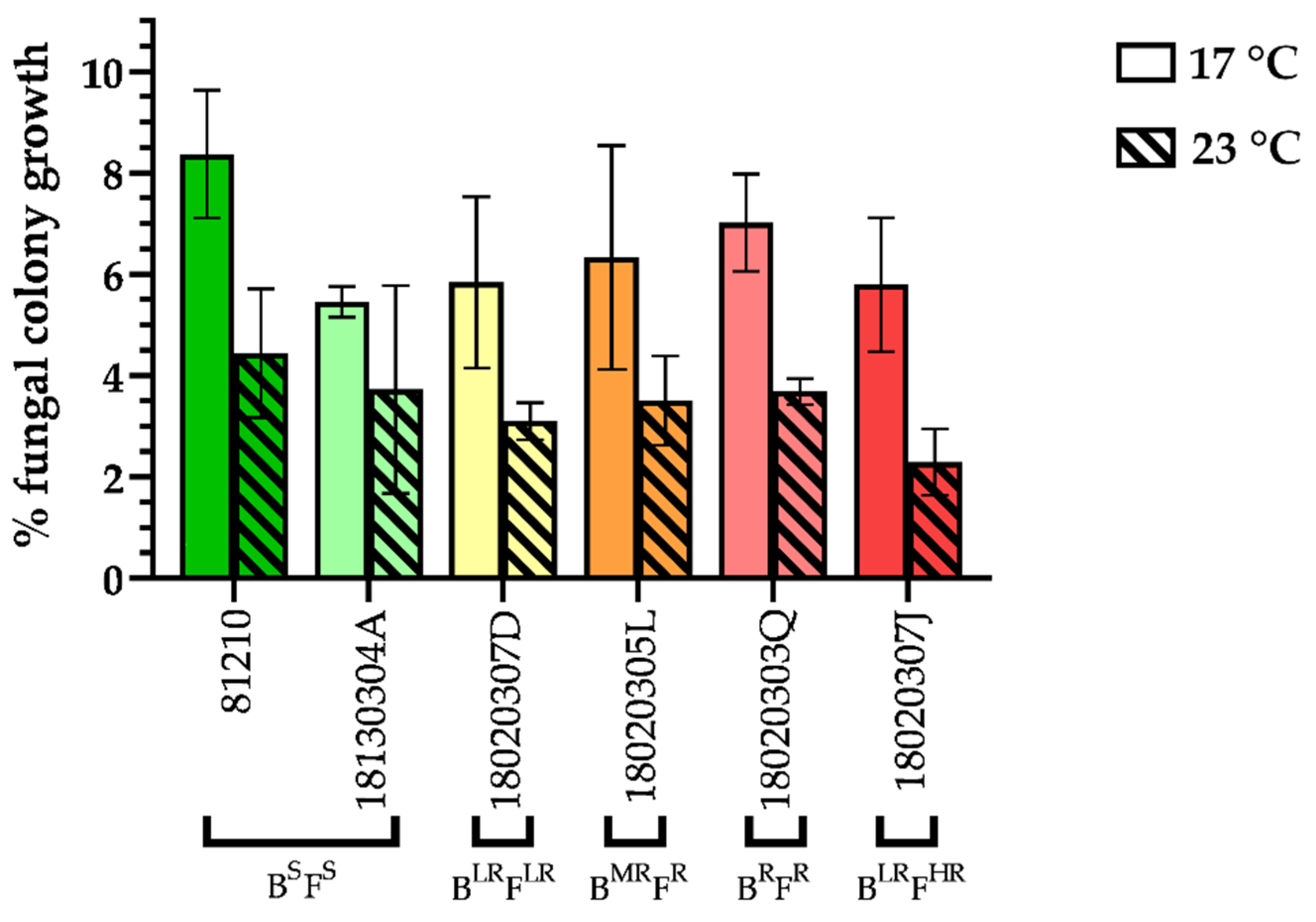
2.5.2. Mycelial Growth
2.6. LAMP Technique
2.6.1. LAMP Primer Design
2.6.2. Mixture and Optimization of LAMP Reaction
2.6.3. Specificity of LAMP
2.6.4. Repeatability of LAMP
2.6.5. Optimization of LAMP Assay Using Spores as Template
2.6.6. Testing LAMP Assay in Field Samples
3. Results
3.1. Determining the Discriminatory Doses to Boscalid and Fluopyram
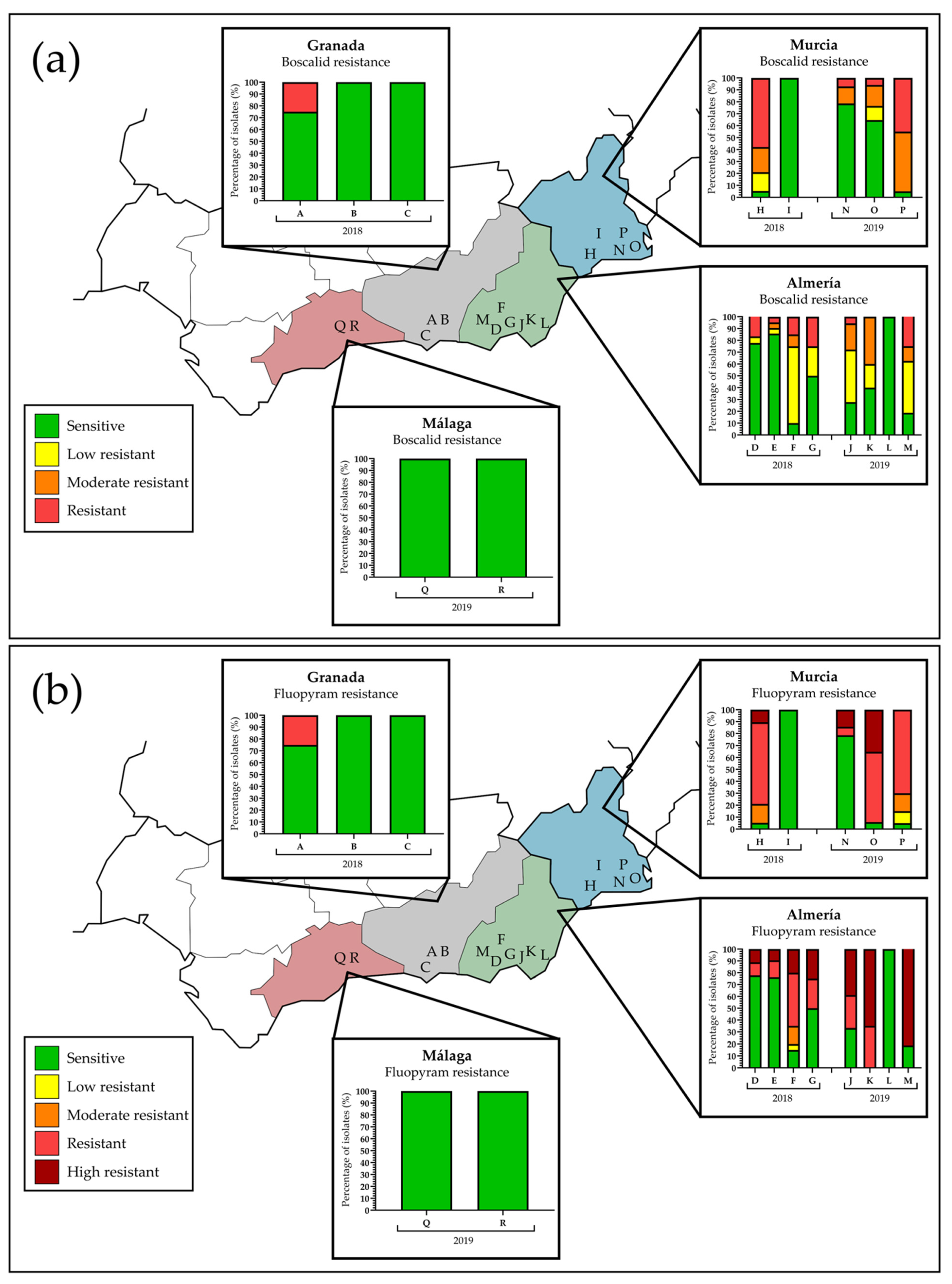
3.2. SDHI Resistance Field Monitoring Studies
3.3. Fungicide Sensitivity Plant Assay
3.4. Analysis of SdhB, SdhC and SdhD Genes in P. xanthii Isolates
3.5. Fitness Cost of SDHI Resistance
3.6. LAMP Technique
3.6.1. Primer Design and Optimization of Lamp Reactions
3.6.2. Specificity of LAMP Using Different Fungal Species
3.6.3. Repeatability of the LAMP Reaction
3.6.4. Optimization of LAMP Assay Using Spores
3.6.5. Testing LAMP Assay in Field Samples
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anuario de Estadística. Estadísticas Agrarias y Alimentación. Capítulo 07: Superficies y Producciones de Cultivos. Hortalizas. 2019. Available online: https://www.mapa.gob.es/es/estadistica/temas/publicaciones/anuario-de-estadistica/2019/default.aspx?parte=3&capitulo=07&grupo=6 (accessed on 18 June 2021).
- Sitterly, W.R. Powdery mildews of cucurbits. In The Powdery Mildews; APS Press: St. Paul, MN, USA, 1978; pp. 359–379. [Google Scholar]
- Pérez-García, A.; Romero, D.; Fernández-Ortuño, D.; López-Ruiz, F.; De Vicente, A.; Torés, J.A. The powdery mildew fungus Podosphaera fusca (synonym Podosphaera xanthii), a constant threat to cucurbits. Mol. Plant Pathol. 2009, 10, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Braun, U.; Cook, R.T.A.; Inman, A.J.; Shin, H.D. The taxonomy of the powdery mildew fungi. In The Powdery Mildews: A Comprehensive Treatis, 1st ed.; Richard, R., Bélanger, R.R., Bushnell, W.R., Dik, A.J., Carver, T.L.W., Eds.; American Phytopathological Society (APS Press): St. Paul, MN, USA, 2001; pp. 13–55. [Google Scholar]
- Torés, J.A.; Cánovas, I.; Velasco, M.V. A note on Sphaerotheca fuliginea (Schlecht. ex Fr.) Poll., causal agent of powdery mildew on Cucurbitaceae in the coastal areas of Malaga and Almeria. Investig. Agrar. Prod. Prot. Veg. 1990, 5, 475–479. [Google Scholar]
- Del Pino, D.; Olalla, L.; Pérez-García, A.; Rivera, M.E.; García, S.; Moreno, R.; de Vicente, A.; Torés, J.A. Occurrence of races and pathotypes of cucurbit powdery mildew in southeastern Spain. Phytoparasitica 2002, 30, 459–466. [Google Scholar] [CrossRef]
- Fernández-Ortuño, D.; Pérez-García, A.; López-Ruiz, F.; Romero, D.; de Vicente, A.; Torés, J.A. Occurrence and distribution of resistance to QoI fungicides in populations of Podosphaera fusca in south central Spain. Eur. J. Plant Pathol. 2006, 115, 215–222. [Google Scholar] [CrossRef] [Green Version]
- López-Ruiz, F.J.; Pérez-García, A.; Fernández-Ortuño, D.; Romero, D.; García, E.; de Vicente, A.; Brown, J.K.; Torés, J.A. Sensitivities to DMI fungicides in populations of Podosphaera fusca in south central Spain. Pest Manag. Sci. 2010, 66, 801–808. [Google Scholar] [CrossRef]
- Bellón-Gómez, D.; Vela-Corcía, D.; Pérez-García, A.; Torés, J.A. Sensitivity of Podosphaera xanthii populations to anti-powdery-mildew fungicides in Spain. Pest. Manag. Sci. 2015, 71, 1407–1413. [Google Scholar] [CrossRef]
- Vielba-Fernández, A.; de Vicente, A.; Pérez-García, A.; Fernández-Ortuño, D. Monitoring methyl benzimidazole carbamate-resistant isolates of the cucurbit powdery mildew pathogen, Podosphaera xanthii, using loop-mediated isothermal amplification. Plant Dis. 2019, 103, 1515–1524. [Google Scholar] [CrossRef]
- Vielba-Fernández, A.; Bellón-Gómez, D.; Torés, J.A.; de Vicente, A.; Pérez-García, A.; Fernández-Ortuño, D. Heteroplasmy for the Cytochrome b Gene in Podosphaera xanthii and its Role in Resistance to QoI Fungicides in Spain. Plant Dis. 2018, 102, 1599–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FRAC Code List. 2021. Available online: https://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2021--final.pdf?sfvrsn=f7ec499a_2 (accessed on 18 June 2021).
- Keon, J.P.; White, G.A.; Hargreaves, J.A. Isolation, characterization, and sequence of a gene conferring resistance to the systemic fungicide carboxin from the maize smut pathogen, Ustilago maydis. Curr. Genet. 1991, 19, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Hägerhäll, C. Succinate: Quinone oxidoreductases: Variations on a conserved theme. Biochim. Biophys. Acta Bioenerg. 1997, 1320, 107–141. [Google Scholar] [CrossRef] [Green Version]
- Sang, H.; Lee, H.B. Molecular mechanisms of Succinate Dehydrogenase Inhibitor resistance in phytopathogenic fungi. Res. Plant Dis. 2020, 26, 1–7. [Google Scholar]
- Avenot, H.F.; Sellam, A.; Karaoglanidis, G.; Michailides, T.J. Characterization of mutations in the iron-sulphur subunit of Succinate Dehydrogenase correlating with boscalid resistance in Alternaria alternata from California pistachio. Phytopathology 2008, 98, 736–742. [Google Scholar] [CrossRef] [Green Version]
- Avenot, H.F.; Sellam, A.; Michailides, T.J. Characterization of mutations in the membrane-anchored subunits AaSDHC and AaSDHD of succinate dehydrogenase from Alternaria alternata isolates conferring field resistance to the fungicide boscalid. Plant Pathol. 2009, 58, 1134–1143. [Google Scholar] [CrossRef] [Green Version]
- Avenot, H.F.; van den Biggelaar, H.; Morgan, D.P.; Moral, J.; Joosten, M.; Michailides, T.J. Sensitivities of baseline isolates and boscalid-resistant mutants of Alternaria alternata from pistachio to fluopyram, penthiopyrad, and fluxapyroxad. Plant Dis. 2014, 98, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avenot, H.F.; Luna, M.; Michailides, T.J. Phenotypic and molecular characterization of resistance to the SDHI fungicide fluopyram in populations of Alternaria alternata from pistachio orchards in California. Crop Prot. 2019, 124, 104838. [Google Scholar] [CrossRef]
- Yang, J.H.; Brannen, P.M.; Schnabel, G. Resistance in Alternaria alternata to SDHI fungicides causes rare disease outbreak in peach orchards. Plant Dis. 2015, 99, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Z.; Yang, J.-H.; Fan, F.; Luo, C.-X.; Schnabel, G. Fitness and competitive ability of Alternaria alternata field isolates with resistance to SDHI, QoI, and MBC fungicides. Plant Dis. 2015, 99, 1744–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroux, P.; Gredt, M.; Leroch, M.; Walker, A.-S. Exploring mechanisms of resistance to respiratory inhibitors in field strains of Botrytis cinerea, the causal agent of gray mold. Appl. Environ. Microbiol. 2010, 76, 6615–6630. [Google Scholar] [CrossRef] [Green Version]
- Veloukas, T.; Leroch, M.; Hahn, M.; Karaoglanidis, G.S. Detection and molecular characterization of boscalid-resistant Botrytis cinerea isolates from strawberry. Plant Dis. 2011, 95, 1302–1307. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.N.; Kim, Y.K.; Xiao, C.L. Molecular characterization of boscalid resistance in field isolates of Botrytis cinerea from apple. Phytopathology 2011, 101, 986–995. [Google Scholar] [CrossRef] [Green Version]
- Amiri, A.; Heath, S.M.; Peres, N.A. Resistance to fluopyram, fluxapyroxad, and penthiopyrad in Botrytis cinerea from strawberry. Plant Dis. 2014, 98, 532–539. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.-J.; Fernández-Ortuño, D.; Schnabel, G. Monitoring resistance to SDHI fungicides in Botrytis cinerea from strawberry fields. Plant Dis. 2016, 100, 959–965. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Ortuño, D.; Pérez-García, A.; Chamorro, M.; de la Peña, E.; de Vicente, A.; Torés, J.A. Resistance to the SDHI fungicides boscalid, fluopyram, fluxapyroxad, and penthiopyrad in Botrytis cinerea from commercial strawberry fields in Spain. Plant Dis. 2017, 101, 1306–1313. [Google Scholar] [CrossRef] [Green Version]
- Fungicide Resistance Action Committee. List of Species Resistant to SDHIs 2015. Available online: https://www.frac.info/docs/default-source/working-groups/sdhi-references/list-of-species-resistant-to-sdhis-april-2015.pdf?sfvrsn=2d144a9a_2 (accessed on 18 June 2021).
- Avenot, H.F.; Thomas, A.; Gitaitis, R.D.; Langston, D.B., Jr.; Stevenson, K.L. Molecular characterization of boscalid- and penthiopyrad-resistant isolates of Didymella bryoniae and assessment of their sensitivity to fluopyram. Pest Manag. Sci. 2012, 68, 645–651. [Google Scholar] [CrossRef]
- Miyamoto, T.; Ishii, H.; Tomita, Y. Occurrence of boscalid resistance in cucumber powdery mildew in Japan and molecular characterization of the iron-sulfur protein of succinate dehydrogenase of the causal fungus. J. Gen. Plant Pathol. 2010, 76, 261–267. [Google Scholar] [CrossRef]
- Rehfus, A.; Miessner, S.; Achenbach, J.; Strobel, D.; Bryson, R.; Stammler, G. Emergence of succinate dehydrogenase inhibitor resistance of Pyrenophora teres in Europe. Pest Manag. Sci. 2016, 72, 1977–1988. [Google Scholar] [CrossRef] [PubMed]
- Mallik, I.; Arabiat, S.; Pasche, J.S.; Bolton, M.D.; Patel, J.S.; Gudmestad, N.C. Molecular characterization and detection of mutations associated with resistance to succinate dehydrogenase inhibiting fungicides in Alternaria solani. Phytopathology 2014, 104, 40–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, T.; Hayashi, K.; Okada, R.; Wari, D.; Ogawara, T. Resistance to succinate dehydrogenase inhibitors in field isolates of Podosphaera xanthii on cucumber: Monitoring, cross-resistance patterns and molecular characterization. Pestic. Biochem. Phys. 2020, 169, 104646. [Google Scholar] [CrossRef] [PubMed]
- Hollomon, D.W.; Ishii, H. Monitoring resistance using molecular methods. In Fungicide Resistance in Plant Pathogens: Principles and a Guide to Practical Management, 1st ed.; Hollomon, D.W., Ishii, H., Eds.; Springer: Tokyo, Japan, 2015; pp. 295–309. [Google Scholar]
- Lichtemberg, P.S.F.; Luo, Y.; Doussoulin, H.; Michailides, T.J. Using Allele-specific PCR for detecting multiple amino acid substitutions associated with SDHI resistance in Alternaria alternata causing Alternaria late blight in pistachio. J. Appl. Microbiol. 2018, 67, 506–512. [Google Scholar] [CrossRef] [PubMed]
- De Miccolis Angelini, R.M.; Masiello, M.; Rotolo, C.; Pollastro, S.; Faretra, F. Molecular characterisation and detection of resistance to succinate dehydrogenase inhibitor fungicides in Botryotinia fuckeliana (Botrytis cinerea). Pest Manag. Sci. 2014, 70, 1884–1893. [Google Scholar] [CrossRef]
- Lee, J.; Elliott, M.; Kim, M.; Yamada, T.; Jung, G. A rapid molecular detection system for SdhB and SdhC point mutations conferring differential SDHI resistance in populations of Clarireedia. Plant Dis. 2020, 105, 660–666. [Google Scholar] [CrossRef]
- Samaras, A.; Madesis, P.; Karaoglanidis, G.S. Detection of sdhB gene mutations in SDHI-resistant isolates of Botrytis cinerea using High Resolution Melting (HRM) analysis. Front. Microbiol. 2016, 7, 1815. [Google Scholar] [CrossRef] [PubMed]
- Chatzidimopoulos, M.; Ganopoulos, I.; Madesis, P.; Vellios, E.; Tsaftaris, A.; Pappas, A.C. High-resolution melting analysis for rapid detection and characterization of Botrytis cinerea phenotypes resistant to fenhexamid and boscalid. Plant Pathol. 2014, 63, 1336–1343. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Zhang, X.; Ge, C.; Wang, Y.; Cao, J.; Jia, X.; Wang, J.; Zhou, M. Development and application of loop-mediated isothermal amplification for detection of the F167Y mutation of carbendazim-resistant isolates in Fusarium graminearum. Sci. Rep. 2014, 4, 7094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, Y.B.; Yang, Y.; Wang, J.X.; Liu, C.C.; He, L.L.; Zhou, M.G. Development and application of loop-mediated isothermal amplification for detecting the highly benzimidazole-resistant isolates in Sclerotinia sclerotiorum. Sci. Rep. 2015, 5, 17278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.H.; Yuan, S.K.; Hu, X.R.; Zhang, C.Q. Shift of sensitivity in Botrytis cinerea to benzimidazole fungicides in strawberry greenhouse ascribing to the rising-lowering of E198A subpopulation and its visual, on-site monitoring by loop-mediated isothermal amplification. Sci. Rep. 2019, 9, 11644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, F.; Hahn, M.; Li, G.-Q.; Lin, Y.; Luo, C.-X. Rapid detection of benzimidazole resistance in Botrytis cinerea by loop-mediated isothermal amplification. Phytopathol. Res. 2019, 1, 10. [Google Scholar] [CrossRef]
- Zhou, D.; Guo, J.; Xu, L.; Gao, S.; Lin, Q.; Wu, Q.; Wu, L.; Que, Y. Establishment and application of a loop-mediated isothermal amplification (LAMP) system for detection of cry1Ac transgenic sugarcane. Sci. Rep. 2014, 4, 4912. [Google Scholar] [CrossRef]
- Goto, M.; Honda, E.; Ogura, A.; Nomoto, A.; Hanaki, K. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques 2009, 46, 167–172. [Google Scholar] [CrossRef]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Tanner, N.A.; Zhang, Y.; Evans, T.C. Visual detection of isothermal nucleic acid amplification using pH-sensitive dyes. Biotechniques 2015, 58, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, F.; Yin, W.X.; Li, G.Q.; Lin, Y.; Luo, C.X. Development of a LAMP method for detecting SDHI fungicide resistance in Botrytis cinerea. Plant Dis. 2018, 102, 1612–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Zhang, L.; Li, H.; Gao, Y.; Mu, W.; Liu, F. Development of a LAMP method for detecting the N75S mutant in SDHI-resistant Corynespora cassiicola. Anal. Biochem. 2020, 597, 113687. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, B.; Torés, J.A. Cultivo in vitro de Sphaerotheca fuliginea (Schlecht. ex Fr.), efecto de diferentes fuentes de carbono sobre su desarrollo. Bol. San. Veg. Plagas 1997, 23, 283–288. [Google Scholar]
- Pérez-García, A.; Mingorance, E.; Rivera, M.E.; del Pino, D.; Romero, D.; Torés, J.A.; de Vicente, A. Long-term preservation of Podosphaera fusca using silica gel. J. Phytopathol. 2006, 154, 190–192. [Google Scholar] [CrossRef] [Green Version]
- Polonio, Á.; Díaz-Martínez, L.; Fernández-Ortuño, D.; de Vicente, A.; Romero, D.; López-Ruiz, F.J.; Pérez-García, A. A hybrid genome assembly resource for Podosphaera xanthii, the main causal agent of powdery mildew disease in cucurbits. Mol. Plant Microbe Interact. 2021, 34, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Bellón-Gómez, D. Nuevas Perspectivas Moleculares y Agronómicas de la Resistencia a Fungicidas en Podospharea fusca. Ph.D. Thesis, University of Malaga, Malaga, Spain, 2014. [Google Scholar]
- Vela-Corcía, D.; Bellón-Gómez, D.; López-Ruiz, F.; Torés, J.A.; Perez-Garcia, A. The Podosphaera fusca TUB2 gene, a molecular “Swiss Army knife” with multiple applications in powdery mildew research. Fungal Biol. 2014, 118, 228–241. [Google Scholar] [CrossRef]
- Martínez-Cruz, J.; Romero, D.; de la Torre, F.N.; Fernández-Ortuño, D.; Torés, J.A.; de Vicente, A.; Pérez-García, A. The functional characterization of Podosphaera xanthii candidate effector genes reveals novel target functions for fungal pathogenicity. Mol. Plant Microbe Interact. 2018, 31, 914–931. [Google Scholar] [CrossRef] [Green Version]
- Whelan, J.A.; Russell, N.B.; Whelan, M.A. A method for the absolute quantification of cDNA using real-time PCR. J. Immunol. Methods 2003, 278, 261–269. [Google Scholar] [CrossRef]
- McGrath, M.T. Fungicide sensitivity in Podosphaera xanthii and efficacy for cucurbit powdery mildew in NY, USA, in 2003–2006. J. Plant Pathol. 2008, 90, 90. [Google Scholar]
- Weber, R.W.; Entrop, A.-P.; Goertz, A.; Mehl, A. Status of sensitivity of Northern German Botrytis populations to the new SDHI fungicide fluopyram prior to its release as a commercial fungicide. J. Plant Dis. Prot. 2015, 122, 81–90. [Google Scholar] [CrossRef]
- Avenot, H.F.; Michailides, T.J. Occurrence and Extent of Boscalid Resistance in Populations of Alternaria alternata from California Pistachio Orchards. Plant Dis. 2020, 104, 306–314. [Google Scholar] [CrossRef]
- Avenot, H.F.; Michailides, T.J. Resistance to boscalid fungicide in Alternaria alternata isolates from pistachio in California. Plant Dis. 2007, 91, 1345–1350. [Google Scholar] [CrossRef] [Green Version]
- Gudmestad, N.C.; Arabiat, S.; Miller, J.S.; Pasche, J.S. Prevalence and impact of SDHI fungicide resistance in Alternaria solani. Plant Dis. 2013, 97, 952–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, A.; Langston, D.B.; Stevenson, K.L. Baseline sensitivity and cross-resistance to succinate-dehydrogenase-inhibiting and demethylation-inhibiting fungicides in Didymella bryoniae. Plant Dis. 2012, 96, 979–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, T.; Ishii, H.; Seko, T.; Kobori, S.; Tomita, Y. Occurrence of Corynespora cassiicola isolates resistant to boscalid on cucumber in Ibaraki Prefecture, Japan. Plant Pathol. 2009, 58, 1144–1151. [Google Scholar] [CrossRef]
- Mueller, D.S.; Wise, K.A.; Dufault, N.S.; Bradley, C.A.; Chilvers, M.I. Introduction. In Fungicides for Field Crops, 1st ed.; Mueller, D.S., Wise, K.A., Dufault, N.S., Bradley, C.A., Chilvers, M.I., Eds.; American Phytopathological Society (APS Press): St. Paul, MN, USA, 2013; pp. 1–13. [Google Scholar]
- Green, E.; Duriatti, A. Sensitivity of Uncinula necator isolates to quinoxyfen: Baseline studies, validation of baseline method, and targeted sensitivity monitoring after several years of commercial use. In Proceedings of the BCPC International Congress Crop Science or Technology 2003, Glasgow, UK, 10–12 November 2003; pp. 163–168. [Google Scholar]
- Fernández-Ortuño, D.; Chen, F.; Schnabel, G. Resistance to cyprodinil and lack of fludioxonil resistance in Botrytis cinerea isolates from strawberry in North and South Carolina. Plant Dis. 2013, 97, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Forster, H.; Su, H.; Vilchez, M.; Gubler, W.; Adaskaveg, J.E. Non-persistent captan and fenhexamid-resistance in Botrytis cinerea populations in California strawberries. Phytopathology 2007, 97, S36. [Google Scholar]
- Weber, R.W.S. Resistance of Botrytis cinerea to multiple fungicides in northern German small-fruit production. Plant Dis. 2011, 95, 1263–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherrad, S.; Hernandez, C.; Vacher, S.; Steva, H. First detection of boscalid-resistant strains of Erysiphe necator in French vineyards: Biological and molecular characterization. In Modern Fungicides and Antifungal Compounds; Deising, H.B., Fraaije, B., Mehl, A., Oerke, E.C., Sierotzki, H., Stammler, G., Eds.; Deutsche Phytomedizinische Gesellschaft: Braunschweig, Germany, 2017; Volume 8, pp. 211–216. [Google Scholar]
- Cherrad, S.; Charnay, A.; Hernandez, C.; Steva, H.; Belbahri, L.; Vacher, S. Emergence of boscalid-resistant strains of Erysiphe necator in French vineyards. Microbiol. Res. 2018, 216, 79–84. [Google Scholar] [CrossRef]
- Mair, W.; Lopez-Ruiz, F.; Stammler, G.; Clark, W.; Burnett, F.; Hollomon, D.; Ishii, H.; Thind, T.S.; Brown, J.K.M.; Fraaije, B.; et al. Proposal for a unified nomenclature for target-site mutations associated with resistance to fungicides. Pest Manag. Sci. 2016, 72, 1449–1459. [Google Scholar] [CrossRef]
- Yamashita, M.; Fraaije, B. Non-target site SDHI resistance is present as standing genetic variation in field populations of Zymoseptoria tritici. Pest Manag. Sci. 2018, 74, 672–681. [Google Scholar] [CrossRef] [Green Version]
- Scalliet, G.; Bowler, J.; Luksch, T.; Kirchhofer-Allan, L.; Steinhauer, D.; Ward, K.; Niklaus, M.; Verras, A.; Csukai, M.; Daina, A.; et al. Mutagenesis and functional studies with Succinate DehydrogenaseInhibitors in the wheat pathogen Mycosphaerella graminicola. PLoS ONE 2012, 7, e35429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stammler, G.A.; Wolf, A.; Klaach, K. Mechanisms of resistance. Respiration inhibitors: Complex II. In Fungicide Resistance in Plant Pathogens; Ishii, H., Hollomon, D.W., Eds.; Springer: Tokyo, Japan, 2015; pp. 104–117. [Google Scholar]
- Popko, J.T., Jr.; Sang, H.; Lee, J.; Yamada, T.; Hoshino, Y.; Jung, G. Resistance of Sclerotinia homoeocarpa field isolates to succinate dehydrogenase inhibitor fungicides. Plant Ducky Dis. 2018, 102, 2625–2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roohparvar, R.; Waard, M.A.D.; Kema, G.H.J.; Zwiers, L.H. MgMfs1, a major facilitator superfamily transporter from the fungal wheat pathogen Mycosphaerella graminicola, is a strong protectant against natural toxic compounds and fungicides. Fungal Genet. Biol. 2007, 44, 378–388. [Google Scholar] [CrossRef]
- Omrane, S.; Audéon, C.; Ignace, A.; Duplaix, C.; Fillinger, S. Plasticity of the MFS1 promoter leads to multidrug resistance in the wheat pathogen Zymoseptoria tritici. mSphere 2017, 2, e00393-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sang, H.; Hulvey, J.; Popko, J.T., Jr.; Lopes, J.; Swaminathan, A.; Chang, T.; Jung, G. A pleiotropic drug resistance transporter is involved in reduced sensitivity to multiple fungicide classes in Sclerotinia homoeocarpa (F.T. Bennett). Mol. Plant Pathol. 2015, 16, 251–261. [Google Scholar] [CrossRef]
- Sang, H.; Hulvey, J.P.; Green, R.; Xu, H.; Im, J.; Chang, T.; Jung, G. A xenobiotic detoxification pathway through transcriptional regulation in filamentous fungi. mBio 2018, 9, e00457-18. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Yan, L.; Ma, Z. Cloning and expression analysis of a putative ABC transporter gene BgABC1 from the biotrophic pathogenic fungus Blumeria graminis f. sp. tritici. J. Phytopathol. 2008, 156, 120–124. [Google Scholar] [CrossRef]
- Avenot, H.F.; Michailides, T.J. Progress in understanding molecular mechanisms and evolution of resistance to succinate dehydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi. Crop Prot. 2010, 29, 643–651. [Google Scholar] [CrossRef]
- Billard, A.; Fillinger, S.; Leroux, P.; Lachaise, H.; Beffa, R.; Debieu, D. Strong resistance to the fungicide fenhexamid entails a fitness cost in Botrytis cinerea, as shown by comparisons of isogenic isolates. Pest Manag Sci. 2012, 68, 684–691. [Google Scholar] [CrossRef]
- Bauske, M.J.; Gudmestad, N.C. Parasitic fitness of fungicide-resistant and -sensitive isolates of Alternaria solani. Plant Dis. 2018, 102, 666–673. [Google Scholar] [CrossRef] [Green Version]
- Vielba-Fernández, A.; Polonio, Á.; Ruiz-Jiménez, L.; de Vicente, A.; Pérez-García, A.; Fernández-Ortuño, D. Fungicide resistance in powdery mildew fungi. Microorganisms 2020, 8, 1431. [Google Scholar] [CrossRef]
- Miyamoto, T.; Hayashi, K.; Ogawara, T. First report of the occurrence of multiple resistance to Flutianil and Pyriofenone in field isolates of Podosphaera xanthii, the causal fungus of cucumber powdery mildew. Eur. J. Plant Pathol. 2020, 156, 953–963. [Google Scholar] [CrossRef]
- McGrath, M.T. First report of resistance to quinoxyfen in Podosphaera xanthii, causal agent of cucurbit powdery mildew, in the United States. Plant Health Prog. 2017, 18, 94. [Google Scholar] [CrossRef] [Green Version]
- Sedláková, B.; Lebeda, A.; Jerabkova, H.; Paulik, R.; Vajdova, M. Resistance to fenarimol, dinocap, benomyl, thiophanate-methyl and azoxystrobin in cucurbit powdery mildew populations in the Czech Republic. Acta Fytotech. Zootech. 2012, 15, 46–49. [Google Scholar]
- Pirondi, A.; Nanni, I.M.; Brunelli, A.; Collina, M. First report of resistance to cyflufenamid in Podosphaera xanthii, causal agent of powdery mildew, from melon and zucchini fields in Italy. Plant Dis. 2014, 98, 1581. [Google Scholar] [CrossRef]
- Farm to Fork Strategy. For a Fair, Healthy and Environmentally Friendly Food System. Available online: https://ec.europa.eu/food/system/files/2020-05/f2f_action-plan_2020_strategy-info_en.pdf (accessed on 16 June 2021).
| Isolate | Year | Location | Host | MIC (mg/L) | EC50 (mg/L) | ||
|---|---|---|---|---|---|---|---|
| Boscalid | Fluopyram | Boscalid | Fluopyram | ||||
| 22014 | 2002 | Almeria | Zucchini | 1 | 0.1 | 0.36 | 0.13 |
| 31430 | 2003 | Murcia | Melon | 10 | 1 | 11.17 | 0.70 |
| 31869 | 2004 | Murcia | Melon | <10 | <10 | 0.62 | 1.01 |
| 44675 | 2003 | Valencia | Watermelon | <10 | 0.1 | 1.78 | 0.04 |
| 64132 | 2002 | Cordoba | Zucchini | <10 | 0.1 | 1.10 | 0.66 |
| 71175 | 2002 | Ciudad Real | Melon | <10 | <10 | 1.27 | 1.66 |
| 72174 | 2002 | Ciudad Real | Zucchini | 10 | 1 | 3.42 | 0.45 |
| 81210 | 2002 | Badajoz | Melon | 1 | 0.1 | 0.03 | 3 × 10−4 |
| 221104 | 2006 | Almeria | Zucchini | <10 | <10 | 1 | 1.91 |
| 311254 | 2008 | Murcia | Melon | 1 | 0.1 | 0.40 | 0.19 |
| 311271 | 2008 | Murcia | Melon | 10 | 0.1 | 2 | 0.77 |
| 711356 | 2008 | Ciudad Real | Melon | 10 | 1 | 1.51 | 0.24 |
| 711419 | 2009 | Ciudad Real | Melon | 1 | 1 | 0.26 | 0.60 |
| 711420 | 2009 | Ciudad Real | Melon | <10 | 0.1 | 3.50 | 2 × 10−3 |
| 811414 | 2009 | Badajoz | Melon | 1 | 0.1 | 0.54 | 2 × 10−3 |
| 811415 | 2009 | Badajoz | Melon | 10 | <10 | 5.46 | 4.42 |
| 1502404 A | 2016 | Almería | Watermelon | 10 | 1 | 5.86 | 0.47 |
| 1503405 C | 2016 | Murcia | Watermelon | 10 | 1 | 2.33 | 0.43 |
| 1509409 C | 2015 | Murcia | Watermelon | 10 | 1 | 1.11 | 1 × 10−4 |
| 1513406 C | 2015 | Granada | Watermelon | 10 | 1 | 2.94 | 0.52 |
| JF01′12 | 2012 | Ciudad Real | Melon | 1 | 1 | 0.97 | 0.77 |
| JF02′11 | 2011 | Ciudad Real | Melon | 1 | 1 | 0.42 | 0.87 |
| JF06′10 | 2010 | Ciudad Real | Melon | 1 | 1 | 0.24 | 0.55 |
| JF09′11 | 2011 | Ciudad Real | Melon | 1 | 1 | 0.52 | 0.35 |
| JF13′10 | 2010 | Ciudad Real | Melon | 10 | 1 | 0.20 | 0.17 |
| SF9 | 1988 | Malaga | Zucchini | 1 | <10 | 0.08 | 2.01 |
| Primer Name | Sequence (5′-3′) | Description | |
|---|---|---|---|
| SdhB_Forward | GCGGGGAGACCTCTGAGATA | Used to amplify a fragment of 1060-bp which contains the 886-bp sdhB ORF of P. xanthii | |
| SdhB_Reverse | GCCAGCAAGGGAGGATGATAA | ||
| SdhC_Forward | CCAATTCTCGCCGATTTCGC | Used to amplify a fragment of 1220-bp which contains the 737-bp sdhC ORF of P. xanthii | |
| SdhC_Reverse | CCCGCATACCCCTGGTATTC | ||
| SdhD_Forward | CGGGTAGGTCGCCTTAGTAC | Used to amplify a fragment of 1079-bp which contains the 695-bp sdhD ORF of P. xanthii | |
| SdhD_Reverse | CGACGTGTCGCATTTGCATT | ||
| LAMP assay | |||
| Set1 | F3 | ATCAACGTGACGACCTGA | Set of primers used in LAMP assays to amplify a fragment of 189-bp of the SdhC allele coding for the A86V amino acid change. |
| B3 | CCACCCGATATGACACAG | ||
| FIP | GGTTCTTACGTTGAGCTATAAGAGTCTCTTAGACCCGTGACAAC | ||
| BIP | GTCCCACATCTCCGCATTTACCCCGTAATGCGATTCAGgA 1 | ||
| Set2 | F3 | ATCAACGTGACGACCTGA | |
| B3 | CCACCCGATATGACACAG | ||
| FIP | GGTTCTTACGTTGAGCTATAAGAGTTTTTCTCTTAGACCCGTGACAAC | ||
| BIP | GTCCCACATCTCCGCATTTACTTTTCCCGTAATGCGATTCAGgA 1 | ||
| Set3 | F3 | CTAGGATTGAAGTCTCTGGT | Set of primers used in LAMP assays to amplify a fragment of the 199-bp of the SdhC allele coding for the G151R amino acid change. |
| B3 | TTTGTAGAGCCTACGTGATT | ||
| FIP | TTGAAAAGGCCTTGCCCAAATCCCTTTCACTTTTCATTCAATAAAaA 1 | ||
| BIP | AGGCAGTTATTAAAACAGGCTGGGTAACCAAAGCTAATGCACT | ||
| Set4 | F3 | CTAGGATTGAAGTCTCTGGT | |
| B3 | TTTGTAGAGCCTACGTGATT | ||
| FIP | TTGAAAAGGCCTTGCCCAAATCTTTTCCTTTCACTTTTCATTCAATAAAaA 1 | ||
| BIP | AGGCAGTTATTAAAACAGGCTGGTTTTGTAACCAAAGCTAATGCACT | ||
| 2018 (N = 143) | 2019 (N = 155) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Field | Location | Phenotype | Field | Location | Phenotype | ||||||||
| S | LR | MR | R | HR | S | LR | MR | R | HR | ||||
| D | Almeria | 14 (77.8%) | 1 (5.6%) | 0 | 3 (16.7%) | 0 | J | Almeria | 5 (27.8%) | 8 (44.4%) | 4 (22.2%) | 1 (5.6%) | 0 |
| E | 18 (85.7%) | 1 (4.8%) | 1 (4.8%) | 1 (4.7%) | 0 | K | 8 (40.0%) | 4 (20.0%) | 8 (40.0%) | 0 | 0 | ||
| F | 2 (10.0%) | 13 (65.0%) | 2 (10.0%) | 3 (15.0%) | 0 | L | 10 (100%) | 0 | 0 | 0 | 0 | ||
| G | 2 (50.0%) | 1 (25.0%) | 0 | 1 (25.0%) | 0 | M | 3 (18.8%) | 7 (43.8%) | 2 (12.5%) | 4 (25.0%) | 0 | ||
| H | Murcia | 1 (5.3%) | 3 (15.8%) | 4 (21.1%) | 11 (57.9%) | 0 | N | Murcia | 11 (78.6%) | 0 | 2 (14.3%) | 1 (7.1%) | 0 |
| I | 13 (100%) | 0 | 0 | 0 | 0 | O | 11 (64.7%) | 2 (11.8%) | 3 (17.6%) | 1 (5.9%) | 0 | ||
| P | 1 (5.0%) | 0 | 10 (50.0%) | 9 (45.0%) | 0 | ||||||||
| A | Granada | 6 (75.0%) | 0 | 0 | 2 (25.0%) | 0 | Q | Malaga | 20 (100%) | 0 | 0 | 0 | 0 |
| B | 20 (100%) | 0 | 0 | 0 | 0 | R | 20 (100%) | 0 | 0 | 0 | 0 | ||
| C | 20 (100%) | 0 | 0 | 0 | 0 | ||||||||
| Total | 96 (67.1%) | 19 (13.3%) | 7 (4.9%) | 21 (14.7%) | 0 | Total | 89 (57.4%) | 21 (13.5%) | 29 (18.7%) | 16 (10.3%) | 0 | ||
| 2018 (N = 143) | 2019 (N = 155) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Field | Location | Phenotype | Field | Location | Phenotype | ||||||||
| S | LR | MR | R | HR | S | LR | MR | R | HR | ||||
| D | Almeria | 14 (77.8%) | 0 | 0 | 2 (11.1%) | 2 (11.1%) | J | Almeria | 6 (33.3%) | 0 | 0 | 5 (27.8%) | 7 (38.9%) |
| E | 16 (76.2%) | 0 | 0 | 3 (14.3%) | 2 (9.5%) | K | 0 | 0 | 0 | 7 (35.0%) | 13 (65.0%) | ||
| F | 3 (15%) | 1 (5.0%) | 3 (15.0%) | 9 (45.0%) | 4 (20.0%) | L | 10 (100%) | 0 | 0 | 0 | 0 | ||
| G | 2 (50%) | 0 | 0 | 1 (25.0%) | 1 (25.0%) | M | 3 (18.8%) | 0 | 0 | 0 | 13 (81.3%) | ||
| H | Murcia | 1 (5.3%) | 0 | 3 (15.8%) | 13 (68.4%) | 2 (10.5%) | N | Murcia | 11 (78.6%) | 0 | 0 | 1 (7.1%) | 2 (14.3%) |
| I | 13 (100%) | 0 | 0 | 0 | 0 | O | 1 (5.9%) | 0 | 0 | 10 (58.8%) | 6 (35.3%) | ||
| P | 1 (5.0%) | 2 (10%) | 3 (15.0%) | 14 (70.0%) | 0 | ||||||||
| A | Granada | 6 (75%) | 0 | 0 | 2 (25.0%) | 0 | Q | Malaga | 20 (100%) | 0 | 0 | 0 | 0 |
| B | 20 (100%) | 0 | 0 | 0 | 0 | R | 20 (100%) | 0 | 0 | 0 | 0 | ||
| C | 20 (100%) | 0 | 0 | 0 | 0 | ||||||||
| Total | 95 (66.4%) | 1 (0.7%) | 6 (4.2%) | 30 (21.0%) | 11 (7.7%) | Total | 72 (46.5%) | 2 (1.3%) | 3 (1.9%) | 37 (23.9%) | 41 (26.5%) | ||
| Isolate | Location | Host | MIC Value (mg/L) | Phenotype | Amino Acid Substitution | ||
|---|---|---|---|---|---|---|---|
| Boscalid | Fluopyram | Boscalid | Fluopyram | ||||
| 18130301D | Granada | Cucumber | >100 1 | >100 1 | R | R | A86V |
| 18130301E | Granada | Cucumber | >100 1 | >100 1 | R | R | A86V |
| 18020303M | Almeria | Cucumber | >100 1 | >100 2 | R | HR | A86V |
| 18020303Q | Almeria | Cucumber | >100 1 | >100 1 | R | R | A86V |
| 18020303S | Almeria | Cucumber | <50 | >100 2 | LR | HR | A86V |
| 18020303T | Almeria | Cucumber | >100 1 | >100 1 | R | R | A86V |
| 18020305A | Almeria | Cucumber | 0 | >100 1 | S | R | A86V |
| 18020305K | Almeria | Cucumber | <50 | >100 2 | LR | HR | A86V |
| 18020305L | Almeria | Cucumber | <100 | >100 1 | MR | R | A86V |
| 18020305P | Almeria | Cucumber | 0 | >100 2 | S | HR | A86V |
| 18020305S | Almeria | Cucumber | >100 1 | >100 1 | R | R | A86V |
| 18030306A | Murcia | Cucumber | <100 | >100 1 | MR | R | A86V |
| 18030306B | Murcia | Cucumber | >100 1 | >100 2 | R | HR | A86V |
| 18030306C | Murcia | Cucumber | >100 1 | >100 1 | R | R | A86V |
| 18030306D | Murcia | Cucumber | <100 | <100 | MR | MR | A86V |
| 18030306E | Murcia | Cucumber | 0 | >100 1 | S | R | A86V |
| 18030306F | Murcia | Cucumber | >100 1 | >100 1 | R | R | A86V |
| 18030306G | Murcia | Cucumber | >100 1 | >100 1 | R | R | A86V |
| 18030306H | Murcia | Cucumber | >100 1 | >100 1 | R | R | A86V |
| 18030306I | Murcia | Cucumber | <100 | <100 | MR | MR | A86V |
| 18030306J | Murcia | Cucumber | >100 1 | >100 1 | R | R | A86V |
| 18030306K | Murcia | Cucumber | >100 1 | <50 | R | LR | G151R |
| 18030306L | Murcia | Cucumber | <50 | >100 1 | LR | R | A86V |
| 18030306M | Murcia | Cucumber | <100 | >100 1 | MR | R | A86V |
| 18030306N | Murcia | Cucumber | <50 | >100 2 | LR | HR | A86V |
| 18030306O | Murcia | Cucumber | >100 1 | >100 1 | R | R | A86V |
| 18030306P | Murcia | Cucumber | >100 1 | >100 1 | R | R | A86V |
| 18030306Q | Murcia | Cucumber | >100 1 | <100 | R | MR | A86V |
| 18030306S | Murcia | Cucumber | <50 | >100 1 | LR | R | A86V |
| 18030306U | Murcia | Cucumber | >100 1 | >100 1 | R | R | A86V |
| 18020307A | Almeria | Cucumber | >100 1 | >100 1 | R | R | A86V |
| 18020307B | Almeria | Cucumber | >100 1 | <100 | R | MR | A86V |
| 18020307C | Almeria | Cucumber | <50 | <100 | LR | MR | A86V |
| 18020307D | Almeria | Cucumber | <50 | <50 | LR | LR | A86V |
| 18020307E | Almeria | Cucumber | <50 | >100 2 | LR | HR | A86V |
| 18020307F | Almeria | Cucumber | <50 | >100 1 | LR | R | A86V |
| 18020307H | Almeria | Cucumber | <100 | >100 1 | MR | R | A86V |
| 18020307I | Almeria | Cucumber | >100 1 | 0 | R | S | G151R |
| 18020307J | Almeria | Cucumber | <50 | >100 2 | LR | HR | A86V |
| 18020307K | Almeria | Cucumber | <50 | >100 2 | LR | HR | A86V |
| 18020307L | Almeria | Cucumber | <50 | >100 1 | LR | R | A86V |
| 18020307M | Almeria | Cucumber | <50 | >100 2 | LR | HR | A86V |
| 18020307N | Almeria | Cucumber | <50 | <100 | LR | MR | A86V |
| 18020307P | Almeria | Cucumber | <50 | >100 1 | LR | R | A86V |
| 18020307Q | Almeria | Cucumber | <50 | >100 1 | LR | R | A86V |
| 18020307R | Almeria | Cucumber | <50 | >100 1 | LR | R | A86V |
| 18020307S | Almeria | Cucumber | <100 | >100 1 | MR | R | A86V |
| 18020307T | Almeria | Cucumber | <50 | >100 1 | LR | R | A86V |
| 18020208B | Murcia | Zucchini | <50 | >100 2 | LR | HR | A86V |
| 18020208D | Murcia | Zucchini | >100 1 | >100 1 | R | R | A86V |
| 19020203A | Almeria | Zucchini | <100 | >100 2 | MR | HR | A86V |
| 19020203B | Almeria | Zucchini | <50 | >100 1 | LR | R | A86V |
| 19020203C | Almeria | Zucchini | <50 | >100 1 | LR | R | A86V |
| 19020203F | Almeria | Zucchini | >100 1 | >100 2 | R | HR | A86V |
| 19020203H | Almeria | Zucchini | <100 | >100 1 | MR | R | A86V |
| 19020203I | Almeria | Zucchini | <50 | >100 2 | LR | HR | A86V |
| 19020203J | Almeria | Zucchini | <50 | >100 1 | LR | R | A86V |
| 19020304A | Almeria | Cucumber | <50 | >100 1 | LR | R | A86V |
| 19020304B | Almeria | Cucumber | 0 | >100 2 | S | HR | A86V |
| 19020304C | Almeria | Cucumber | 0 | >100 2 | S | HR | A86V |
| 19020304D | Almeria | Cucumber | 0 | >100 2 | S | HR | A86V |
| 19020304G | Almeria | Cucumber | 0 | >100 1 | S | R | A86V |
| 19020304I | Almeria | Cucumber | 0 | >100 1 | S | R | A86V |
| 19020304J | Almeria | Cucumber | 0 | >100 1 | S | R | A86V |
| 19020304O | Almeria | Cucumber | 0 | >100 1 | S | R | A86V |
| 19020304S | Almeria | Cucumber | 0 | >100 1 | S | R | A86V |
| Optimization of LAMP Reaction | Repeatability | Spores Assay | Field Samples Assay | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t (min:s) 1 | T (°C) 1 | t (min:s) 1 | T (°C) 1 | Phenotype | Genotype | t (min:s) 1 | T (°C) 1 | t (min:s) 1 | T (°C) 1 | t (min:s) 1 | T (°C) 1 | ||||
| Set 1-A86V | |||||||||||||||
| 19020304D | SF9 | ||||||||||||||
| 60.5 °C | 27:30 | 87.44 | - | - | 18020303T | BRFR | A86V | 16:30 | 85.50 | 19020304D | 20:30 | 85.70 | Leaf 1–3 | 29:15 | 85.99 |
| 61.6 °C | 26:00 | 87.64 | - | - | 18020305L | BMRFR | A86V | 14:00 | 85.79 | SF9 | - | - | Leaf 4–6 | 28:30 | 86.19 |
| 62.7 °C | 23:00 | 87.10 | - | - | 18030306D | BMRFMR | A86V | 14:30 | 85.70 | g19020304D 2 | 15:30 | 85.50 | Leaf 7–8 | 28:45 | 86.39 |
| 63.8 °C | 22:30 | 86.98 | - | - | 18030306M | BMRFR | A86V | 15:45 | 85.79 | gSF9 2 | - | - | Leaf 9–10 | 27:00 | 85.99 |
| 64.9 °C | 22:00 | 87.68 | - | - | 18020307K | BLRFHR | A86V | 14:45 | 85.30 | 19020304D | 23:30 | 86.19 | |||
| 66 °C | 23:15 | 87.68 | - | - | 18020307L | BLRFR | A86V | 15:15 | 85.79 | g19020304D 2 | 15:45 | 85.40 | |||
| 67.1 °C | - | - | - | - | 19020304D | BLRFR | A86V | 14:45 | 85.20 | gSF9 2 | - | - | |||
| 69.5 °C | - | - | - | - | MR03 | BSFS | - | - | - | ||||||
| SF60 | BSFS | - | - | - | |||||||||||
| SF9 | BSFS | - | - | - | |||||||||||
| 311271 | BSFS | - | - | - | |||||||||||
| Set 4-G151R | |||||||||||||||
| 18020307I | SF9 | ||||||||||||||
| 60.5 °C | - | - | - | - | 18030306K | BRFLR | G151R | 22:00 | 85.10 | 18020307I | 22:15 | 85.30 | Leaf 1–3 | - | - |
| 61.6 °C | 25:15 | 87.44 | - | - | 18020307I | BRFS | G151R | 21:45 | 85.30 | SF9 | - | - | Leaf 4–6 | - | - |
| 62.7 °C | 24:30 | 87.64 | - | - | MR03 | BSFS | - | - | - | g18020307I 2 | 20:45 | 85.90 | Leaf 7–8 | - | - |
| 63.8 °C | 25:15 | 87.10 | - | - | SF60 | BSFS | - | - | - | gSF9 2 | - | - | Leaf 9–10 | - | - |
| 64.9 °C | 27:45 | 86.98 | - | - | SF9 | BSFS | - | - | - | 18020307I | 21:00 | 85.50 | |||
| 66 °C | 28:00 | 87.68 | - | - | 311271 | BSFS | - | - | - | g18020307I 2 | 20:30 | 85.70 | |||
| 67.1 °C | - | - | - | - | gSF9 2 | - | - | ||||||||
| 69.5 °C | - | - | - | - | |||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vielba-Fernández, A.; Polonio, Á.; Ruiz-Jiménez, L.; de Vicente, A.; Pérez-García, A.; Fernández-Ortuño, D. Resistance to the SDHI Fungicides Boscalid and Fluopyram in Podosphaera xanthii Populations from Commercial Cucurbit Fields in Spain. J. Fungi 2021, 7, 733. https://doi.org/10.3390/jof7090733
Vielba-Fernández A, Polonio Á, Ruiz-Jiménez L, de Vicente A, Pérez-García A, Fernández-Ortuño D. Resistance to the SDHI Fungicides Boscalid and Fluopyram in Podosphaera xanthii Populations from Commercial Cucurbit Fields in Spain. Journal of Fungi. 2021; 7(9):733. https://doi.org/10.3390/jof7090733
Chicago/Turabian StyleVielba-Fernández, Alejandra, Álvaro Polonio, Laura Ruiz-Jiménez, Antonio de Vicente, Alejandro Pérez-García, and Dolores Fernández-Ortuño. 2021. "Resistance to the SDHI Fungicides Boscalid and Fluopyram in Podosphaera xanthii Populations from Commercial Cucurbit Fields in Spain" Journal of Fungi 7, no. 9: 733. https://doi.org/10.3390/jof7090733
APA StyleVielba-Fernández, A., Polonio, Á., Ruiz-Jiménez, L., de Vicente, A., Pérez-García, A., & Fernández-Ortuño, D. (2021). Resistance to the SDHI Fungicides Boscalid and Fluopyram in Podosphaera xanthii Populations from Commercial Cucurbit Fields in Spain. Journal of Fungi, 7(9), 733. https://doi.org/10.3390/jof7090733






