Trends and Applications of Omics Technologies to Functional Characterisation of Enzymes and Protein Metabolites Produced by Fungi
Abstract
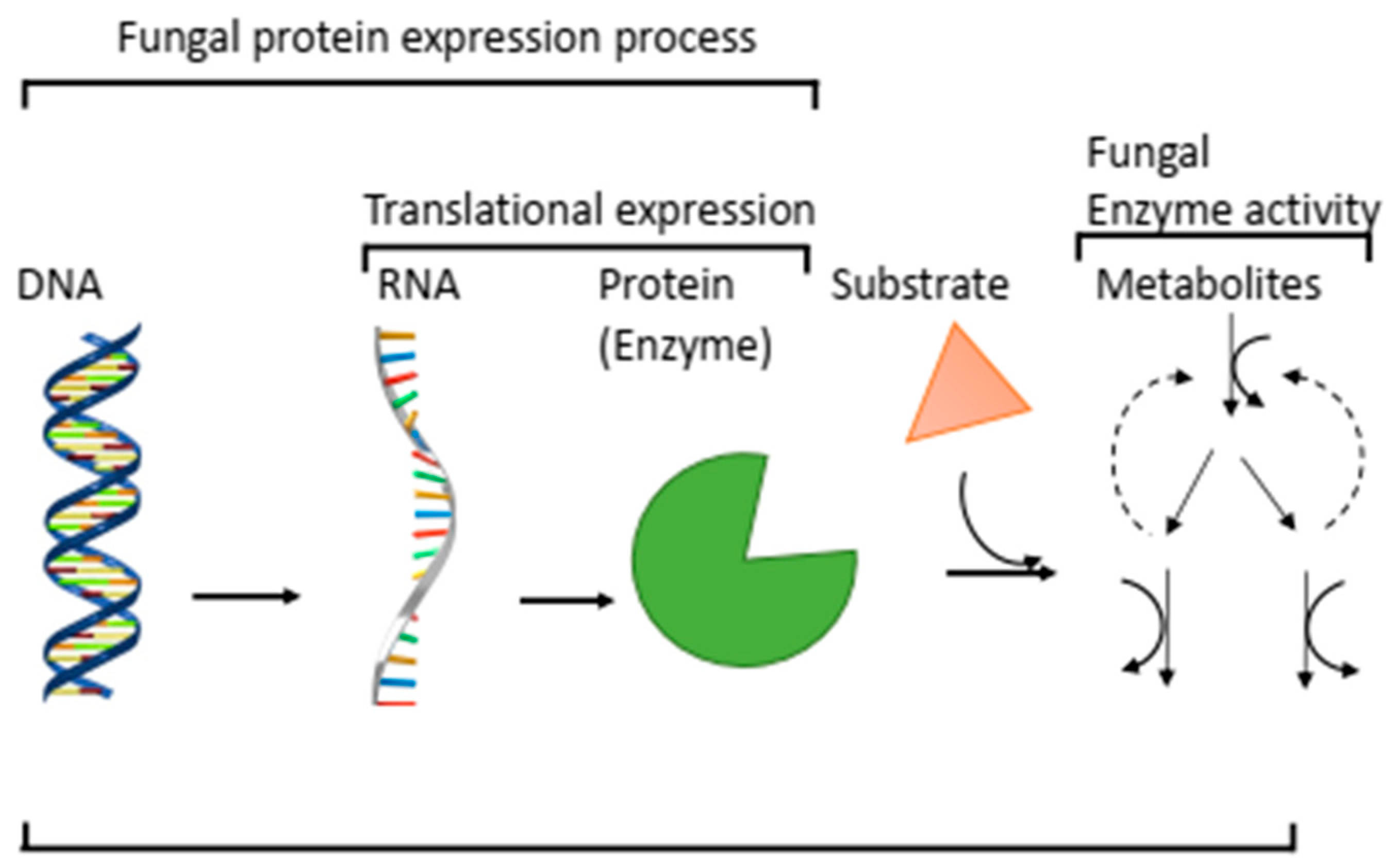
1. Introduction
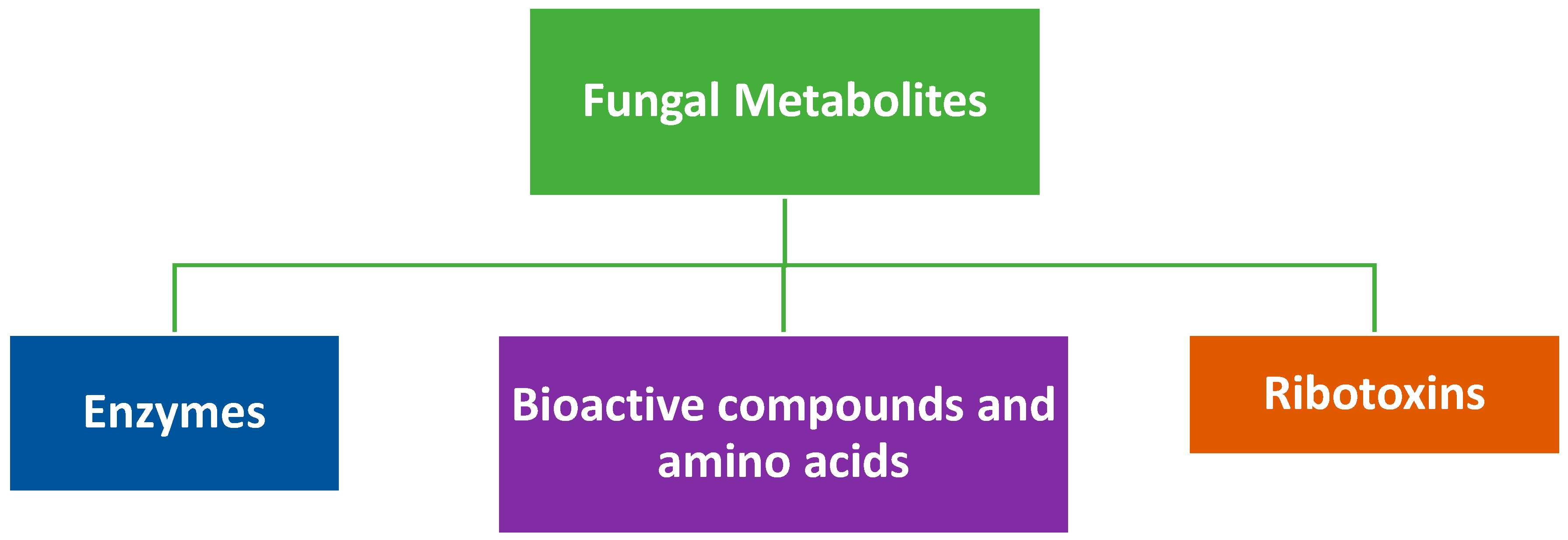
2. Relevant Metabolites Produced by Fungi
| Class of Enzyme | Sub-Category | Enzyme | Industrial Applications | References |
|---|---|---|---|---|
| Oxidoreductases | Heme-containing peroxidases and peroxygenases | Lignin peroxidase | Production of vanillin, textile dye degradation and bleaching, xenobiotic compounds degradation | [37] |
| Manganese peroxidase | Bioethanol production, dye decolourisation, degradation of rubber, distillery waste treatment | [37,39] | ||
| Versatile peroxidase | Novel delignification strategies | [38] | ||
| Chloroperoxidase | Halogenation and oxygenation of organic compounds, lignin cleavage | [41,49] | ||
| Aromatic/Unspecific peroxygenases | Bioremediation of recalcitrant pollutants such as polycyclic aromatic hydrocarbons, halogenated biphenyl esters, and chlorinated benzenes | [40] | ||
| Dye-decolourising peroxidases | Decolourisation of synthetic dyes | [42,43] | ||
| Flavin-containing oxidases and dehydrogenases | Aryl-alcohol oxidase | Synthesis of high value-added chemicals, flavours, production of bio-based polymer precursors, dye decolourisation, pulp bleaching | [50,51] | |
| Copper-containing oxidases and monooxygenases | Laccase | Detoxifier in industries such as pulp and paper, textile, and petrochemical Heavy metal precipitation, biosensor production, food processing, drug preparation | [52,53,54] | |
| Hydrolases | Proteases | Alkaline, acid, and neutral proteases | Fabrication of laundry detergents, beer haze clarification, brewing, cheese making, meat tenderisation, synthesis of bioactive peptides | [33,34] |
| Glycoside hydrolase | Cellulases: endoglucanases, exocellulases, and processive endoglucanases | Juice extraction and clarification, supplementation of livestock, biofuel production, laundry detergent production | [35] | |
| Xylanase | Hemicellulases, endo-1,4-β-D-xylanases, β-D-xylosidases, α-glucuronidase acetylxylan esterase, α-L-arabinofuranosidases, p-coumaric esterase, and ferulic acid esterase | Juice clarification, paper bleaching, and increasing the nutritional value of animal feeds. | [36,55] | |
| Amylase | α-amylase, β-amylase, and glucoamylase | Bread making, detergent manufacture, treatment of digestive disorders | [56] | |
| Lipase | Triacylglycerolacyl hydrolases | Production of detergents and cosmetic products, synthesis of biopolymers and biodiesel, manufacturing of food products | [36] |
3. Advances in Molecular Characterisation of Fungi
4. Transcriptomics in Fungi Functional Characterisation
Bioinformatics in Fungi Transcriptomics Studies
5. Proteomics in Fungi Translational Characterisation
5.1. Protein Production and Analysis
5.2. Enzyme Production through Recombinant DNA Technology
5.3. Expression Analysis
5.4. Protein (Enzyme) Purification and Separation
5.5. Structural Studies and Sequencing
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cerimi, K.; Akkaya, K.C.; Pohl, C.; Schmidt, B.; Neubauer, P. Fungi as source for new bio-based materials: A patent review. Fungal Biol. Biotechnol. 2019, 6, 17. [Google Scholar] [CrossRef]
- Yuvaraj, M.; Ramasamy, M. Role of Fungi in Agriculture. In Biostimulants in Plant Science; Mirmajlessi, S.M., Radhakrishnan, R., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.S.; Brahamanage, R.S.; Brooks, S.; et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers 2019, 97, 1–136. [Google Scholar] [CrossRef]
- Meyer, V.; Basenko, E.Y.; Benz, J.P.; Braus, G.H.; Caddick, M.X.; Csukai, M.; De Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N.; et al. Growing a circular economy with fungal biotechnology: A white paper. Fungal Biol. Biotechnol. 2020, 7, 5. [Google Scholar] [CrossRef]
- Susca, A.; Villani, A.; Moretti, A.; Stea, G.; Logrieco, A. Identification of toxigenic fungal species associated with maize ear rot: Calmodulin as single informative gene. Int. J. Food Microbiol. 2020, 319, 108491. [Google Scholar] [CrossRef]
- Wennig, R.; Eyer, F.; Schaper, A.; Zilker, T.; Andresen-Streichert, H. Mushroom Poisoning. Dtsch. Arztebl. Int. 2020, 117, 701–708. [Google Scholar] [CrossRef]
- Kumar, A.; Chandra, R. Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef]
- Chávez, R.; Fierro, F.; García-Rico, R.O.; Vaca, I. Filamentous fungi from extreme environments as a promising source of novel bioactive secondary metabolites. Front. Microbiol. 2015, 6, 903. [Google Scholar] [CrossRef]
- Murgia, M.; Fiamma, M.; Barac, A.; Deligios, M.; Mazzarello, V.; Paglietti, B.; Cappuccinelli, P.; Al-Qahtani, A.; Squartini, A.; Rubino, S.; et al. Biodiversity of fungi in hot desert sands. MicrobiologyOpen 2019, 8, e595. [Google Scholar] [CrossRef]
- Raghukumar, S.; Raghukumar, C.; Manohar, C.S. Fungi living in diverse extreme habitats of the marine environment. Kavaka 2014, 42, 145–153. [Google Scholar]
- Ostrem Loss, E.M.; Lee, M.-K.; Wu, M.-Y.; Martien, J. Utilization of Benzo [a] Pyrene by Aspergillus Species. Mol. Biol. Physiol. 2019, 10, 1–15. [Google Scholar]
- Asemoloye, M.D.; Tosi, S.; Daccò, C.; Wang, X.; Xu, S.; Marchisio, M.A.; Gao, W.; Jonathan, S.G.; Pecoraro, L. Hydrocarbon degradation and enzyme activities of Aspergillus oryzae and Mucor irregularis isolated from nigerian crude oil-polluted sites. Microorganisms 2020, 8, 1912. [Google Scholar] [CrossRef]
- Henn, C.; Monteiro, D.A.; Boscolo, M.; Da Silva, R.; Gomes, E. Biodegradation of atrazine and ligninolytic enzyme production by basidiomycete strains. BMC Microbiol. 2020, 20, 266. [Google Scholar] [CrossRef]
- Basu, S.; Bose, C.; Ojha, N.; Das, N.; Das, J.; Pal, M.; Khurana, S. Evolution of bacterial and fungal growth media. Bioinformation 2015, 11, 182–183. [Google Scholar] [CrossRef]
- Shi, X.X.; Qiu, H.P.; Wang, J.Y.; Zhang, Z.; Wang, Y.L.; Sun, G.C. A handy method to remove bacterial contamination from fungal cultures. PLoS ONE 2019, 14, e0224635. [Google Scholar] [CrossRef]
- Black, W.D. A comparison of several media types and basic techniques used to assess outdoor airborne fungi in Melbourne, Australia. bioRxiv 2020. [Google Scholar] [CrossRef]
- Cheptsov, V.S.; Tsypina, S.I.; Minaev, N.V.; Yusupov, V.I.; Chichkov, B.N. New microorganism isolation techniques with emphasis on laser printing. Int. J. Bioprint. 2019, 5, 156. [Google Scholar] [CrossRef]
- Mahmood, M.A.; Popescu, A.C. 3D Printing at Micro-Level: Laser-Induced Forward Transfer and Two-Photon Polymerization. Polymers 2021, 13, 2034. [Google Scholar] [CrossRef]
- Hopp, B.; Smausz, T.; Antal, Z.; Kresz, N.; Bor, Z.; Chrisey, D. Absorbing film assisted laser induced forward transfer of fungi (Trichoderma conidia). J. Appl. Phys. 2004, 96, 3478–3481. [Google Scholar] [CrossRef]
- Hopp, B.; Smausz, T.; Barna, N.; Vass, C.; Antal, Z.; Kredics, L.; Chrisey, D. Time-resolved study of absorbing film assisted laser induced forward transfer of Trichoderma longibrachiatum conidia. J. Phys. D Appl. Phys. 2005, 38, 833–837. [Google Scholar] [CrossRef]
- Hopp, B.; Smausz, T.; Nógrádi, A. Absorbing-Film Assisted Laser Induced Forward Transfer of Sensitive Biological Subjects. In Cell and Organ Printing; Ringeisen, B., Spargo, B., Wu, P., Eds.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Nevalainen, H.; Kautto, L.; Te’o, J. Methods for Isolation and Cultivation of Filamentous Fungi. In Environmental Microbiology: Methods and Protocols; Paulsen, I.T., Holmes, A.J., Eds.; Springer Science+Business Media: Berlin, Germany, 2014; Volume 1096, pp. 2–16. ISBN 9781493990047. [Google Scholar]
- Ijoma, G.N.; Selvarajan, R.; Oyourou, J.N.; Sibanda, T.; Matambo, T.; Monanga, A.; Mkansi, K. Exploring the application of biostimulation strategy for bacteria in the bioremediation of industrial effluent. Ann. Microbiol. 2019, 69, 541–551. [Google Scholar] [CrossRef]
- De Vero, L.; Boniotti, M.B.; Budroni, M.; Buzzini, P.; Cassanelli, S.; Comunian, R.; Gullo, M.; Logrieco, A.F.; Mannazzu, I.; Musumeci, R.; et al. Preservation, characterization and exploitation of microbial biodiversity: The perspective of the italian network of culture collections. Microorganisms 2019, 7, 685. [Google Scholar] [CrossRef]
- Toledo, A.V.; Simurro, M.E.; Balatti, P.A. Morphological and molecular characterization of a fungus, Hirsutella sp., isolated from planthoppers and psocids in Argentina. J. Insect Sci. 2013, 13, 18. [Google Scholar] [CrossRef]
- Alsohaili, S.A.; Bani-Hasan, B.M. Morphological and molecular identification of fungi isolated from different environmental sources in the northern eastern Desert of Jordan. Jordan J. Biol. Sci. 2018, 11, 329–337. [Google Scholar]
- Rachmania, M.; Oetari, A.; Fitri, R.; Susetyo-Salim, T.; Sjamsuridzal, W. Isolation and morphological characterization of fungi from deteriorated old Chinese manuscripts from Central Library Universitas Indonesia. AIP Conf. Proc. 2018, 2023, 020144. [Google Scholar] [CrossRef]
- Lutzoni, F.; Kauff, F.; Cox, C.J.; McLaughlin, D.; Celio, G.; Dentinger, B.; Padamsee, M.; Hibbert, D.; James, T.Y.; Baloch, E.; et al. Assembling the fungal tree of life: Progress, classification, and evolution of subcellular traits. Am. J. Bot. 2004, 91, 1446–1480. [Google Scholar] [CrossRef]
- Oates, N.C.; Abood, A.; Schirmacher, A.M.; Alessi, A.M.; Bird, S.M.; Bennett, J.P.; Leadbeater, D.R.; Li, Y.; Dowle, A.A.; Liu, S.; et al. A multi-omics approach to lignocellulolytic enzyme discovery reveals a new ligninase activity from Parascedosporium putredinis NO1. Proc. Natl. Acad. Sci. USA 2021, 118, e2008888118. [Google Scholar] [CrossRef]
- Brenelli, L.B.; Persinoti, G.F.; Cairo, J.P.L.F.; Liberato, M.V.; Gonçalves, T.A.; Otero, I.V.R.; Mainardi, P.H.; Felby, C.; Sette, L.D.; Squina, F.M. Novel redox-active enzymes for ligninolytic applications revealed from multiomics analyses of Peniophora sp. CBMAI 1063, a laccase hyper-producer strain. Sci. Rep. 2019, 9, 17564. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, M. The fungi: 1, 2, 3 … 5.1 million species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef]
- Srilakshmi, J.; Madhavi, J.; Lavanya, S.; Ammani, K. Commercial Potential of Fungal Protease: Past, Present and Future Prospects. J. Pharm. Chem. Biol. Sci. 2015, 2, 218–234. [Google Scholar]
- de Souza, P.M.; de Assis Bittencourt, M.L.; Caprara, C.C.; de Freitas, M.; de Almeida, R.P.C.; Silveira, D.; Fonseca, Y.M.; Filho, E.X.F.; Pessoa Junior, A.; Magalhães, P.O. A biotechnology perspective of fungal proteases. Braz. J. Microbiol. 2015, 46, 337–346. [Google Scholar] [CrossRef]
- da Silva, R.R. Bacterial and Fungal Proteolytic Enzymes: Production, Catalysis and Potential Applications. Appl. Biochem. Biotechnol. 2017, 183, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Bibi, A. Fungal Cellulase; Production and Applications: Minireview. LIFE Int. J. Health Life-Sci. 2018, 4, 19–36. [Google Scholar] [CrossRef]
- McKelvey, S.M.; Murphy, R.A. Biotechnological Use of Fungal Enzymes. In Fungi: Biology and Applications; Kavanagh, K., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 201–225. ISBN 9781119976950. [Google Scholar]
- Maciel, M.J.M.; Castro e Silva, A.; Ribeiro, H.C.T. Industrial and biotechnological applications of ligninolytic enzymes of the basidiomycota: A review. Electron. J. Biotechnol. 2010, 13, 1–13. [Google Scholar] [CrossRef]
- Kong, W.; Fu, X.; Wang, L.; Alhujaily, A.; Zhang, J.; Ma, F.; Zhang, X.; Yu, H. A novel and efficient fungal delignification strategy based on versatile peroxidase for lignocellulose bioconversion. Biotechnol. Biofuels 2017, 10, 218. [Google Scholar] [CrossRef]
- Chowdhary, P.; Shukla, G.; Raj, G.; Ferreira, L.F.R.; Bharagava, R.N. Microbial manganese peroxidase: A ligninolytic enzyme and its ample opportunities in research. SN Appl. Sci. 2019, 1, 45. [Google Scholar] [CrossRef]
- Karich, A.; Ullrich, R.; Scheibner, K.; Hofrichter, M. Fungal unspecific peroxygenases oxidize the majority of organic EPA priority pollutants. Front. Microbiol. 2017, 8, 1463. [Google Scholar] [CrossRef]
- Schmidt-Dannert, C. Biocatalytic Portfolio of Basidiomycota Claudia. Curr. Opin. Chem. Biol. 2016, 31, 40–49. [Google Scholar] [CrossRef]
- Duan, Z.; Shen, R.; Liu, B.; Yao, M.; Jia, R. Comprehensive investigation of a dye-decolorizing peroxidase and a manganese peroxidase from Irpex lacteus F17, a lignin-degrading basidiomycete. AMB Express 2018, 8, 119. [Google Scholar] [CrossRef]
- Qin, X.; Luo, H.; Zhang, X.; Yao, B.; Ma, F.; Su, X. Dye-decolorizing peroxidases in Irpex lacteus combining the catalytic properties of heme peroxidases and laccase play important roles in ligninolytic system. Biotechnol. Biofuels 2018, 11, 302. [Google Scholar] [CrossRef]
- Hoeksma, J.; Misset, T.; Wever, C.; Kemmink, J.; Kruijtzer, J.; Versluis, K.; Liskamp, R.M.J.; Boons, G.J.; Heck, A.J.R.; Boekhout, T.; et al. A new perspective on fungal metabolites: Identification of bioactive compounds from fungi using zebrafish embryogenesis as read-out. Sci. Rep. 2019, 9, 17546. [Google Scholar] [CrossRef]
- Vasundhara, M.; Kumar, A.; Reddy, M.S. Molecular approaches to screen bioactive compounds from endophytic fungi. Front. Microbiol. 2016, 7, 1774. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Lee, Y.L.; Li, R.C.; Mau, J.L. Non-volatile flavour components of Ganoderma tsugae. Food Chem. 2005, 90, 409–415. [Google Scholar] [CrossRef]
- Tagkouli, D.; Kaliora, A.; Bekiaris, G.; Koutrotsios, G.; Christea, M.; Zervakis, G.I.; Kalogeropoulos, N. Free Amino Acids in Three Pleurotus Species Cultivated on Agricultural and Agro-Industrial By-Products. Molecules 2020, 25, 4015. [Google Scholar] [CrossRef] [PubMed]
- Landi, N.; Pacifico, S.; Ragucci, S.; Di Giuseppe, A.M.A.; Iannuzi, F.; Zarreli, A.; Piccolella, S.; Di Maro, A. Pioppino mushroom in the Southern Italy: An undervalued source of nutrients and bioactive compounds. J. Sci. Food Agric. 2017, 97, 5388–5397. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Bermúdez, P.; Srebotnik, E.; Hammel, K.E. Chlorination and cleavage of lignin structures by fungal chloroperoxidases. Appl. Environ. Microbiol. 2003, 69, 5015–5018. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ortega, A.; Ferreira, P.; Martínez, A.T. Fungal aryl-alcohol oxidase: A peroxide-producing flavoenzyme involved in lignin degradation. Appl. Microbiol. Biotechnol. 2012, 93, 1395–1410. [Google Scholar] [CrossRef]
- Urlacher, V.B.; Koschorreck, K. Pecularities and applications of aryl-alcohol oxidases from fungi. Appl. Microbiol. Biotechnol. 2021, 105, 4111–4126. [Google Scholar] [CrossRef]
- Upadhyay, P.; Shrivastava, R.; Agrawal, P.K. Bioprospecting and biotechnological applications of fungal laccase. 3 Biotech 2016, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Khozani, M.; Emtiazi, G.; Aghaei, S.S.; Ghasemi, S.M.; Zolfaghari, M.R. Application of fungal laccase for heavy metals precipitation using tannin as a natural mediator. Int. J. Environ. Sci. Technol. 2020, 18, 2335–2344. [Google Scholar] [CrossRef]
- Senthivelan, T.; Kanagaraj, J.; Panda, R.C. Recent trends in fungal laccase for various industrial applications: An eco-friendly approach—A review. Biotechnol. Bioprocess Eng. 2016, 21, 19–38. [Google Scholar] [CrossRef]
- Walia, A.; Guleria, S.; Mehta, P.; Chauhan, A.; Parkash, J. Microbial xylanases and their industrial application in pulp and paper biobleaching: A review. 3 Biotech 2017, 7, 11. [Google Scholar] [CrossRef]
- Carreras-Sangra, N.; Alvarez-Garcia, E.; Herrero-Galan, E.; Tome, J.; Lacadena, J.; Alegre-Cebollada, J.; Onaderra, M.; Gavilanes, J.; Martinez-del-Pozo, A. The Therapeutic Potential of Fungal Ribotoxins. Curr. Pharm. Biotechnol. 2008, 9, 153–160. [Google Scholar] [CrossRef]
- Saranraj, P.; Stella, D. Fungal Amylase-A Review. Int. J. Microbiol. Res. 2013, 4, 203–211. [Google Scholar] [CrossRef]
- Lacadena, J.; Álvarez-García, E.; Carreras-Sangrà, N.; Herrero-Galán, E.; Alegre-Cebollada, J.; García-Ortega, L.; Oñaderra, M.; Gavilanes, J.G.; Del Pozo, Á.M. Fungal ribotoxins: Molecular dissection of a family of natural killers. FEMS Microbiol. Rev. 2007, 31, 212–237. [Google Scholar] [CrossRef]
- Olombrada, M.; Lázaro-Gorines, R.; López-Rodríguez, J.C.; Martínez-Del-Pozo, Á.; Oñaderra, M.; Maestro-López, M.; Lacadena, J.; Gavilanes, J.G.; García-Ortega, L. Fungal ribotoxins: A review of potential biotechnological applications. Toxins 2017, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, Y. High cell density fed-batch production of insecticidal recombinant ribotoxin hirsutellin A from Pichia pastoris. Microb. Cell Fact. 2018, 17, 145. [Google Scholar] [CrossRef]
- Landi, N.; Pacifico, S.; Ragucci, S.; Iglesias, R.; Piccolella, S.; Amici, A.; Di Giuseppe, A.M.A.; Di Maro, A. Purification, characterization and cytotoxicity assessment of Ageritin: The first ribotoxin from the basidiomycete mushroom Agrocybe aegerita. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1113–1121. [Google Scholar] [CrossRef]
- Landi, N.; Ragucci, S.; Russo, R.; Valletta, M.; Pizzo, E.; Ferreras, J.M.; Di Maro, A. The ribotoxin-like protein Ostreatin from Pleurotus ostreatus fruiting bodies: Confirmation of a novel ribonuclease family expressed in basidiomycetes. Int. J. Biol. Macromol. 2020, 161, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Ragucci, S.; Landi, N.; Russo, R.; Valletta, M.; Pedone, P.V.; Chambery, A.; Di Maro, A. Ageritin from pioppino mushroom: The prototype of ribotoxin-like proteins, a novel family of specific ribonucleases in edible mushrooms. Toxins 2021, 13, 263. [Google Scholar] [CrossRef] [PubMed]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal Identification Using Molecular Tools: A Primer for the Natural Products Research Community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef]
- Bechem, E.T.; Afanga, Y.A. Morphological and molecular identification of fungi associated with corm rot and blight symptoms on plantain (Musa paradisiaca) in macro-propagators. Int. J. Biol. Chem. Sci. 2017, 11, 2793. [Google Scholar] [CrossRef][Green Version]
- Ab Majid, A.H.; Zahran, Z.; Abd Rahim, A.H.; Ismail, N.A.; Abdul Rahman, W.; Mohammad Zubairi, K.S.; Dieng, H.; Satho, T. Morphological and molecular characterization of fungus isolated from tropical bed bugs in Northern Peninsular Malaysia, Cimex hemipterus (Hemiptera: Cimicidae). Asian Pac. J. Trop. Biomed. 2015, 5, 707–713. [Google Scholar] [CrossRef]
- Slatko, B.E.; Gardner, A.F.; Ausubel, F.M. Overview of Next Generation Sequencing technologies. Curr. Protoc. Mol. Biol. 2018, 122, e59. [Google Scholar] [CrossRef] [PubMed]
- Crossley, B.M.; Bai, J.; Glaser, A.; Maes, R.; Porter, E.; Killian, M.L.; Clement, T.; Toohey-Kurth, K. Guidelines for Sanger sequencing and molecular assay monitoring. J. Vet. Diagn. Investig. 2020, 32, 767–775. [Google Scholar] [CrossRef]
- Valones, M.A.A.; Guimarães, R.L.; Brandão, L.A.C.; De Souza, P.R.E.; De Albuquerque, T.C.; Crovela, S. Principles and applications of polymerase chain reaction in medical diagnostic fields: A review. Braz. J. Microbiol. 2009, 40, 1–11. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal Rna Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Hoang, M.T.V.; Irinyi, L.; Chen, S.C.A.; Sorrell, T.C.; Meyer, W.; Arabatzis, M.; Arthur, I.; Cano-Lira, J.F.; Cardinali, G.; Castañón, L.R.; et al. Dual DNA barcoding for the molecular identification of the agents of invasive fungal infections. Front. Microbiol. 2019, 10, 1647. [Google Scholar] [CrossRef] [PubMed]
- Cadez, N.; Raspor, P.; De Cock, A.W.A.M.; Boekhout, T.; Smith, M.T. Molecular identification and genetic diversity within species of the genera Hanseniaspora and Kloeckera. FEMS Yeast Res. 2002, 1, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Consortium, F.B. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Totomoch-Serra, A.; Marquez, M.F.; Cervantes-Barragán, D.E. Sanger sequencing as a first-line approach for molecular diagnosis of Andersen-Tawil syndrome. F1000Research 2017, 6, 1016. [Google Scholar] [CrossRef]
- Hedeler, C.; Wong, H.M.; Cornell, M.J.; Alam, I.; Soanes, D.M.; Rattray, M.; Hubbard, S.J.; Talbot, N.J.; Oliver, S.G.; Paton, N.W. e-Fungi: A data resource for comparative analysis of fungal genomes. BMC Genom. 2007, 8, 426. [Google Scholar] [CrossRef]
- Federhen, S. The NCBI Taxonomy database. Nucleic Acids Res. 2012, 40, D136–D143. [Google Scholar] [CrossRef]
- Meena, B.; Anburajan, L.; Vinithkumar, N.V.; Kirubagaran, R.; Dharani, G. Molecular characterization, phylogenetic and in silico sequence analysis data of trehalose biosynthesis genes; otsA and otsB from the deep sea halophilic actinobacteria, Streptomyces qinglanensis NIOT-DSA03. Data Brief 2021, 35, 106727. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef] [PubMed]
- Kõljalg, U.; Abarenkov, K.; Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S. The UNITE Database for Molecular Identification and for Communicating Fungal Species. Biodivers. Inf. Sci. Stand. 2019, 3, e37402. [Google Scholar] [CrossRef]
- Loeffler, C.; Karlsberg, A.; Martin, L.S.; Eskin, E.; Koslicki, D.; Mangul, S. Improving the usability and comprehensiveness of microbial databases. BMC Biol. 2020, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Lücking, R.; Aime, M.C.; Robbertse, B.; Miller, A.N.; Ariyawansa, H.A.; Aoki, T.; Cardinali, G.; Crous, P.W.; Druzhinina, I.S.; Geiser, D.M.; et al. Unambiguous identification of fungi: Where do we stand and how accurate and precise is fungal DNA barcoding? IMA Fungus 2020, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Finotello, F.; Di Camillo, B. Measuring differential gene expression with RNA-seq: Challenges and strategies for data analysis. Brief. Funct. Genom. 2015, 14, 130–142. [Google Scholar] [CrossRef]
- Choi, J.K.; Kim, S.C. Environmental effects on gene expression phenotype have regional biases in the human genome. Genetics 2007, 175, 1607–1613. [Google Scholar] [CrossRef]
- Guo, J. Transcription: The epicenter of gene expression. J. Zhejiang Univ. B Biomed. Biotechnol. 2014, 15, 409–411. [Google Scholar] [CrossRef]
- Koonin, E.V. Why the Central Dogma: On the nature of the great biological exclusion principle. Biol. Direct 2015, 10, 52. [Google Scholar] [CrossRef]
- Manzoni, C.; Kia, D.A.; Vandrovcova, J.; Hardy, J.; Wood, N.W.; Lewis, P.A.; Ferrari, R. Genome, transcriptome and proteome: The rise of omics data and their integration in biomedical sciences. Brief. Bioinform. 2018, 19, 286–302. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef] [PubMed]
- Elizondo, L.; Jafar-Nejad, P.; Clewing, J.; Boerkoel, C. Gene Clusters, Molecular Evolution and Disease: A Speculation. Curr. Genom. 2009, 10, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Noriega, D.D.; Arias, P.L.; Barbosa, H.R.; Arraes, F.B.M.; Ossa, G.A.; Villegas, B.; Coelho, R.R.; Albuquerque, E.V.S.; Togawa, R.C.; Grynberg, P.; et al. Transcriptome and gene expression analysis of three developmental stages of the coffee berry borer, Hypothenemus hampei. Sci. Rep. 2019, 9, 12804. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, S.W.; Lim, G.H.; Lyu, J.I.; Choi, H.I.; Jo, Y.D.; Kang, S.Y.; Kang, B.C.; Kim, J.B. Transcriptome analysis to identify candidate genes associated with the yellow-leaf phenotype of a Cymbidium mutant generated by γ-irradiation. PLoS ONE 2020, 15, e0228078. [Google Scholar] [CrossRef] [PubMed]
- Pombo, M.A.; Zheng, Y.; Fei, Z.; Martin, G.B.; Rosli, H.G. Use of RNA-seq data to identify and validate RT-qPCR reference genes for studying the tomato-Pseudomonas pathosystem. Sci. Rep. 2017, 7, 44905. [Google Scholar] [CrossRef] [PubMed]
- Adams, G. A beginner’s guide to RT-PCR, qPCR and RT-qPCR. Biochemist 2020, 42, 48–53. [Google Scholar] [CrossRef]
- Bleve, G.; Rizzotti, L.; Dellaglio, F.; Torriani, S. Development of reverse transcription (RT)-PCR and real-time RT-PCR assays for rapid detection and quantification of viable yeasts and molds contaminating yogurts and pasteurized food products. Appl. Environ. Microbiol. 2003, 69, 4116–4122. [Google Scholar] [CrossRef]
- Arya, M.; Shergill, I.S.; Williamson, M.; Gommersall, L.; Arya, N.; Patel, H.R. Basic principles of real-time quantitative PCR. Expert Rev. Mol. Diagn. 2005, 5, 209–219. [Google Scholar] [CrossRef]
- Fernández-Fueyo, E.; Castanera, R.; Ruiz-dueñas, F.J.; López-lucendo, M.F.; Ramírez, L.; Pisabarro, A.G.; Martínez, A.T. Ligninolytic peroxidase gene expression by Pleurotus ostreatus: Differential regulation in lignocellulose medium and effect of temperature and pH. Fungal Genet. Biol. 2014, 72, 150–161. [Google Scholar] [CrossRef]
- Stuardo, M.; Vásquez, M.; Vicuña, R.; González, B. Molecular approach for analysis of model fungal genes encoding ligninolytic peroxidases in wood-decaying soil systems. Lett. Appl. Microbiol. 2004, 38, 43–49. [Google Scholar] [CrossRef]
- Chen, D.M.; Taylor, A.F.S.; Burke, R.M.; Cairney, J.W.G. Identification of genes for lignin peroxidases and manganese peroxidases in ectomycorrhizal fungi. New Phytol. 2001, 152, 151–158. [Google Scholar] [CrossRef]
- Castanera, R.; Pérez, G.; Omarini, A.; Alfaro, M.; Pisabarro, A.G.; Faraco, V.; Amore, A.; Ramírez, L. Transcriptional and enzymatic profiling of Pleurotus ostreatus laccase genes in submerged and solid-state fermentation cultures. Appl. Environ. Microbiol. 2012, 78, 4037–4045. [Google Scholar] [CrossRef] [PubMed]
- Vasina, D.V.; Moiseenko, K.V.; Fedorova, T.V.; Tyazhelova, T.V. Lignin-degrading peroxidases in white-rot fungus Trametes hirsuta 072. Absolute expression quantification of full multigene family. PLoS ONE 2017, 12, e0173813. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Osborn, A.M. Advantages and limitations of quantitative PCR (Q-PCR)-based approaches in microbial ecology. FEMS Microbiol. Ecol. 2009, 67, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Nonis, A.; De Nardi, B.; Nonis, A. Choosing between RT-qPCR and RNA-seq: A back-of-the-envelope estimate towards the definition of the break-even-point. Anal. Bioanal. Chem. 2014, 406, 3533–3536. [Google Scholar] [CrossRef]
- Castanera, R.; López-Varas, L.; Pisabarro, A.G.; Ramíre, L. Validation of reference genes for transcriptional analyses in Pleurotus ostreatus by using reverse transcription-quantitative PCR. Appl. Environ. Microbiol. 2015, 81, 4120–4129. [Google Scholar] [CrossRef] [PubMed]
- Moyerbrailean, G.A.; Davis, G.O.; Harvey, C.T.; Watza, D.; Wen, X.; Pique-Regi, R.; Luca, F. A high-throughput RNA-seq approach to profile transcriptional responses. Sci. Rep. 2015, 5, 14976. [Google Scholar] [CrossRef]
- Costa-Silva, J.; Domingues, D.; Lopes, F.M. RNA-Seq differential expression analysis: An extended review and a software tool. PLoS ONE 2017, 12, e0190152. [Google Scholar] [CrossRef]
- Kukurba, K.R.; Montgomery, S.B. DNA sequencing and analysis. Cold Spring Harb. Protoc. 2015, 2015, 951–969. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Jazayeri, S.M.; Melgarejo Muñoz, L.M.; Romero, H.M. RNA-seq: A glance at technologies and methodologies. Acta Biol. Colomb. 2015, 20, 23–35. [Google Scholar] [CrossRef]
- Loman, N.J.; Misra, R.V.; Dallman, T.J.; Constantinidou, C.; Gharbia, S.E.; Wain, J.; Pallen, M.J. Performance comparison of benchtop high-throughput sequencing platforms. Nat. Biotechnol. 2012, 30, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Quail, M.A.; Smith, M.; Coupland, P.; Otto, T.D.; Harris, S.R.; Connor, T.R.; Bertoni, A.; Swerdlow, H.P.; Gu, Y. A tale of three next generation sequencing platforms: Comparison of Ion Torrent, Pacific Biosciences and Illumina MiSeq sequencers. BMC Genom. 2012, 13, 341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Schug, J.; Lin, J.; Wang, Z.; Kossenkov, A.; Kaestner, K.H. Comparative analysis of commercially available single-cell RNA sequencing platforms for their performance in complex human tissues. bioRxiv 2019, 541433. [Google Scholar] [CrossRef]
- Ravichandran, A.; Kolte, A.P.; Dhali, A.; Gopinath, S.M.; Sridhar, M. Transcriptomic analysis of Lentinus squarrosulus provide insights into its biodegradation ability. bioRxiv 2020. [Google Scholar] [CrossRef]
- Henske, J.K.; Springer, S.D.; O’Malley, M.A.; Butler, A. Substrate-based differential expression analysis reveals control of biomass degrading enzymes in Pycnoporus cinnabarinus. Biochem. Eng. J. 2018, 130, 83–89. [Google Scholar] [CrossRef]
- Ma, L.; Chen, L.; Zhang, L.; Zou, G.; Liu, R.; Jiang, Y.; Zhou, Z. RNA Sequencing Reveals Xyr1 as a Transcription Factor Regulating Gene Expression beyond Carbohydrate Metabolism. Biomed. Res. Int. 2016, 2016, 4841756. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Guo, M.Y.; Gao, Y.H.; Bai, X.H.; Zhou, X.W. Expression and characteristics of manganese peroxidase from Ganoderma lucidum in Pichia pastoris and its application in the degradation of four dyes and phenol. BMC Biotechnol. 2017, 17, 19. [Google Scholar] [CrossRef]
- Korripally, P.; Hunt, C.G.; Houtman, C.J.; Jones, D.C.; Kitin, P.J.; Cullen, D.; Hammel, K.E. Regulation of gene expression during the onset of ligninolytic oxidation by Phanerochaete chrysosporium on spruce wood. Appl. Environ. Microbiol. 2015, 81, 7802–7812. [Google Scholar] [CrossRef]
- Liu, Z.H.; Le, R.K.; Kosa, M.; Yang, B.; Yuan, J.; Ragauskas, A.J. Identifying and creating pathways to improve biological lignin valorization. Renew. Sustain. Energy Rev. 2019, 105, 349–362. [Google Scholar] [CrossRef]
- Minami, M.; Suzuki, K.; Shimizu, A.; Hongo, T.; Sakamoto, T.; Ohyama, N.; Kitaura, H.; Kusaka, A.; Iwama, K.; Irie, T. Changes in the gene expression of the white rot fungus Phanerochaete chrysosporium due to the addition of atropine. Biosci. Biotechnol. Biochem. 2009, 73, 1722–1731. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hernández-Domínguez, E.M.; Castillo-ortega, L.S.; García-esquivel, Y.; Mandujano-gonzález, V.; Díaz-godínez, G.; Álvarez-cervantes, J. Bioinformatics as a Tool for the Structural and Evolutionary Analysis of Proteins. In Computational Biology and Chemistry; Behzadi, P., Bernabò, N., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Merino, G.A.; Fresno, C.; Netto, F.; Netto, E.D.; Pratto, L.; Fernández, E.A. The impact of quality control in RNA-seq experiments. J. Phys. Conf. Ser. 2016, 705, 012003. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Li, W. RSeQC: Quality control of RNA-seq experiments. Bioinformatics 2012, 28, 2184–2185. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Schlapbach, R.; Rehrauer, H. RNA-Seq Data Analysis: From Raw Data Quality Control to Differential Expression Analysis. Methods Mol. Biol. 2017, 1669, 295–307. [Google Scholar] [CrossRef]
- Zhou, Q.; Su, X.; Jing, G.; Chen, S.; Ning, K. RNA-QC-chain: Comprehensive and fast quality control for RNA-Seq data. BMC Genom. 2018, 19, 144. [Google Scholar] [CrossRef] [PubMed]
- Schaarschmidt, S.; Fischer, A.; Zuther, E.; Hincha, D.K. Evaluation of Seven Di ff erent RNA-Seq Alignment Tools Based on Experimental Data from the Model Plant Arabidopsis thaliana. Int. J. Mol. Sci. 2020, 21, 1720. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Gingeras, T.R. Optimizing RNA-Seq Mapping with STAR. In Data Mining Techniques for the Life Sciences; Carugo, O., Eisenhaber, F., Eds.; Springer Science+Business Media: New York, NY, USA, 2016; Volume 1415, pp. 245–262. ISBN 9781493935727. [Google Scholar]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Philip, D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Macmanes, M.D.; et al. Reference generation and analysis with Trinity. Nat. Protoc. 2014, 8, 1494–1512. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Jin, H.; Wan, Y.; Liu, Z. Comprehensive evaluation of RNA-seq quantification methods for linearity. BMC Bioinform. 2017, 18, 51–59. [Google Scholar] [CrossRef]
- Conesa, A.; Madrigal, P.; Tarazona, S.; Gomez-cabrero, D.; Cervera, A.; Mcpherson, A.; Szcze, W.; Gaffney, D.J.; Elo, L.L.; Zhang, X.; et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016, 17, 13. [Google Scholar] [CrossRef]
- Assefa, A.T.; De Paepe, K.; Everaert, C.; Mestdagh, P.; Thas, O.; Vandesompele, J. Differential gene expression analysis tools exhibit substandard performance for long non-coding RNA-sequencing data. Genome Biol. 2018, 19, 96. [Google Scholar] [CrossRef]
- Wang, C.; Schröder, M.S.; Hammel, S.; Butler, G. Using RNA-seq for Analysis of Differential Gene Expression in Fungal Species. In Methods in Molecular Biology; Devaux, F., Ed.; Springer Science+Business Media: New York, NY, USA, 2016; Volume 1361, pp. 1–40. ISBN 9781493930791. [Google Scholar]
- Pawlik, A.; Mazur, A.; Wielbo, J.; Koper, P.; Zebracki, K.; Kubik-Komar, A.; Janusz, G. RNA Sequencing Reveals Differential Gene Expression of Cerrena Unicolor in Response to Variable Lighting Conditions. Int. J. Mol. Sci. 2019, 20, 290. [Google Scholar] [CrossRef]
- Wang, Y.C.; Peterson, S.E.; Loring, J.F. Protein post-translational modifications and regulation of pluripotency in human stem cells. Cell Res. 2014, 24, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Basit, M.; Nisar, M.A.; Khurshid, M.; Rasool, M.H. Proteomics: Technologies and their applications. J. Chromatogr. Sci. 2017, 55, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Graves, P.R.; Haystead, T.A.J. Molecular Biologist’s Guide to Proteomics. Microbiol. Mol. Biol. Rev. 2002, 66, 39–63. [Google Scholar] [CrossRef]
- Amiri-dashatan, N.; Koushki, M.; Abbaszadeh, H.; Rostami-nejad, M.; Rezaei-Tavirani, M. Proteomics Applications in Health: Biomarker and Drug Discovery and Food Industry. Iran. J. Pharm. Res. 2018, 17, 1523–1536. [Google Scholar] [PubMed]
- Yokota, H. Applications of proteomics in pharmaceutical research and development. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 17–21. [Google Scholar] [CrossRef]
- Champer, J.; Ito, J.I.; Clemons, K.V.; Stevens, D.A.; Kalkum, M. Proteomic Analysis of Pathogenic Fungi Reveals Highly Expressed Conserved Cell Wall Proteins. J. Fungi 2016, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Quecine, M.C.; Leite, T.F.; Bini, A.P.; Regiani, T.; Franceschini, L.M.; Budzinski, I.G.F.; Marques, F.G.; Labate, M.T.V.; Guidetti-Gonzalez, S.; Moon, D.H.; et al. Label-free quantitative proteomic analysis of Puccinia psidii uredospores reveals differences of fungal populations infecting eucalyptus and guava. PLoS ONE 2016, 11, e0145343. [Google Scholar] [CrossRef]
- Bianco, L.; Perrotta, G. Methodologies and perspectives of proteomics applied to filamentous fungi: From sample preparation to secretome analysis. Int. J. Mol. Sci. 2015, 16, 5803–5829. [Google Scholar] [CrossRef] [PubMed]
- Ball, B.; Bermas, A.; Carruthers-lay, D.; Geddes-McAlister, J. Mass Spectrometry-Based Proteomics of Fungal Pathogenesis, Host—Fungal Interactions, and Antifungal Development. J. Fungi 2019, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.D.; Brown, A.J.P. Posttranslational modifications of proteins in the pathobiology of medically relevant fungi. Eukaryot. Cell 2012, 11, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Ramazi, S.; Zahiri, J. Posttranslational modifications in proteins: Resources, tools and prediction methods. Database 2021, 2021, baab012. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhong, C.; Xiao, H. Genetic Engineering of Filamentous Fungi for Efficient Protein Expression and Secretion. Front. Bioeng. Biotechnol. 2020, 8, 293. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Gai, J.; Tian, X.; Zhang, X.; Lv, Y.; Jian, Y. Evolution of protein N-glycosylation process in Golgi apparatus which shapes diversity of protein N-glycan structures in plants, animals and fungi. Sci. Rep. 2017, 7, 40301. [Google Scholar] [CrossRef]
- Goto, M. Protein O-glycosylation in fungi: Diverse structures and multiple functions. Biosci. Biotechnol. Biochem. 2007, 71, 1415–1427. [Google Scholar] [CrossRef]
- Deshpande, N.; Wilkins, M.R.; Packer, N.; Nevalainen, H. Protein glycosylation pathways in filamentous fungi. Glycobiology 2008, 18, 626–637. [Google Scholar] [CrossRef]
- Tokmakov, A.A.; Kurotani, A.; Takagi, T.; Toyama, M.; Shirouzu, M.; Fukami, Y.; Yokoyama, S. Multiple post-translational modifications affect heterologous protein synthesis. J. Biol. Chem. 2012, 287, 27106–27116. [Google Scholar] [CrossRef] [PubMed]
- Cruz, E.R.; Nguyen, H.; Nguyen, T.; Wallace, I.S. Functional analysis tools for post-translational modification: A post-translational modification database for analysis of proteins and metabolic pathways. Plant J. 2019, 99, 1003–1013. [Google Scholar] [CrossRef]
- Rugabber, T.P.; Talley, J.W. Enhancing bioremediation with enzymatic processes: A review. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2006, 10, 73–85. [Google Scholar] [CrossRef]
- Ellouze, M.; Sayadi, S. White-Rot Fungi and their Enzymes as a Biotechnological Tool for Xenobiotic Bioremediation. In Management of Hazardous Wastes; IntechOpen: London, UK, 2016; pp. 103–120. [Google Scholar]
- Arnau, J.; Yaver, D.; Hjort, C.M. Strategies and Challenges for the Development of Industrial Enzymes Using Fungal Cell Factories. In Grand Challenges in Biology and Biotechnology; Nevalainen, H., Ed.; Springer Nature: Basel, Switzerland, 2020; pp. 179–210. ISBN 9783030295417. [Google Scholar]
- Duzenli, O.F.; Okay, S. Promoter engineering for the recombinant protein production in prokaryotic systems. AIMS Bioeng. 2020, 7, 62–81. [Google Scholar] [CrossRef]
- Sevillano, L.; Vijgenboom, E.; van Wezel, G.P.; Díaz, M.; Santamaría, R.I. New approaches to achieve high level enzyme production in Streptomyces lividans. Microb. Cell Fact. 2016, 15, 28. [Google Scholar] [CrossRef]
- Che, Z.; Cao, X.; Chen, G.; Liang, Z. An effective combination of codon optimization, gene dosage, and process optimization for high-level production of fibrinolytic enzyme in Komagataella phaffii (Pichia pastoris). BMC Biotechnol. 2020, 20, 63. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, M.W.; Siddique, R.; Nabi, G.; Manan, S.; Yousaf, M.; Hou, H. Role of recombinant DNA technology to improve life. Int. J. Genom. 2016, 2016, 2405954. [Google Scholar] [CrossRef]
- Gupta, V.; Sengupta, M.; Prakash, J.; Tripathy, B.C. Production of Recombinant Pharmaceutical Proteins. In Basic and Applied Aspects of Biotechnology; Springer Science+Business Media: Singapore, 2017; pp. 77–100. ISBN 9789811008757. [Google Scholar]
- Gifre, L.; Arís, A.; Bach, À.; Garcia-Fruitós, E. Trends in recombinant protein use in animal production. Microb. Cell Fact. 2017, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.K.; Shrivastava, A. Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development. Front. Bioeng. Biotechnol. 2019, 7, 420. [Google Scholar] [CrossRef] [PubMed]
- von Schaewen, A.; Jeong, I.S.; Rips, S.; Fukudome, A.; Tolley, J.; Nagashima, Y.; Fischer, K.; Kaulfuerst-Soboll, H.; Koiwa, H. Improved recombinant protein production in Arabidopsis thaliana. Plant Signal. Behav. 2018, 13, e1486149. [Google Scholar] [CrossRef]
- Jia, B.; Jeon, C.O. High-throughput recombinant protein expression in Escherichia coli: Current status and future perspectives. Open Biol. 2016, 6, 160196. [Google Scholar] [CrossRef]
- Nishikawa, R.; Yoshida, M.; Noda, T.; Okuhara, T.; Taguchi, G.; Inatomi, S.; Shimosaka, M. pFungiway: A series of plasmid vectors used for gene manipulation in fungi. Ann. Microbiol. 2016, 66, 825–832. [Google Scholar] [CrossRef]
- Hartley, J.L. Cloning technologies for protein expression and purification. Curr. Opin. Biotechnol. 2006, 17, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Rocco, C.J.; Dennison, K.L.; Klenchin, V.A.; Rayment, I.; Escalante-Semerena, J.C. Construction and use of new cloning vectors for the rapid isolation of recombinant proteins from Escherichia coli. Plasmid 2008, 59, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Hohnholz, R.; Pohlmann, K.J.; Achstetter, T. A set of isomeric episomal plasmids for systematic examination of mitotic stability in Saccharomyces cerevisiae. Yeast 2017, 34, 267–275. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; Choksi, A.; Smolke, C.D. A system for multi-locus chromosomal integration and transformation-free selection marker rescue. FEMS Yeast Res. 2014, 14, 1171–1185. [Google Scholar] [CrossRef]
- Amen, T.; Kaganovich, D. Integrative modules for efficient genome engineering in yeast. Microb. Cell 2017, 4, 182–190. [Google Scholar] [CrossRef]
- Falcon, A.A.; Rios, N.; Aris, J.P. 2-micron circle plasmids do not reduce yeast life span. FEMS Microbiol. Lett. 2005, 250, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Żymańczyk-Duda, E.; Brzezińska-Rodak, M.; Klimek-Ochab, M.; Duda, M.; Zerka, A. Yeast as a Versatile Tool in Biotechnology. In Yeast-Industrial Applications; Morata, A., Loira, I., Eds.; IntechOpen: London, UK, 2017; pp. 3–40. [Google Scholar]
- Gnügge, R.; Rudolf, F. Saccharomyces cerevisiae Shuttle vectors. Yeast 2017, 34, 205–221. [Google Scholar] [CrossRef]
- Larionov, V.; Kouprina, N.; Graves, J.; Resnick, M.A. Highly selective isolation of human DNAs from rodent-human hybrid cells as circular yeast artificial chromosomes by transformation-associated recombination cloning. Proc. Natl. Acad. Sci. USA 1996, 93, 13925–13930. [Google Scholar] [CrossRef]
- Jung, H.M.; Kim, Y.H. Simultaneous overexpression of integrated genes by copy number amplification of a mini-yeast artificial chromosome. J. Microbiol. Biotechnol. 2018, 28, 821–825. [Google Scholar] [CrossRef]
- Alharbi, R.A. Proteomics approach and techniques in identification of reliable biomarkers for diseases. Saudi J. Biol. Sci. 2020, 27, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Syahir, A.; Usui, K.; Tomizaki, K.; Kajikawa, K.; Mihara, H. Label and Label-Free Detection Techniques for Protein Microarrays. Microarrays 2015, 4, 228–244. [Google Scholar] [CrossRef]
- Braitbard, O.; Bishara-Shieban, J.; Glickstein, H.; Kott-Gutkowski, M.; Pace, U.; Rund, D.G.; Stein, W.D. An ELISA-based procedure for assaying proteins in digests of human leukocytes and cell lines, using specifically selected peptides and appropriate antibodies. Proteome Sci. 2006, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Jellison, J.; Goodell, B.; Tracy, V.; Chandhoke, V. The Use of ELISA for the Detection of White- and Brown-Rot Fungi. Holzforschung 1991, 45, 403–406. [Google Scholar] [CrossRef]
- Martin-Souto, L.; Buldain, I.; Areitio, M.; Aparicio-Fernandez, L.; Antoran, A.; Bouchara, J.P.; Martin-Gomez, M.T.; Rementeria, A.; Hernando, F.L.; Ramirez-Garcia, A. ELISA Test for the Serological Detection of Scedosporium/Lomentospora in Cystic Fibrosis Patients. Front. Cell. Infect. Microbiol. 2020, 10, 602089. [Google Scholar] [CrossRef]
- Santana, A.E.; Taborda, C.P.; Severo, J.S.; Gomes Rittner, G.M.; Muñoz, J.E.; Larsson, C.E.; Larsson, C.E. Development of enzyme immunoassays (ELISA and Western blot) for the serological diagnosis of dermatophytosis in symptomatic and asymptomatic cats. Med. Mycol. 2018, 56, 95–102. [Google Scholar] [CrossRef]
- Kim, Y.; Bigelow, L.; Borovilos, M.; Dementieva, I.; Duggan, E.; Hatzos, C.; Joachimiak, G.; Li, H.; Maltseva, N.; Mulligan, R.; et al. High-Throughput Protein Purification for X-Ray Crystallography and NMR. Adv. Protein Chem. Struct. Biol. 2008, 75, 85–105. [Google Scholar] [CrossRef] [PubMed]
- Thiemann, J.; Jankowski, J.; Rykl, J.; Kurzawski, S.; Pohl, T.; Wittmann-Liebold, B.; Schlüter, H. Principle and applications of the protein-purification-parameter screening system. J. Chromatogr. A 2004, 1043, 73–80. [Google Scholar] [CrossRef] [PubMed]
- More, S.S.; Renuka, P.S.; Pruthvi, K.; Swetha, M.; Malini, S.; Veena, S.M. Isolation, purification, and characterization of fungal laccase from Pleurotus sp. Enzym. Res. 2011, 2011, 248735. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Banerjee, R. Purification and biochemical characterization of a newly produced yellow laccase from Lentinus squarrosulus MR13. Biotechniques 2015, 5, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Mehmood, S.; Irshad, M.; Anwar, Z. Optimized production, purification and molecular characterization of fungal laccase through Alternaria alternata. Turk. J. Biochem. 2018, 43, 613–622. [Google Scholar] [CrossRef]
- Carrasco, M.; Alcaíno, J.; Cifuentes, V.; Baeza, M. Purification and characterization of a novel cold adapted fungal glucoamylase. Microb. Cell Fact. 2017, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Duong-Ly, K.C.; Gabelli, S.B. Salting out of proteins using ammonium sulfate precipitation. In Methods in Enzymology; Elsevier Inc.: Baltimore, MD, USA, 2014; Volume 541, pp. 85–94. ISBN 9780124201194. [Google Scholar]
- Bhat, E.; Abdalla, M.; Rather, I. Key Factors for Successful Protein Purification and Crystallization. Glob. J. Biotechnol. Biomater. Sci. 2018, 4, 001–007. [Google Scholar] [CrossRef]
- Ó’Fágáin, C.; Cummins, P.M.; O’Connor, B.F. Gel-Filtration Chromatography. In Protein Chromatography; Walls, D., Loughran, S.T., Eds.; Springer Science+Business Media: New York, NY, USA, 2017; Volume 1485, pp. 15–25. ISBN 9781493964109. [Google Scholar]
- Acikara, Ö.B. Ion-Exchange Chromatography and Its Applications. In Column Chromatography; IntechOpen: London, UK, 2013; pp. 31–58. [Google Scholar]
- Kosanović, M.; Milutinović, B.; Goč, S.; Mitić, N.; Janković, M. Ion-Exchange Chromatography Purification of Extracellular Vesicles. Biotechniques 2017, 63, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.L.; Poddar, S.; Iftekhar, S.; Suh, K.; Woolfork, A.G.; Ovbude, S.; Pekarek, A.; Walters, M.; Lott, S.; Hage, D.S. Affinity chromatography: A review of trends and developments over the past 50 years. J. Chromatogr. B 2020, 122332. [Google Scholar] [CrossRef] [PubMed]
- Mant, C.T.; Chen, Y.; Yan, Z.; Popa, T.V.; Kovacs, J.M.; Mills, J.B.; Tripet, B.P.; Hodges, R.S. HPLC analysis and purification of peptides. In Methods in Molecular Biology; Fields, G., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2007; Volume 386, pp. 3–55. [Google Scholar]
- Encarnación, S.; Hernández, M.; Martínez-Batallar, G.; Contreras, S.; del Carmen Vargas, M.; Mora, J. Comparative proteomics using 2-D gel electrophoresis and mass spectrometry as tools to dissect stimulons and regulons in bacteria with sequenced or partially sequenced genomes. Biol. Proced. Online 2005, 7, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.L.C.; Balbuena, T.S.; Silveira, V.; Santa-Catarina, C.; Schevchenko, A.; Floh, E.I.S. Two-dimensional gel electrophoretic protein profile analysis during seed development of Ocotea catharinensis: A recalcitrant seed species. Braz. J. Plant Physiol. 2010, 22, 23–33. [Google Scholar] [CrossRef]
- Najmanovich, R.J.; Torrance, J.W.; Thornton, J.M. Prediction of protein function from structure: Insights from methods for the detection of local structural similarities. Biotechniques 2005, 38, 847–851. [Google Scholar] [CrossRef]
- Yee, A.A.; Savchenko, A.; Ignachenko, A.; Lukin, J.; Xu, X.; Skarina, T.; Evdokimova, E.; Liu, C.S.; Semesi, A.; Guido, V.; et al. NMR and X-ray crystallography, complementary tools in structural proteomics of small proteins. J. Am. Chem. Soc. 2005, 127, 16512–16517. [Google Scholar] [CrossRef]
- Bryn Fenwick, R.; Van Den Bedem, H.; Fraser, J.S.; Wright, P.E. Integrated description of protein dynamics from room-temperature X-ray crystallography and NMR. Proc. Natl. Acad. Sci. USA 2014, 111, E445–E454. [Google Scholar] [CrossRef]
- Chen, C.; Huang, H.; Wu, C.H. Protein Bioinformatics Databases and Resources. Methods Mol. Biol. 2017, 1558, 3–39. [Google Scholar] [CrossRef]
- Mills, C.L.; Beuning, P.J.; Ondrechen, M.J. Biochemical functional predictions for protein structures of unknown or uncertain function. Comput. Struct. Biotechnol. J. 2015, 13, 182–191. [Google Scholar] [CrossRef]
- Miyashita, M.; Presley, J.M.; Buchholz, B.A.; Lam, K.S.; Lee, Y.M.; Vogel, J.S.; Hammock, B.D. Attomole level protein sequencing by Edman degradation coupled with accelerator mass spectrometry. Proc. Natl. Acad. Sci. USA 2001, 98, 4403–4408. [Google Scholar] [CrossRef] [PubMed]
- Steen, H.; Mann, M. The ABC’s (and XYZ’s) of peptide sequencing. Nat. Rev. Mol. Cell Biol. 2004, 5, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Restrepo-Pérez, L.; Joo, C.; Dekker, C. Paving the way to single-molecule protein sequencing. Nat. Nanotechnol. 2018, 13, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Pundir, S.; Magrane, M.; Martin, M.J.; O’Donovan, C.; Consortium, T.U. Searching and navigating UniProt databases. Curr. Protoc. Bioinform. 2015, 50, 1.27.1–1.27.10. [Google Scholar] [CrossRef]
- Pruitt, K.D.; Tatusova, T.; Maglott, D.R. NCBI reference sequences (RefSeq): A curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2007, 35, 61–65. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, K.M.; Goldfarb, T.; Cox, E.; Farrell, C.M.; Gupta, T.; Joardar, V.S.; Kodali, V.K.; Murphy, M.R.; O’Leary, N.A.; Pujar, S.; et al. Mouse genome annotation by the RefSeq project. Mamm. Genome 2015, 26, 379–390. [Google Scholar] [CrossRef] [PubMed]
| Host | Vector | References |
|---|---|---|
| Yeast |
| [164] |
| [165,166] | |
| [167,168] | |
| [169] | |
| [168,170,171] | |
| Agrobacterium tumefaciens |
| [161] |
| Technique | Principle | References |
|---|---|---|
| Salting in/out | Exploiting protein solubility by increasing salt concentration in the solution | [184] |
| Dialysis | Using size exclusion to separate proteins from small molecules and ions that pass through a semi-permeable membrane | [185] |
| Gel filtration chromatography | Separation of proteins based on size | [186] |
| Ion-exchange chromatography | Separation of proteins based on their net charge | [187,188] |
| Affinity chromatography | Exploiting the affinity of proteins for given chemical groups to separate them | [189] |
| High-Pressure Liquid Chromatography (HPLC) | Can use different principles of column chromatography to separate proteins using high pressure to give better resolution | [190] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ijoma, G.N.; Heri, S.M.; Matambo, T.S.; Tekere, M. Trends and Applications of Omics Technologies to Functional Characterisation of Enzymes and Protein Metabolites Produced by Fungi. J. Fungi 2021, 7, 700. https://doi.org/10.3390/jof7090700
Ijoma GN, Heri SM, Matambo TS, Tekere M. Trends and Applications of Omics Technologies to Functional Characterisation of Enzymes and Protein Metabolites Produced by Fungi. Journal of Fungi. 2021; 7(9):700. https://doi.org/10.3390/jof7090700
Chicago/Turabian StyleIjoma, Grace N., Sylvie M. Heri, Tonderayi S. Matambo, and Memory Tekere. 2021. "Trends and Applications of Omics Technologies to Functional Characterisation of Enzymes and Protein Metabolites Produced by Fungi" Journal of Fungi 7, no. 9: 700. https://doi.org/10.3390/jof7090700
APA StyleIjoma, G. N., Heri, S. M., Matambo, T. S., & Tekere, M. (2021). Trends and Applications of Omics Technologies to Functional Characterisation of Enzymes and Protein Metabolites Produced by Fungi. Journal of Fungi, 7(9), 700. https://doi.org/10.3390/jof7090700









